Abstract
Coccidiosis is known to affect a wide range of animals including chickens. This study was designed to determine the nation-wide prevalence and clinico-histopathological changes associated with Eimeria infections in commercial laying birds in Nigeria. One Thousand eight hundred and forty-three (1843) commercial laying chickens from 28 states and the Federal Capital Territory (FCT) of Nigeria, between 2007 and 2016 were examined for the presence of Eimeria parasites and for clinicopathological lesions. Simple flotation and the McMaster techniques were used to confirm the presence of Eimeria oocysts and estimate the oocyst load. Positive samples were sporulated for the purpose of species differentiation. Gross and histopathology were carried out accordingly. Two hundred and seven (207) faecal samples/carcasses were positive for Eimeria infections (11.23%; 95% CI = 9.87–12.75). Eimeria tenella and E. necatrix were the most prevalent species. The highest mean oocyst per gram (OPG) was recorded in E. acervulina (5260.87 (± 1838.35)). The prevalence of Eimeria infections in commercial laying birds within the states ranged between 4.4% (Kaduna State), and 33% (Ebonyi and Lagos States), and 21.1% in FCT. There was a higher prevalence of Eimeria infections in commercial laying birds that are less than 1 year compared to those above a year. Eimeria infections was 2.19 times more likely to occur during the wet season compared to the dry season (95% CI = 1.59–3.06; χ2 = 23.29; P = < 0.01). One hundred and eighty-five (185) carcasses showed moderate petechiae to ecchymotic intestinal/caecal mucosa, while 22 carcasses showed severe petechiae to ecchymotic intestinal/caecal mucosa with bloody intestinal lumen. This study appears to be the first nation-wide study on the prevalence and clinico-histopathological changes associated with Eimeria infections in commercial laying chickens in Nigeria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poultry production in the world has increased dramatically in the last 20 years with more than 90 million tons of chicken meat and 1.1 trillion eggs produced yearly (FAO Stat 2015; Prakashbabu et al. 2017). Poultry constitutes an important component of the agricultural and household economy in most parts of the world, and it plays an important role in providing employment, income, animal protein for urban and rural dwellers as well as manure for crop production (Abebe and Gugsa 2018; Ola-Fadunsin et al. 2019a). In Nigeria, the poultry subsector is one of the most important components of the agricultural sector, providing animal proteins such as meat and eggs to man, help in alleviating problems associated with poverty, as well as contributing greatly to the national income through revenue (Lawal et al. 2015; Ola-Fadunsin 2017). Nigeria records the largest poultry population in Africa with an estimated poultry population of about 130–150 million chickens (Mshelia et al. 2016).
Coccidiosis is a disease condition caused by a protozoan parasite of the genus Eimeria, Family Eimeridae, Order Eimeriorina and Phylum Apicomplexa (Taylor et al. 2016). It is known to affect a wide range of animals including chickens (Soutter et al. 2021). Chicken coccidiosis is known to be caused by seven different species of Eimeria (Eimeria acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox and E. tenella) (Chapman 2014; Prakashbabu et al. 2017). Chicken coccidiosis is cosmopolitan in nature and it is found anywhere chickens are reared. This disease condition remains one of the most economically important parasitic disease in the poultry industry worldwide (Ola-Fadunsin and Ademola 2014; Ola-Fadunsin et al. 2019b). Chicken coccidiosis is associated with different clinical signs such as anorexia, dysentery, enteritis, diarrhea (which may be bloody with certain Eimeria species), emaciation, pale comb, lower feed conversion rate, delayed sexual maturity, drooping wings, poor growth rate, low production, and even death (Ola-Fadunsin 2017; Hamid et al. 2018). Coccidiosis is responsible for an annual global loss of approximately £10.4 billion in the poultry industry (Blake et al. 2020) and this economic loss covers the cost of prophylaxis, treatment, losses due to death, and development of novel anticoccidial vaccines (Blake et al. 2020).
With the devastating economic impact of the disease caused by these protozoan parasites in chickens, there is need to determine the nation-wide prevalence, the species diversity, and the clinico- pathological changes associated with the infection among commercial laying chickens in Nigeria.
Materials and methods
This study was conducted by using faecal samples and carcasses of laying commercial chickens from different parts of Nigeria. Nigeria covers 909,890 square kilometers of land area and 13,879 square kilometers of water area, and is situated between 3° and 14° East Longitude and 4° and 14° North Latitude. Nigeria is bordered on the east by the Republic of Cameroon; on the west by the Republics of Benin and Niger; on the north by Niger and Chad Republics and on the south by the Gulf of Guinea. There are two climatic seasons in Nigeria; the wet and dry seasons. The wet season lasts from April to October, while the dry season from November through March (NBS 2016).
