Abstract
Coccidiosis is one of the most notable diseases in chickens having a high economic impact on the poultry industry worldwide. The present study is the first attempt to epidemiologically investigate Eimeria spp. distribution and associated risk factors under different housing and production systems in three major regions in Greece. Faecal samples were obtained from 42 operations (broilers, floor housed, free range and organic layers, backyard farms). A questionnaire was obtained from included operations to acquire additional information regarding farm management, location, production rate and diseases history. Positivity level was 85.7%. All seven Eimeria species were identified, and the most prevalent ones were E. acervulina (79.3%) and E. tenella (65.5%). Single-species and mixed infections were detected in 20.7% and 79.3% of the flocks, respectively. Flock size, type of outdoor area, production system and presence of respiratory disease proved significant risk factors. Flock size up to 10,000 birds correlated strongly (p = 0.02) with higher E. tenella quantities. A very strong correlation (p < 0.001) was found between the presence of respiratory disease and the average OPG level in broiler farms. Organic flocks showed higher prevalence of E. tenella (p = 0.023), while presence of vegetation at the outdoor area correlated strongly with E. brunetti (p < 0.001). Molecular analysis and correlation results in this survey give strong indications although more studies are needed to further understand the involvement of different Eimeria species in various husbandry, production and management systems, to gain more knowledge about the sustainable control of coccidia in poultry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coccidiosis is one of the most notable diseases in chickens and has a high economic impact on the poultry industry worldwide (Haug et al. 2008; Quiroz-Castañeda and Dantan-Gonzalez 2015). Blake et al. (2020) recently estimated that losses due to coccidiosis amount to more than USD 15 billion across the world, equalling USD 0.24 for each single chicken produced. This global cost has seemingly increased by USD 12 billion over the last decade, as Dalloul and Lillehoj (2006) had previously reported it USD 3 billion. Apicomplexan protozoan parasites of the genus Eimeria are the causative agent of avian coccidiosis. The infective agent was identified early (Railliet, 1913), and since then, it has been studied worldwide. Tyzzer et al. (1932) had shown that species of chicken Eimeria develop in different regions of the intestine. In chickens, there are seven recognized species of Eimeria that develop in certain locations within the intestine, each causing a different clinical manifestation (Williams et al. 2009), namely, E. acervulina, E. mitis, E. brunetti, E. maxima, E. necatrix, E. praecox, and E. tenella.
Chicken Eimeria species display varying degrees of pathogenicity. Depending on the respective species, infection dose and Eimeria species specific site of infection, coccidiosis can result in more or less severe disease. It can range from limited enteritis with malabsorption of nutrients and reduced growth rate (e.g. E. praecox and E. mitis), over inflammation of the intestinal wall with pinpoint haemorrhage and epithelial demolition (e.g. E. brunetti, E. acervulina and E. maxima), to complete villar destruction resulting in extensive haemorrhage, high morbidity and death (e.g. E. necatrix and E. tenella) (Iacob and Duma 2009; Morris et al. 2007; Williams et al. 2009). Mixed species infections are common (Callow 1984). In addition to direct Eimeria-related damages, co-infections with other pathogens are common and lead to aggravated disease and economic losses (Alnassan et al. 2013; Van Immerseel et al. 2004).
The genetic diversity of the Eimeria species and the lack of new anticoccidial drugs for decades have led to the development of widespread resistance for all the drugs approved for use in chickens (Chapman 1997; Peek and Landman 2011; Tan et al. 2017). Drug-resistant Eimeria strains can cause subclinical coccidiosis and low body weight gain and feed conversion ratio (Shirzad et al. 2011). Anticoccidial live vaccines have been used efficiently to prevent coccidiosis in the last decades (Marugan-Hernandez et al. 2016); however, they are expensive, and thus, they are not applied exhaustively on a global scale (Blake and Tomley 2014). Recent studies highlight the potential use of recombinant vaccines as an antimicrobial control measure (Clark et al. 2016; Kundu et al. 2017; Lin et al. 2017; Tian et al. 2017), but these tools are not commercially available yet. In addition to control programs based upon chemotherapy or vaccination, satisfactory control of coccidiosis in poultry requires strict attention to hygiene and sanitation, as well as biosecurity measures that limit human access to poultry facilities (Chapman 2018).
To efficiently control coccidiosis, it is important to understand the Eimeria species that are circulating and infecting the different types of poultry farming (i.e. backyard and commercial), as well as potential risk factors associated with the occurrence and burden of different Eimeria species. As far as we know, in Greece, there is no accurate data or previously published information about the prevalence of different Eimeria species in different types of poultry. The purpose of the present study was (1) to analyse the epidemiology of Eimeria species in Greece, using molecular methods, and (2) to investigate risk factors associated with the presence of Eimeria spp. for different housing systems and production types. This data will not only support the knowledge about occurrence and control options for Eimeria infections in poultry in Greece but also enhance our general knowledge about potential risk factors in poultry production under different husbandry conditions.
Materials and methods
Study design and sampling
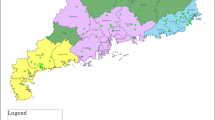
Selection of poultry operations was based on the number of commercial farms in three major Greek regions. Particularly, the geographical location of poultry farms in Greece is concentrated in Epirus (in North-Western Greece), Central Macedonia and Central Greece (Hellenic Ministry of Rural Development & Food, www.minagric.gr). For the purpose of the study, sampling from both commercial operations (broiler and layer flocks) and backyard farms was conducted proportionately to their frequency. (Fig. 1)
The study was conducted on 42 poultry operations in Greece, between January 2016 and March 2017. Fifteen of them were raising broilers, fourteen were backyard farms, and thirteen were layer operations (conventional floor housed, free range and organic flocks) (Table 1). Broiler flocks sampled for this study were slow-growing chicken or commercial broiler breeds kept under conventional (n = 10), free-range (n = 3) or organic (n = 2) production conditions, due to concurrent studies performed. In these flocks, slaughter age had a 75–100-day range, and animals were sampled twice, to record Eimeria occurrence (OPGs and Eimeria species). The first sampling was done at the age of 3–4 weeks, in the operation by collecting faeces from the litter and the second one at process by collecting the whole gut. As gut sampling relied on farm availability and our earlier notification to follow up the flocks at process, gut samples were collected only from six broiler farms in total, three located in Epirus and three located in Central Macedonia. Three out of six farms were employing conventional, two farms free-range and one farm organic production conditions. In layers and backyard flocks, faecal samples were collected once. On layer farms, sampling was performed at the age of 10–12 months, whereas in backyard flocks, according to the flock composition, chickens of different ages were sampled by the described pool sample technique.
