Abstract
Neurocysticercosis is a heterogeneous disease, and the patient’s sex seems to play a role in this heterogeneity. Hosts’ sexual dimorphism in cysticercosis has been largely explored in the murine model of intraperitoneal Taenia crassiceps cysticercosis. In this study, we investigated the sexual dimorphism of inflammatory responses in a rat model of extraparenchymal neurocysticercosis caused by T. crassiceps. T. crassiceps cysticerci were inoculated in the subarachnoid space of Wistar rats (25 females, 22 males). Ninety days later, the rats were euthanized for histologic, immunohistochemistry, and cytokines studies. Ten animals also underwent a 7-T magnetic resonance imaging (MRI). Female rats presented a higher concentration of immune cells in the arachnoid-brain interface, reactive astrogliosis in the periventricular region, in situ pro-inflammatory cytokine (interleukin [IL]-6) and anti-inflammatory cytokine (IL-10), and more intense hydrocephalus on MRI than males. Intracranial hypertension signals were not observed during the observational period. Overall, these results suggest sexual dimorphism in the intracranial inflammatory response that accompanied T. crassiceps extraparenchymal neurocysticercosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurocysticercosis (NCC) is a common parasitic disease of the central nervous system in developing countries. Despite the development of tools and knowledge to control the disease, it remains a neglected disease with a significant burden in endemic countries. In contrast, it reappears in developed countries because of migratory flows (Bhattarai et al. 2019; Singh et al. 2017; Abraham et al. 2020). Data from Latin America suggest that the prevalence of the disease is decreasing in some countries while it persists in others (Rodríguez-Rivas et al. 2022). In Africa and parts of Asia, the situation is difficult to assess owing to the lack of diagnostic tools; however, NCC is still an important cause of seizures in these regions (Stelzle et al. 2022; Sahu et al. 2017).
NCC is a heterogeneous disease. Infected patients may remain asymptomatic or experience mild symptoms (such as headaches or single seizures) for long periods. Severe symptoms with increased intracranial pressure may appear suddenly in some patients, probably triggered by some damage caused by the parasite with the release of cysticercal antigens that may trigger an exacerbated neuroinflammation (Fleury et al. 2010). This heterogeneity is linked to several factors, such as the number and location of cysts within the central nervous system, the genetic particularities of the parasite and patients across different geographical regions, and the differences in infection pressure in different populations (Ito & Budke 2021; Hamamoto Filho et al. 2020). Regarding the location of cysts, the presence of cysts within the cerebrospinal fluid (CSF) compartments (extraparenchymal NCC) is the most severe form of the disease. It may cause vasculitis, hydrocephalus, and increased intracranial pressure. In addition, this form of disease is less responsive to medical treatment and has higher mortality rates (Fleury et al. 2011; Marcin-Sierra et al., 2017; Hamamoto Filho et al. 2019a).
Another factor contributing to NCC heterogeneity is the patient’s sex. When parasites are present in the parenchyma, the female sex is associated with more intense pericystic oedema and contrast enhancement on neuroimaging examinations, and worse outcomes (Brutto et al. 1988; Kelvin et al., 2009). Additionally, in one study including patients with parenchymal and extraparenchymal NC, higher CSF cytokine levels (interleukin [IL]-6, IL-10) were observed among women (Chavarría et al. 2005), while a higher lymphocyte CSF count was reported among female patients with extraparenchymal NC (Marcin-Sierra et al. 2017).
Hosts’ sexual dimorphism in cysticercosis has been largely explored in the murine model of intraperitoneal Taenia crassiceps cysticercosis. These studies showed that cysticerci grow in larger numbers in female mice than males. Sex steroids play a significant role in regulating these differences in the parasite load (Huerta et al. 1992; Escobedo et al. 2009). Moreover, drastic endocrinological changes occur during the infection suggesting the complexity of the host-parasite relationship (Gomez et al. 2000).
In this context, we aimed to gain further insights into sexual dimorphism in the inflammatory responses accompanying the murine extraparenchymal experimental NCC caused by T. crassiceps.