The laboratory studies were conducted at the National Veterinary Research Institute (NVRI), Nigeria. The National Veterinary Research Institute, is the major Veterinary reference Research Institute in Nigeria. The Institute is located in Vom, Jos South Local Government Area of Plateau State (Akanbi et al. 2022).
Study animals
A total of 1843 laying commercial chickens (moribund and dead) were used for this study. These birds were brought from 28 of the 36 states of Nigeria, and from the Federal Capital Territory (FCT) of Nigeria to the Central Diagnostic Laboratory of the NVRI. Commercial laying chickens were brought over a ten years period (2007–2016) for different veterinary health challenges. The age of each commercial layer was determined. Also, the year and the month the birds were presented were taken into consideration.
Sample collection
Freshly passed faecal samples were collected from moribund commercial laying chickens, while intestinal and caecal contents and the entire intestinal tracts were collected from moribund and dead laying chickens following gross pathological examination. The faecal samples, intestinal and caecal contents were collected into separate sterile and well-labeled polyethylene bags and then taken to the Parasitology laboratory for parasitological studies, while the intestinal tracts were used for pathological studies in the Pathology laboratory. Both laboratories are part of the Central Diagnostic Laboratory of the NVRI, Vom, Plateau State.
Gross pathology
Carcasses were examined fully and the entire intestines including the caeca of all the presented commercial laying birds were pathologically examined. The intestines and caeca of the carcasses were examined as described by Akanbi et al. (2021). No lesion was scored 0 and the most severe lesion was scored 4 according to the gross lesion score criteria for E. tenella (Johnson and Reid 1970). A gross score of 1, described caeca with petechiation, and few scattered lesions, but with normal wall of the caeca and content. Score 2 described caeca petechiation, with more numerous lesions, thickened caeca wall, and bloody mucosa. A score of 3 described a bloody caecum with coalescing lesions and greatly thickened caeca wall, fibrin clots with little or no faecal debris. While a score of 4 described a bloody caecum, with bluish-black colouration, coalescent lesions, and swollen and thickened caeca wall with bloody, caseous clots, and no faecal debris.
Histopathology
Histopathological examination of the intestines including the caeca were carried out immediately after processing. Briefly, intestinal and caecal samples were fixed in 10% buffered formalin, embedded in paraffin wax, sectioned at 5 µm thickness, stained with haematoxylin and eosin (H&E) stain, cleared in xylene, and mounted in a mountant as previously described by Akanbi et al. (2021). Additionally, tissue sections were stained with periodic acid Schiff (PAS) to demonstrate the Eimeria oocysts.
Parasitological analysis
Faecal samples, intestinal and caecal contents were examined for the presence of Eimeria oocyst using the simple flotation technique. Positive samples were further subjected to the McMaster counting technique so as to determine the intensity of Eimeria infections. Both aforementioned techniques were carried out as described by Soulsby (1982). Samples positive for Eimeria oocysts were subjected to sporulation as described by Al-Quraishy et al. (2009) with slight modifications. Faecal samples, intestinal and caecal contents positive for Eimeria were placed in petri dishes and made damp (not wet). About 2.5% potassium dichromate (K2Cr2O7) solution was then added to the sample and allowed to stand for 5–7 days at room temperature away from sun rays to permit the coccidian oocysts to sporulate. After sporulation, flotation concentration technique was used to examine the sporulated oocyst using the X40 objective lenses of the OLYMPUS (B-Bran) light microscope (Olympus Co, Japan). Identification of sporulated Eimeria oocysts was carried out based on the oocyst size and shape using morphological keys by Conway and McKenzie (2007) and Taylor et al. (2007).
Statistical analyses
Data were entered in Microsoft® office Excel version 2016 for the determination of prevalence (%), mean, and standard error of mean (SEM). The Statistical Package for the Social Sciences (SPSS, Chicago, Illinois, USA) for windows version 22.0 was used to analyze the Chi-Square value, the odds ratio with 95% confidence interval (CI), and the statistical association between each epidemiological factor and the presence/absence of Eimeria oocysts. The odds ratios were calculated with respect to a reference category as indicated in the respective tables. Significant level was set at P < 0.05.