Two hundred fifty-two faecal samples were collected from the litter of the chicken housing according to a W shape, in order to collect one fresh dropping every two to five paces according to the floor size (Fornace et al. 2013). Sampling was performed only indoors for all type of farms, and maximum three houses were sampled from all farms. A total of 18 samples were collected from each house, pooled in six samples of three subsamples, placed in labelled sterile bottles and stored in a cool box. From broiler flocks that were followed up to the slaughterhouse, the whole gut was extracted during evisceration and placed in labelled zipped plastic bags. All the samples were transferred immediately to the laboratory of Parasitology of the Veterinary Research Institute – Hellenic Agricultural Organization Demeter, (Thessaloniki, Greece), where they were stored at 4 °C for further parasitological examination. The total number of gastrointestinal tracts collected per broiler flock was calculated based on the flock size using SampSize (http://sampsize.sourceforge.net/iface) and OpenEpi (version 3.01, http://www.openepi.com/SampleSize/SSPropor.htm). According to the flock sizes available for our study, for conventional slow-growing flocks with capacities higher than 5000 birds, 19 guts were collected per flock, while for free-range and organic flocks with capacities higher than 3000 birds, 35 guts were collected per flock.
Data collection by questionnaire
Furthermore, a questionnaire was obtained from all included operations in order to acquire additional information for each farm. It included details about the farm management, location, production rate, disease history and poultry health (Table 2 and Supplemental Table 1), including anticoccidial measures, disinfection measures and perceived flock performance. Farms employing live vaccination protocols have been included to identify potential impacts on Eimeria species infection intensity. If applicable, coccidiostat use was classified as either rotation program or shuttle program; drugs applied were chemicals (nicarbazin) or ionophores (narasin, lasalocid, salinomycin).
Disease history was asking about perceived respiratory disease (sneezing, coughing, laboured breathing, nasal discharge, swollen heads) and perceived intestinal disease (diarrhoea), respectively.
Faecal oocyst counts
Faecal samples were quantitatively examined, by a modified McMaster technique (Roepstorff and Nansen, 1998) with two counts per sample that were averaged. Results were expressed as oocyst content per gram of faeces (OPGs) with a lower detection limit of 50 OPG. For every flock, six pooled samples were processed and 12 counts were performed (2 averaged counts per sample). From the positive faecal samples, oocysts were collected by a combined sedimentation and flotation method according to Daugschies et al. (2002). The purified oocysts were pooled back in one sample per farm for PCR analysis. All samples were afterwards transferred to a 2.5% potassium dichromate solution for sporulation at room temperature for up to 2 weeks and then stored at – 20 °C for further molecular analysis. For the six broiler farms (three in Central Macedonia and three in Epirus), where the whole gut was collected at the slaughterhouse during process, faecal samples were collected from the rectum or the lower part of the intestine and the same steps were followed in regards of McMaster examination, oocysts purification and storage.
DNA extraction
DNA was extracted from sporulated oocysts of Eimeria spp. using the QIAamp® DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions after initial sonication (6 min, pulse 0.5, amplitude 60%) using a Bandelin sonopuls HD70 (Bandelin electronics, Berlin, Germany) to disintegrate the Eimeria oocysts’ wall. DNA concentrations were measured at 260 nm using a spectrophotometer (Eppendorf BioPhotometer, Hamburg, Germany). The extracted DNA was analysed directly by Eimeria spp.–specific polymerase chain reactions (PCR) or kept at – 20 °C until use.
Molecular analyses by PCR
Purified Eimeria spp. oocyst isolates from the different operations were identified to species level by multiple PCR assays (Schnitzler et al. 1998 and 1999) using species-specific primers (Table 3). The multiple PCRs were performed for each sample in a final volume of 25 µl using 1 U of Taq DNA polymerase (5 U/µl Promega, USA), 1 × PCR buffer (10X Promega, USA), 200 µM of each deoxyribonucleotide (Peqlab, Erlangen, Germany), 300 nM of each primer (MWG-Biotech, Ebersberg, Germany), 2 mM MgCl2 (25 mM, Promega, USA), 30–50 ng template DNA and deionized DEPC-H2O (Roth, Karlsruhe, Germany). The reaction in the PCR cycler (iCycler®, Bio-Rad, München, Germany) was programmed for initial denaturation at 94 °C for 3 min, followed by 35 cycles of denaturation (45 s at 94 °C), annealing (40 s at 61 °C) and amplification (1 min at 72 °C) and a final extension at 72 °C for 10 min. PCR products were separated by electrophoresis (Biometra, Göttingen, Germany) by loading 10 µl of PCR product onto 1.5% agarose gel (preqGold Universal Agarose, Peqlab, Erlangen, Germany). The gels were transferred to be stained in an aqueous ethidium bromide solution (0.5 µg/ml) for 15 min, and DNA bands were visualized under UV light (transilluminator; UV wavelength, 254 nm; TFX-20 M, Vilber Lourmat, France) and photographed by digital camera (CSE-0028, Cybertech, Berlin, Germany). Bands were categorized by intensity semiquantitatively as described before (Antiabong et al. 2016).
Statistical analysis
Statistical analysis of OPG counts and PCR findings and correlations with the questionnaire data was done using the PSPP statistical program (GNU PSPP 1.3.0). Following a Kolmogorov–Smirnov normality test that revealed all data were non-normally distributed, Kruskal–Wallis tests and bivariate correlations were used to determine if there were any statistically relevant correlations between findings within the samples and the husbandry conditions on operations and flock size. The data was separated out between data on the individual production types (layer operations, broiler operations and backyard farms) and also analysed combined for all operation types. The influence of production type (conventional versus organic, as well as different conventional husbandry systems for both layer and broiler flocks, as listed in Table 2) on Eimeria species infection intensity and extensity was tested using a bivariate correlation. Statistical significance was assumed for p values < 0.05.