Materials and methods
Animals
Wistar rats (Rattus norvegicus, 25 females and 22 males) aged 6 to 7 weeks were used. The animals were handled according to ethical guidelines and current legislation. The research project and all protocols were approved by the Ethics Committee on the Use of Animals (CEUA) of the Botucatu Medical School (CEUA 1318/2019). During the 90 days of the experiment, the animals were kept in polyethylene boxes (40 × 30 × 15 cm), under controlled conditions of light (12 h light/12 h dark) and temperature (24 ± 2 °C), with water and food provided ad libitum. They were examined weekly for signs of illness or altered behaviour.
Experimental design
According to a previously described technique, the animals were subjected to cisternal inoculation of T. crassiceps (Hamamoto Filho et al. 2019b). Ninety days post-inoculation, the rats were euthanized to harvest the brain. Animals without visible cysts during euthanasia were discarded. By simple randomization, half of the animals were subjected to morphological analysis and immunohistochemistry, and the other half were subjected to enzyme-linked immunosorbent assay (ELISA) to measure some cytokines.
Sample size
According to a pilot study, with 5–6 animals in each experimental group, it is possible to determine statistically significant differences between the groups. As each animal was subjected to morphological analysis or measurement of inflammatory features, each group should have a minimum of 10–12 animals. Furthermore, based on our experience, considering a 66% success rate in inducing the disease, 22 animals would be needed in each experimental group. This sample size was estimated assuming simple random sampling; type I and II errors were equal to 0.05 and 0.20, respectively. Additional inoculations were performed in cases of deaths or a higher number of uninfected animals. Therefore, 25 female and 22 male rats were used.
Parasites and inoculations
Cysts of T. crassiceps (ORF strain), maintained in the peritoneal cavity of mice, were aseptically removed and selected according to their size (0.5 mm in diameter) and viability (intact membrane).
For inoculation, Wistar rats were anaesthetized with ketamine (87 mg/kg) and xylazine (13 mg/kg). Inoculation was performed after a 1-cm skin incision at the occipito-cervical transition, with the suboccipital puncture of the cisterna magna using a 24-G needle and inoculation of 50 viable T. crassiceps cysts in 0.2 ml of saline. The skin was then sutured with 4.0 mononylon thread.
Histological analysis
After euthanasia with an overdose of ketamine and intraperitoneal xylazine, cardiac perfusion was performed with a peristaltic pump for perfusion and fixation (30 ml/min), initially with 0.9% saline and then 4% paraformaldehyde. Subsequently, the brains were removed and sectioned in the transverse plane in the topography of the optic chiasm with a brain matrix. The sections were kept in paraformaldehyde overnight at room temperature and dehydrated in increasing concentrations of alcohol baths, clarified in xylene, and paraffinized.
The paraffin blocks were sectioned at 5-μm slices stained with haematoxylin and eosin. Morphometric analysis was performed to quantify immune cells (lymphocytes and plasmacytes) in the basal arachnoid and periventricular regions (striatum and lateral septal nucleus).
The glial fibrillary acid protein (GFAP) expression level was estimated by immunohistochemistry to evaluate astrocytes’ activation within the periventricular zone. The blocks were sectioned at 3-μm slices, and the slices were dewaxed in xylene and rehydrated in decreasing concentrations of alcohol. For antigen recovery, the slides were heated at 98 °C with citrate buffer (pH 6.0) for 30 min and then incubated in 0.3% hydrogen peroxide at room temperature for endogenous tissue peroxidase block. Furthermore, they were washed with Tris-buffered saline/Tween 20 (TBST), pH 7.5, and incubated with primary antibodies against GFAP (mouse monoclonal IgG1 [2A5] to GFAP, Abcam, Cambridge, MA, USA) in a humid chamber at room temperature overnight. The slides were washed again with TBST, incubated with a secondary peroxidase horseradish polymer-conjugated antibody for 30 min at room temperature, washed with TBST, incubated with 3,3′ diaminobenzidine stain for 5 min, and counterstained with haematoxylin.