Results
Of the 1843 commercial laying birds examined, 207 were positive for Eimeria infections, representing 11.23% of the studied birds, with a 95% confidence interval of 9.87–12.75.
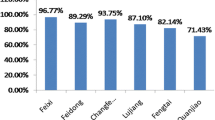
Eimeria oocyst were detected in 9 of the 28 states (32.14%) where samples were brought from, and the FCT. The prevalence of Eimeria infections in commercial laying chickens within the states in Nigeria ranged between 4.4% (Kaduna State), and 33% (Ebonyi and Lagos States), and 21.1% in FCT (Fig. 1).
Seven Eimeria species were recorded in this study (E. tenella, E. maxima, E. necatrix, E. acervulina, E. mitis, E. brunetti, and E. praecox), with E. tenella and E. necatrix been the most prevalent with 3.15% and 2.06% prevalence respectively. Eimeria praecox (0.81%; 95% CI = 0.47–1.31) and E. mitis (1.09%; 95% CI = 0.68–1.64) were the least prevalent. The highest mean oocyst per gram (OPG) of faeces was recorded in E. acervulina (5260.87 (± 1838.35)), and the lowest OPG was recorded in E. tenella (2872.41 (± 505.92)) (Table 1).
The yearly distribution of Eimeria infections among commercial laying birds in Nigeria (2007–2016) shows that the infection was present all through the years under review. The highest prevalence was recorded in 2007 having a prevalence of 64.29% (36/56), and the lowest prevalence was recorded in 2008 with a prevalence of 3.57% (6/168). Other yearly prevalence ranged between 4.35% in 2009 and 20.43% in 2016. There was a significant difference (P < 0.05) in the prevalence of Eimeria parasites within the studied years compared to 2016 with exception of 2012. The detection of Eimeria species was about 7 times more in 2007 compared to 2016, while its detection was more likely to occur in 2016 compared to the other years (2008–2015) (Table 2).
Eimeria oocysts were detected in all the months with the highest prevalence observed in July (24.00%), August (16.67%), and June (15.13%), while the lowest prevalence was recorded in March (2.73%), April (7.48%), and February (8.02%). There was a significant difference in the detection of Eimeria protozoan in March and July compared to December, with a Chi Square value of 60.73 (Table 3).
There was a higher prevalence of Eimeria infections in commercial laying birds that are less than 1 year compared to those that are above a year old, although the difference was not significant (P = 0.18). The occurrence of Eimeria infections was higher during the wet season compared to the dry season, and the difference was significant (P = < 0.01). Eimeria infections was 2.19 times more likely to occur during the wet season compared to the dry season (95% CI = 1.59–3.06; χ2 = 23.29) (Table 4).
Of the 1843 commercial laying birds’ carcasses examined, and the 207 which fecal samples/intestinal and caecal contents were positive for Eimeria species, 185 carcasses showed moderate petechiae to ecchymotic intestinal/caecal mucosa, while 22 carcasses showed severe petechiae to ecchymotic intestinal/caecal mucosa with bloody intestinal lumen. Clinical signs of anorexia, bloody diarrhea, emaciation, pale comb, lower feed intake and low conversion rate, drooping wings, low production, and even death were reported in the history from the various farms of confirmed coccidia positive flocks. In addition to the above lesions, caeca of commercial laying chicken were ballooned and filled with bloody content due to Eimeria tenella infection. Histopathological examination of the intestines and caeca of infected chickens revealed intestinal mucosa with ecchymotic haemorrhages and intestinal crypts, laden with and displaced by medium to large sized Eimeria schizonts, merozoites, oocysts and severe infiltration and intestinal villi and enterocytes are displaced by Eimeria oocysts and polymorphonuclear inflammatory cells at low (X100) and high (X400) powered field of Carl Zeiss binocular microscope. Detailed gross and histopathological presentations are shown in Fig. 2.