Results
Operation parameters
Farms selected included backyard farms, slow-growth, free-range or organic broilers, floor-housed conventional layer farms and free-range or organic layer farms. Backyard farms had up to 50 hens or chickens per flock. Seven out of 14 backyard farms (50%) had grass soil as outdoor area, while the rest 50% had bush land. Poultry from all 14 farms had access to the outdoor area for free grazing, whereas most of these farms (64.3%) did not perform any kind of disinfections. Only three out of 14 farms (21.4%) had taken some control measures against coccidiosis, through dietary supplements like oregano oil or garlic extract. In 35.7% of the backyard operations (five out of 14 farms), poor performance in terms of egg production was reported or observed, with two of these farms having intestinal disease or clinical disorders and one farm showing signs of respiratory disease, with nasal discharge and swollen heads.
Broiler flocks’ samples had a flock size ranging from 4500 to 50,000 chickens per farm and were mainly (73.3%) multi-age farms with an average of 22,447.7 chickens and 2.8 sheds per farm. 80% of the farms’ outdoor area was concrete floor, 13.3% was bush land and 6.6% grass soil. Access to the outdoor area in a well-fragmented area, especially designed for free grazing in some hours within the day, was given to 26.7% of these flocks; 46.6% reported intestinal disease or disorders. Sixty percent of the farms were applying rotation programs for the control of coccidiosis, and 26.7% had adopted a shuttle program. Only one farm was applying vaccination against Eimeria spp. with a live attenuated vaccine.
Layer farms sampled for this study were located in Central Macedonia (53.85%), Epirus (15.38%) and Central Greece (30.77%). Of these, 23.08% were single-age, floor-housed layers, with an average farm flock size of 11,000 hens and an average shed number of 1.66. Of the flocks examined, 15.38% were conventional free-range farms with single-age hens that had partial access to a fenced outdoor area. The average flock size was 3750 hens housed in one shed per farm. Organic layer farms were 61.54%, with an average flock size of 4375 hens per flock and 2.63 sheds per farm on average. Of the organic farms, 62.5% were multi-age ones. In total, 38.5% of the layer farms had bush land as surroundings, 53.85% had grass soil outdoor area and only one farm (7.7%) had concrete floor in the outdoor environment. All farms were applying disinfections on a regular basis, while 46.15% of them had also visitor books with daily records. Of the layer farms, 23.08% and 15.38% reported intestinal disorders or respiratory clinical signs, respectively. However, none of the farms reported significant losses in productivity, although in 53.85% of the farms, daily egg production and performance were reported below the optimal age-related standards of the layer hybrids housed. Regarding coccidiosis control, 38.46% of the layer farms had performed vaccination against coccidiosis during rearing, 30.77% were adding essential oils or herbs with anticoccidial indications as dietary supplements, and 7.7% had used coccidiostats in the ratio during the rearing period. Of the layer farms, 23.07% did not apply any control measures against coccidiosis, or farmers were not aware of any treatments or vaccination against Eimeria spp. during rearing, as in most cases, hens were placed in the poultry houses as pullets.
Faecal oocyst counts
McMaster flotation results revealed the presence of Eimeria species oocysts in 36 out of 42 operations sampled, demonstrating a level of 85.7% positivity for the presence of Eimeria oocysts. Twelve out of 14 backyard flocks were found positive (85.7%), with an average mean OPG of 774.7. Broiler flocks demonstrated 80% positivity (12 out of 15 farms) during sampling at the age of 3–4 weeks, but with a significantly higher average mean OPG level of 33,502.2. Layers showed the highest positivity level (92.3%) with 12 out of 13 farms testing positive for the presence of Eimeria oocysts and 577.0 average mean OPG (Table 4). The highest mean OPG was recorded in broiler farm 2 (271,900) and the lowest mean OPG was observed in backyard farm 6 (6.66). Epirus showed the highest average mean OPG level of 29,840.6, followed by Central Macedonia (4,228.6) and Central Greece (300). A noteworthy result is the big difference observed in average mean OPG for broilers in Epirus (80,178.3) compared to broilers in Central Macedonia (10,164.2). As for layers, average mean OPG between conventional floor-housed and free-range or organic flocks was 521.7 and 588, respectively, with layer farms located in Central Macedonia showing a much higher average mean OPG (914.7) compared to layer farms located in Central Greece (384.4). Average OPG level ranked higher in broiler farms versus layer farms (p < 0.001).
Molecular analyses by PCR
Sufficiently high numbers of oocysts for purification and DNA extraction were present in samples from 33 out of the sampled 42 farms (78.6%). PCR-positive findings for any Eimeria spp. were obtained from DNA extracts out of 29 farms. All seven Eimeria species were identified over all 29 operations: E. tenella, E. acervulina, E. maxima, E. brunetti, E. necatrix, E. mitis and E. praecox. The distribution of Eimeria species in poultry farms is presented in Table 5. Overall, the most prevalent species were E. acervulina (23/29; 79.3%), followed by E. tenella (19/29; 65.5%), E. brunetti (8/29; 27.6%) and E. maxima (7/29; 24.1%), while the less abundant were E. necatrix (4/29; 13.8%), E. mitis (3/29; 10.3%) and E. praecox (3/29; 10.3%).
In broiler farms, the most prevalent species were equally E. tenella and E. acervulina (13/15; 86.7%), in layer flocks E. brunetti and E. necatrix (4/7; 57.1%) equally, while in backyard flocks E. acervulina represented the most frequently found species (8/11; 72.7). In backyard flocks, no E. brunetti was detected. E. praecox and E. mitis were absent in layer flocks. E. praecox was found in broilers only on two operations at the second sampling during slaughter, while it could not be detected on any operation at the age of 3–4 weeks when the first sampling was performed.
E. tenella and E. acervulina were significantly more prevalent in broilers compared to layers (p = 0.01 and p = 0.04, respectively). On layer farms, E. brunetti and E. necatrix were more prevalent than on broiler farms (p = 0.019 and p = 0.02, respectively). The two latter species had also a strong correlation with each other (r = 0.76, p = 0.001) for layer flocks, indicating that they are commonly occurring together.