The morphometric analysis was based on Weibel counting reticle (Weibel et al. 1966), a mathematical model that quantitatively transforms two-dimensional to three-dimensional acquisition. The studied structures intersecting with the reticle lines were counted and divided by the total number of lines.
Cytokine measurement
After euthanasia with an overdose of xylazine and ketamine intraperitoneally, a craniectomy was performed to remove the brains, which were then transported in liquid nitrogen and frozen at −80 °C. The brains were thawed under temperature control, and the cerebral hemispheres were dissected. The latter were then sonicated with a solution of Tris and HCl, 1% NP40, and protease inhibitor. IL-6, IL-10, and interferon-gamma (IFN-γ) levels were quantified in the homogenized solution of brain tissues using the sandwich ELISA according to the manufacturer’s instructions (BD OptEIATM). IL-6 and IL-10 expression levels were determined using IL-6 ELISA - BD OptEIATM (BD Biosciences, San Diego, CA) and IL-10 ELISA - BD OptEIATM, respectively. IFN-γ assay was performed using high-binding ELISA microplates sensitized with 80 μl of IFN-γ monoclonal antibody (5 μg/μl of clone XMG in phosphate-buffered saline). The measurement was determined using a Thermo/Labsystems microplate reader with specific filters for each cytokine. The assays were performed in duplicate.
Magnetic resonance imaging
For illustrative purposes, five male and five female rats were randomly selected to undergo magnetic resonance imaging (MRI) to assess ventricle enlargement. The animals were anaesthetized with 1.5% inhaled isoflurane. MRI was performed using 7-T equipment (Siemens Magnetom scanner, Siemens, Erlanger, Germany), with a T2-TSE sequence (parameters: plane resolution = 270 μm, FOV = 64 × 128 mm, matrix = 240 × 480 mm, thickness = 700 μm, TR/TE = 6000/60 ms, eco train length = 5, total time of acquisition = 4 min 43 s). A normal ventricle size was considered at 4.5 mm of the distance between the frontal horns of the lateral ventricles at the level of the interventricular foramen. Mild ventricle dilatation was considered for a distance between 4.5 and 5.5 mm, and severe dilatation was considered when the distance was higher than 5.5 mm.
Statistical analysis
To determine the normality of the data, the Shapiro–Wilk test was used. The Mann–Whitney test was used to compare two independent groups with non-parametric data. For variables with normal distribution, Student’s t-test was used to compare the groups. Differences with p < 0.05 were considered statistically significant. Statistical Package for the Social Sciences (SPSS) v. 24.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism v. 8.2.0 (GraphPad Software, La Jolla, CA, USA) were used for the analyses.
Results
Experimental infection
Based on the observation of cysts during necropsy, 13/22 animals developed neurocysticercosis (59.1%) in the male group, and 15/25 animals developed neurocysticercosis (60%) in the female group. Seven males and eight females were used for morphological study, and six males and seven females were used to measure cytokine levels. Throughout the observation period, no animal developed signs of illness, discomfort, or abnormal behaviour.
Female rats presented more inflammatory infiltrate in the arachnoid than male rats
On histologic examination, lymphocytes and plasmacytes infiltrating the basal arachnoid region (near the optic chiasm) and periventricular zone (nearby the basal ganglia) were observed (Fig. 1). The ratio of lymphocytes/μm2 was higher among female than male rats in the arachnoid (p=0.023), but there was no statistically significant difference in the periventricular zone (Fig. 2). The ratio of plasmacytes/area was not different between the groups either in the arachnoid or the periventricular zone (p = 0.093 and 0.102, respectively). The ratio of reactive astrocytes (immunopositive cells) in the periventricular zone was also higher in the female group, with a statistically significant difference (p=0.001).