The number of Eimeria spp. recovered per operation is listed in Table 5. Infection with only one species was detected in backyard and layer chickens only. Mixed infections with two or more species were found in 23 (79.3%) flocks. Out of these co-infections, thirteen flocks were infected by two Eimeria species, seven flocks by three species, two flocks by four species and only one by six Eimeria species. On average, largest Eimeria spp. variety was seen in broiler flocks (Table 5).
Regional differences
There were some regional differences observed in animal husbandry parameters and Eimeria occurrence. For both E. tenella (p = 0.003) and E. acervulina (p = 0.029), higher prevalence rates were observed in Epirus compared to Central Macedonia. In Central Greece, E. brunetti (p = 0.019) and E. necatrix (p = 0.001) were significantly more prevalent than in Epirus. No statistically significant differences were observed between Central Macedonia and Central Greece.
Correlation of Eimeria oocyst counts and species identity with questionnaire data
For both broilers and layers, some Eimeria species and OPGs have been found to be significantly linked with the respective production or housing system parameters assessed by the questionnaire (Table 6).
Broilers
In broilers, E. tenella was more prevalent in organic flocks or flocks with partial outdoor area access compared to flocks that were housed only indoors on litter (p = 0.023). Smaller flocks of up to 10,000 birds per flock had significantly (p = 0.02) higher E. tenella quantities, as compared to larger sized flocks. Eimeria species and oocyst quantities (per semiquantitative PCR, as described by Antiabong et al.2016)), also correlated with the type of the outdoor area; presence of soil and vegetation were correlated with higher oocyst quantities of E. tenella (p = 0.06), E. brunetti (p < 0.001), and E. praecox (p = 0.07), respectively. The presence of neighbouring broiler farms correlated moderately with lower quantities of E. maxima (p = 0.012) and E. praecox (p = 0.021). Per producers’ performance and productivity evaluation for their flocks, E. tenella quantity correlated with perceived, however not quantified, economic losses (p = 0.034). Additionally, a very strong correlation (p < 0.001) was found between the presence of respiratory disease and the average OPG level in broiler farms. The type of on-site coccidiosis control measures on broiler operations influenced both E. tenella (p = 0.034) and E. brunetti (p = 0.063) quantities. Vaccination, which was defined as the maximum control level in our study’s questionnaire (Supplemental Table 1), seems more likely to reduce the quantities of E. tenella and E. brunetti when compared to anticoccidial drug rotation or shuttle programs.
Layers
For layers, E. necatrix was more prevalent in conventional floor housed flocks (p = 0.03), free-range flocks (p = 0.046) and organic flocks (p = 0.015), compared to backyard flocks. Floor-housed layers were also more likely to be infected with E. brunetti (p = 0.018) and E. necatrix (p = 0.02) than free-range and organic layers, respectively. Larger layer flock size showed a positive correlation with the quantity of E. brunetti (p = 0.05) and E. necatrix (p < 0.001) oocysts excreted, respectively. The presence of neighbouring farms was linked with higher presence of E. acervulina (p = 0.04). Absence of a routine disinfection program correlated with higher E. tenella oocyst quantities (p = 0.019). Both E. tenella (p < 0.001) and E. maxima (p < 0.001) presence were found to be associated with observed production losses. Interestingly, respiratory diseases on layer operations correlated with higher oocyst excretion of E. acervulina (p = 0.024). Intestinal disorders observed on layer farms displayed a weak but statistically significant correlation with the average Eimeria OPGs (p = 0.026). Ground feeding of layers was linked with higher oocyst excretion of E. necatrix (p = 0.017). From a farm management perspective, floor-housed layers and large size flocks were more likely to employ regular disinfection measures compared to free-range, organic (p = 0.013) and smaller sized flocks (p < 0.001).
Overall, flock size, as well as type of outdoor area, was found to impact Eimeria species presence and OPG significantly stronger on broiler farms compared to layer farms (p < 0.001).
Discussion
Epidemiological studies on the prevalence of Eimeria species are useful tools for the prevention and control of coccidiosis (Gyorke et al. 2013). Traditionally, Eimeria species have been identified using morphometry of the sporulated oocysts (McDougald et al. 1997) as well as intestinal lesion evaluation during necropsy (Johnson and Reid 1970). Molecular techniques can overcome morphometry limitations (Andrews and Chilton 1999; Gasser 1999) and do not require euthanasia of animals for diagnostic necropsies. This study appears to be the first epidemiological survey to be conducted in a wider area of Greece and in different types of poultry farming. For the current study, we used species-specific primers in multiple conventional PCRs to determine which Eimeria species are circulating in Greek poultry farms under different production conditions. Caged layers were not included in the study design, as Eimeria spp. and coccidiosis are believed to be a less prominent problem in hens housed in battery cages (Lunden et al. 2000). Indeed, after indicative faecal sampling in caged layers with both presence and absence of automatic manure belt, no Eimeria oocyst excretion was detected, which is in contrast with reports by Soares et al.2004. Coproscopy revealed the presence of Eimeria species in 36 out of 42 operations sampled, demonstrating a level of 85.7% positivity for the presence of Eimeria oocysts, similar to what was reported in other countries worldwide such as Romania, France, Jordan, India, Argentina and Turkey where prevalence ranged between 70 and 96% (Al-Natour et al. 2002; Gyorke et al. 2013; Karaer et al. 2012; Kumar et al. 2015; McDougald et al. 1997; Williams et al. 1996). We detected less Eimeria-positive findings by PCR than by McMaster analysis. In samples from McMaster positive and PCR negative flocks, we detected a low OPG count. Our samples may not have contained enough oocysts to be detected in the small faecal volume subjected to DNA extraction (Haug et al. 2007; Gyorke et al. 2013). Though we employed an ultrasound disintegration of oocysts before using the DNA extraction kit, our DNA extraction protocol may have a limited sensitivity as Haug et al. (2007) described reduced efficiency of their method of oocyst cracking by grinding if oocyst concentration was low, leading to reduced amounts of extracted parasite DNA.