Inflammatory infiltrates (dashed lines) within the arachnoid-brain interface (A, B) and the periventricular zone (C, D). A higher number of inflammatory cells were observed in the female rats. In the periventricular zone, there was also a higher immune positivity for GFAP (arrowheads, E, F) in female rats. Forty times magnification
Comparison of histologic patterns between the groups of male and female rats. The ratio of lymphocytes/μm2 within the basal arachnoid-brain interface was higher in the female group (A). The ratio of lymphocytes/μm2 within the periventricular zone (B) and the ratio of plasmocytes/μm2 in the arachnoid-brain interface (C) and the periventricular zone (D) did not reach a statistically significant difference. The immune positivity of astrocytes for GFAP was higher in the female group (E)
IL-6 and IL-10 levels were significantly higher in females than in male rats
The levels of IL-6, IL-10, and IFN-γ are presented in Table 1. A significantly higher level of IL-6 and IL-10 was observed in females than in males. The numerical mean level of IFN-γ was also higher in females, but the difference was not statistically significant.
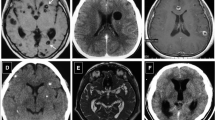
Detection of hydrocephalus (ventricle enlargement) was more frequent in females
On MRI, marked ventricle enlargement was observed in 2/5 males (40%) and 3/5 females (60%). No ventricle enlargement was observed in 2/5 males (40%) and 1/5 females (20%, Fig. 3).
Discussion
This study presents the first evidence of sexual dimorphism in the neuroinflammation accompanying murine experimental extraparenchymal NCC (ExP-NCC). In humans, some differences between men and women in ExP-NCC have been previously reported, particularly the higher cellularity in the cerebral spinal fluid in women (Marcin-Sierra et al. 2017). However, it is difficult to describe intracranial inflammation in humans except for autopsy studies. In contrast, in experimental models, it is possible to evaluate the presence of inflammatory cells in different parts of the central nervous system using histopathological analysis, the status of astrocytes, and the profile of pro- and anti-inflammatory cytokines (Alvarez et al. 2010; Moura et al. 2016; de Lange et al. 2019; Sitali et al. 2022). In our study, we observed that the inflammatory reaction associated with subarachnoid infection with T. crassiceps was more severe in female than in male rats. Female rats presented a higher concentration of immune cells in the arachnoid, higher reactive astrogliosis in the periventricular region, higher in situ pro-inflammatory cytokine (IL-6) and anti-inflammatory cytokine (IL-10), and more intense hydrocephalus on MRI. The higher inflammatory profile in females resembles what was reported in human NCC (Fleury et al. 2004). This finding should be further explored in ExP-NCC in humans. In addition, these findings provide new alternatives to modulate neuroinflammation through hormones that can be systematically explored using the experimental model of ExP-NCC.
There was no significant difference in inflammatory cells in the periventricular zone. This is probably because the cysts were placed in the subarachnoid space without direct contact with the ventricles. We have previously observed the migration of subarachnoid cysts to the ventricles in our model (Hamamoto Filho et al. 2017); however, this was not observed in the present study.
The observation of more intense immune positivity to GFAP, i.e. reactive astrogliosis, in the periventricular area in female rats is noteworthy. GFAP is overexpressed in animals with hydrocephalus according to the degree of ventricle enlargement (Del Bigio et al., 2003; Santos et al. 2016). In addition to the MRI results in 10 animals, this finding suggests that hydrocephalus is more intense in females than males, probably due to the more intense inflammatory reaction.
Higher levels of IL-6 were observed in females, demonstrating a more pro-inflammatory pattern. Accordingly, IL-10 levels increased in females to control the inflammatory reaction (Sciutto et al. 2013). This mixed response may be related to endocrinal regulation, since cytokine production can be regulated by hormones, with direct implications to central nervous system diseases (Bornstein et al. 2004; Morales-Montor & Larralde 2005; Członkowska et al. 2006). However, the levels of IFN-γ were not different between the groups. Future studies with larger setting of cytokines may help understand the balance of pro- and anti-inflammatory cytokines involved in this infection model. The cytokine profile accompanying this murine model closely resembles that reported in severe human ExP-NCC (Chavarría et al. 2005). This is another similarity between a human and murine infection that consolidates the use of this model to study the modulation of neuroinflammation.
Regarding the clinical consequences of the infection, we did not observe any signs consistent with intracranial hypertension, such as abnormal behaviour, malaise, or anorexia/vomiting, throughout the study period. However, we have previously demonstrated that this model of neurocysticercosis-induced hydrocephalus requires a long time to cause changes in behavioural patterns (Hamamoto Filho et al. 2019b). A longer follow-up period can verify whether females will present more intracranial hypertension symptoms than males.
This sexual dimorphism of inflammation in extraparenchymal neurocysticercosis seems to be related to the endocrine status, particularly the steroid levels. Experimentally, it has been proposed that cysticerci may use steroids to improve their ability to synthesize androgens and oestrogens, thereby improving their reproductive capacity (Toledo et al. 2018; Romano et al. 2015; Hinojosa et al. 2012). Furthermore, in vivo studies with T. crassiceps demonstrated that the cysts may cause changes in the host’s neuroimmunoendocrine profile as a survival strategy (Morales-Montor & Larralde 2005; Arteaga-Silva et al. 2009; Nava-Castro et al. 2022). Recently, sexual hormones were shown to change the expression and morphology of T. crassiceps’ flame cells, thereby determining the survival (oestrogens and progesterone) or death (androgens) of the parasites (Ambrosio et al. 2014; Ambrosio et al. 2015).
As a limitation of the present study, it is worth mentioning that we did not include a sham group to exclude the possible effect of the surgical procedure on inflammation, even though it was not observed previously (Hamamoto Filho et al. 2019b). Besides, future investigations with systematic evaluation of the cysts’ developmental stages (viable, degenerating, or dead) will provide relevant information about the characteristics of inflammatory infiltrates, as well as the role of intrinsic differences between male and female brain structures on the susceptibility to infection.
In conclusion, inflammatory responses are more severe in female than male rats infected with T. crassiceps in the subarachnoid space. Sexual dimorphism is a critical variable in infectious diseases, and a better comprehension of this difference is a critical step for developing personalized approaches (Gay et al. 2021; Wesołowska 2022). The mechanisms underlying sexual dimorphism in the neuroinflammation accompanying murine ExP-NCC, including the relevance of endocrinological components, will be further studied to identify new therapeutic targets for immunomodulation. This is particularly important considering that the potent steroid anti-inflammatory agents currently used to treat patients with ExP-NCC decrease the effectiveness of the cysticidal treatment in approximately 70% of the patients.
References
Abraham A, Schmidt V, Kaminski M, Stelzle D, De Meijere R, Bustos J, Sahu PS, Garcia HH, Bobić B, Cretu C, Chiodini P, Deksne G, Dermauw V, Devleesschauwer B, Dorny P, Fonseca A, Gabriël S, Gómez-Morales MA, Kucsera I et al (2020) Epidemiology and surveillance of human (neuro)cysticercosis in Europe: is enhanced surveillance required? Trop Med Int Health 5:566–578. https://doi.org/10.1111/tmi.13384
Alvarez JI, Mishra BB, Gundra UM, Mishra PK, Teale JM (2010) Mesocestoides corti intracranial infection as a murine model for neurocysticercosis. Parasitol 137(3):359–372. https://doi.org/10.1017/S0031182009991971
Ambrosio JR, Ostoa-Saloma P, Palacios-Arreola MI, Ruíz-Rosado A, Sánchez-Orellana PL, Reynoso-Ducoing O, Nava-Castro KE, Martínez-Velázquez N, Escobedo G, Ibarra-Coronado EG, Valverde-Islas L, Morales-Montor J (2014) Oestradiol and progesterone differentially alter cytoskeletal protein expression and flame cell morphology in Taenia crassiceps. Int J Parasitol 44(10):687–696. https://doi.org/10.1016/j.ijpara.2014.04.004