In our study, 38.5% of the layer farms sampled had performed vaccination against coccidiosis during rearing, while 7.7% had used in-feed coccidiostats during the rearing period. Interestingly, 80% of the layer farms that performed vaccination had established organic production settings, where certain limitations for the use of anticoccidial drugs apply, based on the EU organic regulation (EC No 834/2007). For 23.1% of the layer farms, owners did not apply any control measures against coccidiosis and farmers were not aware of any treatments or vaccination against Eimeria spp. on a previous rearing operation. These findings are not in accordance with coccidiosis management in the floor-housed layer flocks in the USA, where the majority (83%) of the flocks used a coccidiostat-based anticoccidial program versus only 12% that used vaccination (Soares et al. 2004). In broilers, 60% of the farms were applying rotation programs for the control of coccidiosis. Generally, our observed OPG levels were significantly higher than described for some other European countries; e.g. a study in Norway revealed a median OPG of less than 14,000 OPG for indoor floor pen housed broilers (Haug et al. 2008). While Kaboudi et al. (2016) found a weak correlation between diarrhoea and Eimeria presence, our study could not establish a significant correlation between intestinal disease (per questionnaire) and high oocyst quantities on Greek broiler operations. Potentially, the actual Eimeria species present and their virulence may determine the clinical signs to a higher degree than the total OPG number observed, especially since the OPG numbers are prone to variation over time based on the Eimeria life cycle. This may explain the lacking consistency in findings from different studies.
Eimeria oocysts are considered omnipresent, spreading rapidly in the poultry house environment and showing resistance to various disinfection practices (Hadipour et al. 2011; López-Osorio et al. 2020). In our study, outdoor access obviously contributed in favour of Eimeria species circulation in both broiler and layer production, fostering faecal-oral transmission, particularly for E. tenella (p = 0.06), E. brunetti (p < 0.001) and E. praecox (p = 0.07). One notable result was that we did not find any E. brunetti in backyard flocks. This is in accordance with Godwin and Morgan (2015), who also reported E. brunetti as the least common species in backyard flocks. There are reports about persistence of cattle Eimeria oocysts on pastures over different seasons (Lassen et al. 2013, 2014). Any unpaved outdoor premises are hard to disinfect, which increases the outdoor area contamination level (Meroz and Samberg 1995). Hence, disinfection is an important risk management factor in coccidiosis control. In our study, floor-housed layers had a more regular disinfection protocol compared to free-range or even organic flocks. In the European Union, organic poultry producers are allowed to use selected approved cleansers, disinfectants and sanitizers only (EC 834/2007), which obviously limits the application of efficient anticoccidial disinfectants (e.g. cresol-based products). On backyard farms, coccidiosis control measures were not common. Only three out of the 14 backyard farms (21.4%) were attempting some control measures through alternative dietary supplements like oregano essential oil or garlic extract, and there was no clear impact of these measures on the observed Eimeria OPG count. Oregano (Origanum vulgare subsp. hirtum) is used as a bioactive compound that is thought to act as a natural growth enhancer with potent anticoccidial properties and has been studied for several years. Garlic (Allium sativum) metabolites have been reported to display potential performance-enhancing, antioxidant and anticoccidial activities (Burt et al 2013; Partheniadis et al. 2019; Sheoran et al. 2017). In vivo studies performed on herbal formulas based on garlic and wild thyme have previously failed to control E. tenella burden and pathology (Pop et al. 2019), highlighting the necessity for further research into dietary supplements use. In total, five layer farms reported poor performance, including one farm that used oregano oil, coinciding with high Eimeria OPG counts and PCR-based identification of up to four Eimeria species in four out of these five farms (80%).
On broiler chicken farms, all typical Eimeria species were found except for E. necatrix, while studies from other European countries like Sweden, France and former Czechoslovakia showed the presence of all seven species (Kucera 1990; Williams et al. 1996). In our observations, the most prevalent species in broilers were E. acervulina and E. tenella, similar to findings in Romania and Norway (Gyorke et al. 2013, Haug et al. 2008). Mixed species infections were common and found in 79.3% of the flocks. This is in accordance with previously reported studies from Europe, Africa, Asia and America (Aarthi et al. 2010; Gadelhaq et al. 2015; Gyorke et al. 2013; Huang et al. 2017; Kaboudi et al. 2016, Jenkins et al. 2017).
Interestingly, average OPG levels were higher in broiler farms than in layer farms. This may be due to the young age of broilers, as younger birds are more susceptible while older birds like layers are more likely to be protected by immunity resulting from previous pathogen contact. Moreover, in broilers like E. acervulina and E. tenella are prevailing, as species of a particularly high reproductive potential (Williams 2001), which results in particularly high OPG.
In our study, smaller sized broiler flocks were more likely to exhibit higher E. tenella burdens, which is attributed to a better hygienic management on larger operations. Particularly in broilers, E. tenella was most common in organic flocks and flocks with partial outdoor area access that is often related to poorer sanitation. Anyway, consumers nowadays show increased interest in organic poultry production and the number of organic farms has increased significantly in the EU (Agency for the Development and Promotion of Organic Farming 2019). Although literature about prevalence of Eimeria species in organic poultry farming is still limited, the need for sustainable alternative control methods against coccidiosis compatible with organic production is considered high.
E. tenella quantity correlates with losses in performance and productivity in broilers and layers, while E. maxima primarily alters productivity in layers. E. tenella is highly pathogenic and specifically infects epithelial cells of the caecal crypts of Lieberkühn, resulting, along with E. maxima, in the induction of a range of pro- and anti-inflammatory cytokines including interleukin IL-6, IL-17A, IL-10 and interferon IFN-γ (Hong et al. 2006; Laurent et al. 2001; MacDonald et al. 2017). Infection may also result in haemorrhagic lesions of varying severity that affect performance parameters for both broilers and layers. A very strong correlation has also been found between the presence or history of respiratory disease signs and the average OPG level in broiler farms, while for layers, respiratory disease history correlated with a stronger presence of E. acervulina. According to Chapman (2014), this is not a surprising finding since interactions with co-infecting pathogens via immunosuppression and thus mutual aggravation of clinical signs are commonly seen.
In broilers, anticoccidial drug rotation and shuttle programs seemed more efficient for E. brunetti and E. tenella control than the adoption of anticoccidial vaccination. Introduction of attenuated live vaccine strains may have confounded these results and thus findings have to be interpreted with caution. However, overall Eimeria excretion was lower in vaccinated (averaging at 1,136 OPG) versus drug treated flocks (averaging at 33,121 OPG). Medicated flocks have been compared before with vaccinated flocks, observing higher peak oocyst shedding in drug treated birds as compared to vaccinated flocks (Snyder et al. 2021).
E. tenella and E. acervulina were more prevalent in Epirus (North-western Greece) than in other examined regions, whereas E. brunetti and E. necatrix were more frequently found in Central Greece. This can be explained by the main type of poultry production in the respective region. Epirus is the region where 45% of Greek broiler farms are located (Andreopoulou et al. 2019), with E. acervulina and E. tenella being the dominant species in broilers according to our PCR results. Central Greece has a particularly high number of layer farms with 50% of Greek layer farms being located in this prefecture, and consequently, E. brunetti and E. necatrix were more frequently found (Koutoulis 2012). In Central Macedonia, intestinal disorders were more frequently recorded than in other prefectures, which is in line with more Eimeria co-infections seen in this region. Apart from these correlations, farm management practices, level of biosecurity and type of coccidiosis control programs play an important role that should be further investigated. Intestinal disorders were more frequently observed in organic layer flocks when compared to floor housed and free-range flocks. This is most probably attributed to the regulatory limitations regarding the use of antimicrobials and anticoccidial feed additives in organic farms. However, apart from coccidiosis, other unfavourable factors may as well contribute to reduced productivity and higher frequency of disease under such circumstances.
In conclusion, the present study demonstrates a high prevalence of chicken Eimeria in Greece that may vary under different production systems. Circulation of all seven Eimeria species in both broiler and layer flocks and across the country is obvious from our data. The current study appears to be the first attempt to investigate Eimeria species distribution within Greece and associated risk factors in relation with type of farm, flock size, general management practices, disease history and farm location. Due to limited owner consent and thus lacking availability, we did not take samples from intensively reared broilers and a more even farm distribution could not be achieved. Therefore, the molecular analysis of Eimeria species and correlation results should be further evaluated to increase understanding of the presence, transmission and relevance of different Eimeria species in different systems of husbandry, production and management, in order to develop sustainable control of coccidia in poultry production in general and in Greece in particular.
References
Aarthi S, Raj GD, Raman M, Gomathinayagam S, Kumanan K (2010) Molecular prevalence and preponderance of Eimeria spp among chickens in Tamil Nadu India. Parasitol Res 107(4):1013–7
Agency for the development and promotion of organic farming (2019) Organic Farming and Market in the European Union. France, National observatory of organic farming. 2019 Edition. www.agencebio.org. Accessed 16 Feb 2021
Alnassan AA, Shehata AA, Kotsch M, Schrödl W, Krüger M, Daugschies A, Bangoura B (2013) Efficacy of early treatment with toltrazuril in prevention of coccidiosis and necrotic enteritis in chickens. Avian Pathol 42(5):482–490. https://doi.org/10.1080/03079457.2013.823476
Al-Natour MQ, Suleiman MM, Abo-Shehada MN (2002) Flock-level prevalence of Eimeria species among broiler chicks in northern Jordan. Prev Vet Med 53(4):305–310
Andreopoulou M, Franzo G, Tucciarone CM, Prentza Z, Koutoulis KC, Cecchinato M, Chaligiannis I (2019) Molecular epidemiology of infectious bronchitis virus and avian metapneumovirus in Greece. Poult Sci 98(11):5374–5384. https://doi.org/10.3382/ps/pez360.PMID:31264704;PMCID:PMC7107232
Andrews RH, Chilton NB (1999) Multilocus enzyme electrophoresis: a valuable technique for providing answers to problems in parasite systematics. Int J Parasitol 29:213–253
Antiabong JF, Ngoepe MG, Abechi AS (2016) Semi-quantitative digital analysis of polymerase chain reaction-electrophoresis gel: Potential applications in low-income veterinary laboratories. Vet World 9(9):935–939. https://doi.org/10.14202/vetworld.2016.935-939
Bachaya HA, Raza MN, Khan Z, Iqbal RZ, Murtaza AS, Badar N (2012) Predominance and detection of different Eimeria species causing coccidiosis in layer chicken. J Anim Plant Sci 22(3):597–600
Blake DP, Tomley FM (2014) Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol 30(1):12–19. https://doi.org/10.1016/j.pt.2013.10.003
Blake DP, Knox J, Dehaeck B, Huntington B, Rathinam T, Ravipati V, Ayoade S, Gilbert W, Adebambo AO, Jatau ID, Raman M, Parker D, Rushton J, Tomley FM (2020) Re-calculating the cost of coccidiosis in chickens. Vet Res 51:115
Burt SA, Tersteeg-Zijderveld MHG, Jongerius-Gortemaker BGM, Vervelde L, Vernooij JC (2013) In vitro inhibition of Eimeria tenella invasion of epithelial cells by phytochemicals. Vet Parasitol 191:374–378. https://doi.org/10.1016/j.vetpar.2012.09.001
Callow LL (1984) Animal health in Australia. Protozoal and Rickettsial Diseases, vol. 5. Australian Government Publishing Service, Canberra.
Chapman HD (1997) Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol 26(2):221–244. https://doi.org/10.1080/03079459708419208
Chapman HD (2014) Milestones in avian coccidiosis research: a review citing articles via. Poult Sci 93:501–511
Chapman HD (2018) Applied strategies for the control of coccidiosis in poultry. Cab Rev 13:1–1
Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Kumar S, Ayoade S, Fornace KM, Jatau ID, Moftah A, Nolan MJ, Sudhakar NR, Adebambo AO, Lawal IA, Zapata RA, Awuni JA, Chapman HD, Karimuribo E, Mugasa CM, Namangala B, Rushton J, Suo X, Thangaraj K, Srinivasa Rao AS, Tewari AK, Banerjee PS, Raj GD, Raman M, Tomley FM, Blake DP (2016) Cryptic Eimeria genotypes are common across the southern but not northern hemisphere. Int J Parasitol. 46(9):537–44. https://doi.org/10.1016/j.ijpara.2016.05.006
Council Regulation (EC) No 834/2007 of 28 June 2007 on organic production and labelling of organic products and repealing Regulation (EEC) No 2092/91. [Online]. Available from: http://eur-lex.europa.eu/. Accessed 18 Feb 2021
Dalloul RA, Lillehoj HS (2006) Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines 5(1):143–163. https://doi.org/10.1586/14760584.5.1.1
Daugschies A, Bose R, Marx J, Teich K, Friedhoff KT (2002) Development and application of a standardized assay for chemical disinfection of coccidia oocysts. Vet Parasitol 103:299–308
Fornace KM Clark EL Macdonald SE Namangala B Karimuribo E Awuni JA Thieme O Blake DP Rushton J (2013) Occurrence of Eimeria Species Parasites on Small-Scale Commercial Chicken Farms in Africa and Indication of Economic Profitability. Plos One 8(12).