Ambrosio JR, Valverde-Islas L, Nava-Castro KE, Palacios-Arreola MI, Ostoa-Saloma P, Reynoso-Ducoing O, Escobedo G, Ruíz-Rosado A, Dominguez-Ramírez L, Morales-Montor J (2015) Androgens exert a cysticidal effect upon Taenia crassiceps by disrupting flame cell morphology and function. PLoS One 10(6):e0127928. https://doi.org/10.1371/journal.pone.0127928
Arteaga-Silva M, Vargas-Villavicencio JA, Vigueras-Villaseñor RM, Rodríguez-Dorantes M, Morales-Montor J (2009) Taenia crassiceps infection disrupts estrous cycle and reproductive behavior in BALB/c female mice. Acta Trop 109(2):141–145. https://doi.org/10.1016/j.actatropica.2008.10.011
Bhattarai R, Carabin H, Proaño JV, Flores-Rivera J, Corona T, Flisser A, León-Maldonado L, Budke CM (2019) The monetary burden of cysticercosis in Mexico. PLoS Negl Trop Dis 13(7):e0007501. https://doi.org/10.1371/journal.pntd.0007501
Bornstein SR, Rutkowski H, Vrezas I (2004) Cytokines and steroidogenesis. Mol Cell Endocrinol 215(1-2):135–141. https://doi.org/10.1016/j.mce.2003.11.022
Brutto OHD, García E, Talámas O, Sotelo J (1988) Sex-related severity of inflammation in parenchymal brain cysticercosis. Arch Intern Med 148(3):544–546. https://doi.org/10.1001/archinte.148.3.544
Chavarría A, Fleury A, García E, Márquez C, Fragoso G, Sciutto E (2005) Relationship between the clinical heterogeneity of neurocysticercosis and the immune-inflammatory profiles. Clin Immunol 116(3):271–278. https://doi.org/10.1016/j.clim.2005.04.008
Członkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I (2006) Gender differences in neurological disease: role of estrogens and cytokines. Endocr 29(2):243–256. https://doi.org/10.1385/ENDO:29:2:243
de Lange A, Mahanty S, Raimondo JV (2019) Model systems for investigating disease processes in neurocysticercosis. Parasitol 146(5):553–562. https://doi.org/10.1017/S0031182018001932
Del Bigio MR, Wilson MJ, Enno T (2003) Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann Neurol 53(3):337–346. https://doi.org/10.1002/ana.1
Escobedo G, López-Griego L, Morales-Montor J (2009) Neuroimmunoendocrine modulation in the host by helminth parasites: a novel form of host-parasite coevolution? Neuroimmunomodulat 16(2):78–87. https://doi.org/10.1159/000180262
Fleury A, Dessein A, Preux PM, Dumas M, Tapia G, Larralde C, Sciutto E (2004) Symptomatic human neurocysticercosis--age, sex and exposure factors relating with disease heterogeneity. J Neurol 251(7):830–837
Fleury A, Escobar A, Fragoso G, Sciutto E, Larralde C (2010) Clinical heterogeneity of human neurocysticercosis results from complex interactions among parasite, host and environmental factors. Trans R Soc Trop Med Hyg 104(4):243–250. https://doi.org/10.1016/j.trstmh.2010.01.005
Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T (2011) Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Rev Anti Infect Ther 9(1):123–133. https://doi.org/10.1586/eri.10.150
Gay L, Melenotte C, Lakbar I, Mezouar S, Devaux C, Raoult D, Bendiane MK, Leone M, Mège JL (2021) Sexual dimorphism and gender in infectious diseases. Front Immunol 22(12):698121. https://doi.org/10.3389/fimmu.2021.698121
Gomez Y, Valdez RA, Larralde C, Romano MC (2000) Sex steroids and parasitism: Taenia crassiceps cisticercus metabolizes exogenous androstenedione to testosterone in vitro. J Steroid Biochem Mol Biol 74(3):143–147. https://doi.org/10.1016/s0960-0760(00)00099-6