Gadelhaq SM, Arafa WM, Aboelhadid SM (2015) Molecular characterization of eimeria species naturally infecting egyptian baldi chickens. Iran J Parasitol 10(1):87–95
Gasser RB (1999) PCR-based technology in veterinary parasitology. Vet Parasitol 84:229–258
Godwin MR, Morgan JAT (2015) A molecular survey of Eimeria in chickens across Australia. Vet Parasitol 214:16–21
Gyorke A, Pop L, Cozma V (2013) Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite 20:50–57
Hadipour MM, Olyaie A, Naderi M, Azad F, Nekouie O (2011) Prevalence of Eimeria species in scavenging native chickens of Shiraz. Iran African J Micro Res 5:3296–3299
Haug A, Thebo P, Mattsson JG (2007) A simplified protocol for molecular identification of Eimeria species in field samples. Vet Parasitol 146(1–2):35–45
Haug A, Gjevre AG, Thebo P, Mattsson JG, Kaldhusdal M (2008) Coccidial infections in commercial broilers: epidemiological aspects and comparison of Eimeria species identification by morphometric and polymerase chain reaction techniques. Avian Pathol 37(2):161–170
Hong YH, Lillehoj HS, Lee SH, Dalloul RA, Lillehoj EP (2006) Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet Immunol Immunopathol 114(3–4):209–223. https://doi.org/10.1016/j.vetimm.2006.07.007
Huang Y, Ruan X, Li L, Zeng M (2017) Prevalence of Eimeria species in domestic chickens in Anhui province, China. J Parasit Dis 41:1014–1019. https://doi.org/10.1007/s12639-017-0927-1
Iacob OC, Duma V (2009) Clinical paraclinical and morphopathological aspects in cecal eimeriosis of broilers. Scientia Parasitologica 10:43–50
Jenkins MC, Parker C, Ritter D (2017) Eimeria oocyst concentrations and species composition in litter from commercial broiler farms during anticoccidial drug or live Eimeria oocyst vaccine control programs. Avian Dis 61:214–220
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28(1):30–36. https://doi.org/10.1016/0014-4894(70)90063-9
Kaboudi K, Umar S, Munir MT (2016) Prevalence of Coccidiosis in Free-Range Chicken in Sidi Thabet. Tunisia Scientifica 2016:7075195. https://doi.org/10.1155/2016/7075195
Karaer Z, Guven E, Akcay A, Kar S, Nalbantoglu S, Cakmak A (2012) Prevalence of subclinical coccidiosis in broiler farms in Turkey. Trop Anim Health Prod 44(2):589–594
Khalafalla RE, Daugschies A (2010) Single oocyst infection: a simple method for isolation of Eimeria spp from the mixed field samples. Parasitol Res 107(1):187–8
Koutoulis K (2012). Present and future of poultry industry in Greece. Conference: 12th Pan-Hellenic Veterinary Congress.
Kucera J (1990) Identification of Eimeria species in Czechoslovakia. Avian Pathol 19:59–66
Kumar S, Garg R, Ram H, Maurya PS, Banerjee PS (2015) Gastrointestinal parasitic infections in chickens of upper gangetic plains of India with special reference to poultry coccidiosis. J Parasit Dis 39(1):22–26
Kundu K, Garg R, Kumar S, Mandal M, Tomley FM, Blake DP, Banerjee PS (2017) Humoral and cytokine response elicited during immunisation with recombinant Immune Mapped protein-1 (EtIMP-1) and oocysts of Eimeria tenella. Vet Parasitol 244:44–53. https://doi.org/10.1016/j.vetpar.2017.07.025
Lassen B, Lepik T, Bangoura B (2013) Persistence of Eimeria bovis in soil. Parasitol Res 112:2481–2486. https://doi.org/10.1007/s00436-013-3413-4
Lassen B, Lepik T, Jarvis T (2014) Seasonal recovery of Eimeria oocysts from soil on naturally contaminated pastures. Parasitol Res 113:993–999
Laurent F, Mancassola R, Lacroix S, Menezes R, Naciri M (2001) Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect Immun 69(4):2527–2534
Lin RQ, Lillehoj HS, Lee SK, Oh S, Panebra A, Lillehoj EP (2017) Vaccination with Eimeria tenella elongation factor-1α recombinant protein induces protective immunity against E. tenella and E. maxima infections. Vet Parasitol. 243:79–84. https://doi.org/10.1016/j.vetpar.2017.06.003
López-Osorio S, Chaparro-Gutiérrez JJ, Gómez-Osorio LM (2020) Overview of Poultry Eimeria Life Cycle and Host-Parasite Interactions. Front Vet Sci 7:384. https://doi.org/10.3389/fvets.2020.00384
Lunden A, Thebo P, Gunnarsson S, Hooshmand-Rad P, Tauson R, Uggla A (2000) Eimeria infections in litter-based, high stocking density systems for loose-housed laying hens in Sweden. Br Poult Sci 41(4):440–447. https://doi.org/10.1080/713654973
Macdonald SE, Nolan MJ, Harman K, Boulton K, Hume DA, Tomley FM, Stabler RA, Blake DP (2017) Effects of Eimeria tenella infection on chicken caecal microbiome diversity, exploring variation associated with severity of pathology. Plos One 12(9):e0184890. https://doi.org/10.1371/journal.pone.0184890
Marugan-Hernandez V, Cockle C, Macdonald S, Pegg E, Crouch C, Blake DP, Tomley FM (2016) Viral proteins expressed in the protozoan parasite Eimeria tenella are detected by the chicken immune system. Parasit Vectors 9(1):463. https://doi.org/10.1186/s13071-016-1756-2
McDougald LR, Fuller L, Mattiello R (1997) A survey of Coccidia on 43 poultry in Argentina. Avian Dis 41(4):923–929
Meroz M, Samberg Y (1995) Disinfecting poultry production premises. Rev Sci Tech off Int Epiz 14(2):273–291
Morris GM, Woods WG, Richards DG, Gasser RB (2007) Investigating a persistent coccidiosis problem on a commercial broiler-breeder farm utilising PCR-coupled capillary electrophoresis. Parasitol Res 101(3):583–589