Hamamoto Filho PT, Fabro AT, Rodrigues MV, Bazan R, Vulcano LC, Biondi GF, Zanini MA (2017) Taenia crassiceps injection into the subarachnoid space of rats simulates radiological and morphological features of racemose neurocysticercosis. Childs Nerv Syst 33(1):119–123
Hamamoto Filho PT, Fogaroli MO, Oliveira MAC, Oliveira CC, Batah SS, Fabro AT, Vulcano LC, Bazan R, Zanini MA (2019b) A rat model of neurocysticercosis-induced hydrocephalus: chronic progressive hydrocephalus with mild clinical impairment. World Neurosurg 132:e535–e544. https://doi.org/10.1016/j.wneu.2019.08.085
Hamamoto Filho PT, Zanini MA, Fleury A (2019a) Hydrocephalus in neurocysticercosis: challenges for clinical practice and basic research perspectives. World Neurosurg 126:264–271. https://doi.org/10.1016/j.wneu.2019.03.071
Hamamoto Filho PT, Singh G, Winkler AS, Carpio A, Fleury A (2020) Could differences in infection pressure be involved in cysticercosis heterogeneity? Trends Parasitol 36(10):826–834. https://doi.org/10.1016/j.pt.2020.07.003
Hinojosa L, Valdez RA, Salvador V, Rodríguez AG, Willms K, Romano MC (2012) The effect of glucocorticoids on sex steroid synthesis in cultured Taenia crassiceps Wake Forest University (WFU) cysticerci. J Helminthol 86(4):465–469. https://doi.org/10.1017/S0022149X11000708
Huerta L, Terrazas LI, Sciutto E, Larralde C (1992) Immunological mediation of gonadal effects on experimental murine cysticercosis caused by Taenia crassiceps metacestodes. J Parasitol 78(3):471–476
Ito A, Budke CM (2021) Genetic diversity of Taenia solium and its relation to clinical presentation of cysticercosis. Yale J Biol Med 94(2):343–349
Kelvin EA, Carpio A, Bagiella E, Leslie D, Leon P, Andrews H, Hauser WA, Ecuadorian Neurocysticercosis Group (2009) The association of host age and gender with inflammation around neurocysticercosis cysts. Ann Trop Med Parasitol 103(6):487–499. https://doi.org/10.1179/000349809X12459740922291
Marcin-Sierra M, Arroyo M, Torres MC, Cruz NR, Hernández FG, Taboada D, Martínez ÁG, Govezensky T, Sciutto E, Toledo A, Fleury A (2017) Extraparenchymal neurocysticercosis: demographic, clinicoradiological, and inflammatory features. PLoS Negl Trop Dis 11(6):e0005646. https://doi.org/10.1371/journal.pntd.0005646
Morales-Montor J, Larralde C (2005) The role of sex steroids in the complex physiology of the host-parasite relationship: the case of the larval cestode of Taenia crassiceps. Parasitol 131(Pt 3):287–294. https://doi.org/10.1017/s0031182005007894
Moura VB, Lima SB, Matos-Silva H, Vinaud MC, Loyola PR, Lino RS (2016) Cellular immune response in intraventricular experimental neurocysticercosis. Parasitol 143(3):334–342. https://doi.org/10.1017/S0031182015001572
Nava-Castro KE, Pavón L, Becerril-Villanueva LE, Ponce-Regalado MD, Aguilar-Díaz H, Segovia-Mendoza M, Morales-Montor J (2022) Sexual dimorphism of the neuroimmunoendocrine response in the spleen during a helminth infection: a new role for an old player? Pathog 11(3):308. https://doi.org/10.3390/pathogens11030308
Rodríguez-Rivas R, Flisser A, Norcia LF, Hamamoto Filho PT, Bonilla-Aldana DK, Rodriguez-Morales AJ, Carpio A, Romo ML, Fleury A (2022) Neurocysticercosis in Latin America: current epidemiological situation based on official statistics from four countries. PLoS Negl Trop Dis 16(8):e0010652. https://doi.org/10.1371/journal.pntd.0010652
Romano MC, Jiménez P, Miranda-Brito C, Valdez RA (2015) Parasites and steroid hormones: corticosteroid and sex steroid synthesis, their role in the parasite physiology and development. Front Neurosci 30(9):224. https://doi.org/10.3389/fnins.2015.00224
Sahu PS, Lim YAL, Mahmud R, Somanath SD, Tan CT, Ramachandran CP (2017) Needs of exploring the burden of recent onset seizures due to neurocysticercosis and challenges in southeast Asia focusing on scenario in Malaysia. Asian Pac J Trop Med 10(4):332–340. https://doi.org/10.1016/j.apjtm.2017.03.024
Santos MV, Garcia CA, Jardini EO, Romeiro TH, Lopes LS, Machado HR, de Oliveira RS (2016) Ventricular-subcutaneous shunt for the treatment of experimental hydrocephalus in young rats: technical note. Childs Nerv Syst 32(8):1507–1511. https://doi.org/10.1007/s00381-016-3042-1
Sciutto E, Cárdenas G, Adalid-Peralta L, Fragoso G, Larralde C, Fleury A (2013) Human neurocysticercosis: immunological features involved in the host’s susceptibility to become infected and to develop disease. Microbes Infect 15(6-7):524–530. https://doi.org/10.1016/j.micinf.2013.03.007
Singh BB, Khatkar MS, Gill JP, Dhand NK (2017) Estimation of the health and economic burden of neurocysticercosis in India. Acta Trop 165:161–169. https://doi.org/10.1016/j.actatropica.2016.01.017
Sitali MC, Schmidt V, Mwenda R, Sikasunge CS, Mwape KE, Simuunza MC, da Costa CP, Winkler AS, Phiri IK (2022) Experimental animal models and their use in understanding cysticercosis: a systematic review. PLoS One 17(7):e0271232. https://doi.org/10.1371/journal.pone.0271232
Stelzle D, Schmidt V, Keller L, Ngowi BJ, Matuja W, Escheu G, Hauke P, Richter V, Ovuga E, Pfausler B, Schmutzhard E, Amos A, Harrison W, Kaducu J, Winkler AS (2022) Characteristics of people with epilepsy and Neurocysticercosis in three eastern African countries-a pooled analysis. PLoS Negl Trop Dis 16(11):e0010870. https://doi.org/10.1371/journal.pntd.0010870
Toledo A, Osorio R, Matus C, Lopez YM, Cruz NR, Sciutto E, Fragoso G, Arauz A, Carrillo-Mezo R, Fleury A (2018) Human extraparenchymal neurocysticercosis: the control of inflammation favors the host…but also the parasite. Front Immunol 9:2652. https://doi.org/10.3389/fimmu.2018.02652
Weibel ER, Kistler GS, Scherle WF (1966) Practical stereological methods for morphometric cytology. J Cell Biol 30(1):23–38. https://doi.org/10.1083/jcb.30.1.23
Wesołowska A (2022) Sex-the most underappreciated variable in research: insights from helminth-infected hosts. Vet Res 53(1):94. https://doi.org/10.1186/s13567-022-01103-3
Acknowledgements
We thank Mr Khallil Taverna Chaim, MSc — from Universidade de São Paulo (USP) — for the support on magnetic resonance imaging protocol and acquisition.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author upon request.
Author information
Authors and Affiliations
Contributions
Conceptualization: PTHF. Experimental procedures and data curation: CAAM, LHVM, TCM, VTO, DG. Formal analysis: VMVM, SSB, ATF. Investigation: RB, MAZ, ES, AF, PTHF. Project administration: CAAM, LHVM, PTHF. Visualization: CAAM, LHVM, TCM, VTO, DG, VMVM, SSB, ATF, RB, MAZ, ES, AF. Manuscript draft: PTHF. Review and editing: SSB, AF, RB, MAZ, ES, AF. Approval of final version: all.
Corresponding author
Ethics declarations
Ethical approval
All procedures were conducted under accepted guidelines for the care and use of laboratory animals for research and approved by Ethics Committee on the Use of Animals (CEUA) of the Botucatu Medical School (CEUA 1318/2019).
Consent to participate
NA
Consent for publication
NA
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Christoph Grevelding
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moreira, C.A.A., Murayama, L.H.V., Martins, T.d.C. et al. Sexual dimorphism in the murine model of extraparenchymal neurocysticercosis. Parasitol Res 122, 2147–2154 (2023). https://doi.org/10.1007/s00436-023-07913-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07913-4