Partheniadis I, Vergkizi S, Lazari D, Reppas C, Nikolakakis I (2019) Formulation, characterization and antimicrobial activity of tablets of essential oil prepared by compression of spray-dried powder. J Drug Deliv Sci Technol 50:226–236. https://doi.org/10.1016/j.jddst.2019.01.031
Peek HW, Landman WJM (2011) Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet Q 31(3):143–161
Pop LM, Varga E, Coroian M, Nedisan ME, Mircean V, Dumitrache MO, Farczádi L, Fülöp Ι, Croitoru MD, Fazakas M, Gyӧrke A (2019) Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasites Vectors 12:343. https://doi.org/10.1186/s13071-019-3595-4
Quiroz-Castañeda RE, Dantán-González E (2015) Control of avian coccidiosis: future and present natural alternatives. Biomed Res Int 2015:430610. https://doi.org/10.1155/2015/430610
Railliet A (1913) Quel nom doit-on donner à la coccidie intestinale de la poule? Arch Parasitol 16:147–148
Roepstorff A, Nansen P (1998) Epidemiology, diagnosis and control of helminth parasites of swine. FAO Animal Health Manual, Rome
Sachs L (1992) Angewandte Statistik, 7th edn. Springer-Verlag, Berlin Heidelberg New York
Schnitzler BE, Thebo PL, Mattsson JG, Tomley FM, Shirley MW (1998) Development of a diagnostic PCR assay for the detection and discrimination of four pathogenic Eimeria species of the chicken. Avian Pathol 27:490
Schnitzler BE, Thebo PL, Tomley FM, Uggla A, Shirley MW (1999) PCR identification of chicken Eimeria: a simplified read-out. Avian Pathol 28:89–93
Sheoran N, Kumar R, Kumar A, Batra K, Sihag S, Maan S, Maan NS (2017) Nutrigenomic evaluation of garlic (Allium sativum) and holy basil (Ocimum sanctum) leaf powder supplementation on growth performance and immune characteristics in. broilers Vet World 10:121–9. https://doi.org/10.14202/vetworld.2017.121-129
Shirzad MR, Seifi S, Gheisari HR, Hachesoo BA, Habibi H, Bujmehrani H (2011) Prevalence and risk factors for subclinical coccidiosis in broiler chicken farms in Mazandaran province. Iran Trop Anim Health Prod 43(8):1601–1604. https://doi.org/10.1007/s11250-011-9876-3
Snyder RP, Guerin MT, Hargis BM, Page G, Barta JR (2021) Monitoring coccidia in commercial broiler chicken flocks in Ontario: comparing oocyst cycling patterns in flocks using anticoccidial medications or live vaccination. Poult Sci 100:110–118
Soares R, Cosstick T, Lee EH (2004) Control of coccidiosis in caged egg layers: A paper plate vaccination method. J Appl Poult Res 13:360–363
Su YC, Fei ACY, Tsai FM (2003) Differential diagnosis of five avian Eimeria species by polymerase chain reaction using primers derived from the internal transcribed spacer 1 (ITS-1) sequence. Vet Parasitol 117(3):221–7. https://doi.org/10.1016/j.vetpar.2003.07.028
Tan L, Yalin L, Yang X, Ke Q, Lei W, Mughal MN, Fang R, Zhou Y, Shen B, Zhao J (2017) Genetic diversity and drug sensitivity studies on Eimeria tenella field isolates from Hubei Province of China. Parasit Vectors 10(1):137. https://doi.org/10.1186/s13071-017-2067-y
Tian L, Li W, Huang X, Tian D, Liu J, Yang X, Liu L, Yan R, Xu L, Li X, Song X (2017) Protective Efficacy of Coccidial Common Antigen Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) against Challenge with Three Eimeria Species. Front Microbiol 8:1245. https://doi.org/10.3389/fmicb.2017.01245
Tyzzer EE, Theiler H, Jones EE (1932) Coccidiosis in gallinaceous birds II. A comparative study of species of Eimeria of the chicken. Am J Hyg 15:319–393
Van Immerseel F, De Buck J, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R (2004) Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol 33(6):537–549. https://doi.org/10.1080/03079450400013162
Williams RB (2001) Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int J Parasitol 31(10):1056–1069
Williams RB, Bushell AC, Reperant JM, Doy TG, Morgan JH, Shirley MW, Yvore P, Carr MM, Fremont Y (1996) A survey of Eimeria species in commercially-reared chickens in France during 1994. Avian Pathol 25:113–130
Williams RB, Marshall RN, Pagés M, Dardi M, del Cacho E (2009) Pathogenesis of Eimeria praecox in chickens: virulence of field strains compared with laboratory strains of E praecox and E acervulina. Avian Pathology 38(5):359–66. https://doi.org/10.1080/03079450903186028
Acknowledgements
The authors gratefully acknowledge all the veterinarians and farmers for accepting to participate in this study. Furthermore, we are grateful for the support provided by Sandra Gawlowska and the staff of the laboratory of the Institute of Parasitology, Faculty of Veterinary Medicine, University of Leipzig.
Funding
This research was supported by the Onassis Foundation-Scholarship ID: F ZL 007–1/2015–2016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Una Ryan
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Andreopoulou, M., Chaligiannis, I., Sotiraki, S. et al. Prevalence and molecular detection of Eimeria species in different types of poultry in Greece and associated risk factors. Parasitol Res 121, 2051–2063 (2022). https://doi.org/10.1007/s00436-022-07525-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07525-4