A Commercial laying chickens infected with coccidiosis with bloody diarrhoea. B Intestine, commercial laying chicken with widespread serosal petechiae haemorrhages (arrowheads). C Caeca, commercial laying chicken, ballooned caeca filled with bloody content (arrowhead) due to Eimeria tenella infection (caecal coccidiosis). D Intestine, and caecum, chicken, intestinal mucosa with ecchymotic haemorrhages. E intestinal crypts, chicken, crypts are laden and displaced by medium to large sized Eimeria schizonts (arrows) and severe infiltration by polymorphonuclear inflammatory cells (asterisks), H&E X100. F Intestinal villi, enterocytes are displaced by Eimeria oocysts (arrow) H&E X100
Discussion
This study appears to be the first nation-wide study on the prevalence and clinico-histopathological changes associated with Eimeria infections in commercial laying birds in Nigeria. A better understanding of the diversity and pathologies of Eimeria species in Nigeria will reinforce the management and control of coccidiosis in the poultry industry of the country. The total prevalence of 11.23% recorded among commercial laying birds in our nation-wide study is within the reported prevalence of 3.30%, 29.00%, and 49.02% reported among laying birds in Borno State, north-west Nigeria (Lawal et al. 2016), Osun State, south-west, Nigeria (Ola-Fadunsin 2017) and Kwara State, north-central Nigeria (Ola-Fadunsin et al. 2019b) respectively. Higher prevalence of 25.00% and 59.60% has been reported among laying birds in Nepal (Adhikari et al. 2008) and Pakistan (Bachaya et al. 2012) respectively. The disparity in the prevalence of Eimeria infections among commercial laying birds could be multifactorial, as climatic conditions, geographical area, sample size, sampling periods, age of birds, production systems, and study design, could be responsible for the differences (Lawal et al. 2016; Ola-Fadunsin 2017; Ola-Fadunsin et al. 2019b). The detection of Eimeria oocysts in different regions in Nigeria, suggest that the protozoan is of great economic importance to the poultry sector of the country.
We reported seven different species of Eimeria (E. tenella, E. maxima, E. necatrix, E. acervulina, E. mitis, E. brunetti, and E. praecox) in this study. In similar manner, Jatau et al. (2012), Agishi et al. (2016), and Ola-Fadunsin et al. (2019b) reported the same number and species of Eimeria in their studies conducted in different parts of Nigeria. Eimeria tenella and E. necatrix were the most prevalent species detected in this study. These species have been documented to be the most prevalent Eimeria species of laying birds within and outside Nigeria (Adhikari et al. 2008; Agishi et al. 2016). The mean oocyst per gram of Eimeria species ranging from 2872.41 to 5260.87 we observed in this study is an indication that the protozoan is a major colonist of the gastrointestinal tracts of chickens.
The monthly and yearly distribution of Eimeria infections among commercial laying birds in this study showed no defined pattern in its prevalence, as the protozoan was detected in all the months and years of study. The undefined monthly and yearly pattern of Eimeria infections could be attributed to the fact that the protozoan is cosmopolitan in nature and that it can be found anywhere and at every time in places where chickens are reared (Ola-Fadunsin et al. 2019b).
Eimeria oocysts can be found in all ages of birds (Lawal et al. 2016), with higher prevalence recorded among younger birds compared to older birds (Bachaya et al. 2012; Adang and Isah 2016; Wondimu et al. 2019). Similarly, we detected the enteric protozoan in both young and old laying birds, with higher prevalence detected in the former.
Significantly higher prevalence of Eimeria infections was observed during the wet season compared to the dry season. Awais et al. (2012), Grema et al. (2014) and Ola-Fadunsin (2017) documented higher prevalence of Eimeria infections of poultry during the wet season compared to the dry season, with the difference been statistically significant. Warm and humid environmental conditions favour the sporulation of Eimeria oocyst, and in turn increases the infection rate of the enteric protozoan in animals including chickens.
Clinical signs of anorexia, bloody diarrhea, emaciation, pale comb, lower feed intake and low conversion rate, drooping wings, low production, and even death reported in the history from the various farms of confirmed Eimeria positive flocks are consistent with the disease (Taylor et al. 2016). Farm history of signs of coccidiosis correlates the clinico-pathological changes observed in the examined carcasses from Eimeria infected flocks. Pathological lesions of coccidiosis were observed in the confirmed Eimeria infected flocks. Only the 207 confirmed coccidiosis infected carcasses showed lesions consistent with coccidiosis.
Conclusion
All seven Eimeria species causing chicken coccidiosis (Eimeria acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox and E. tenella) were isolated and are responsible for coccidiosis in Nigeria. Rainfall and higher humidity predispose to the increased prevalence of eimeriosis during the wet season compared to the dry season. This study appears to be the first nation-wide study on the prevalence and clinico-histopathological changes associated with Eimeria infections in commercial laying birds in Nigeria. There is need for concerted efforts in controlling coccidiosis among laying birds in Nigeria.
References
Abebe E, Gugsa G (2018) A review on poultry coccidiosis. Abyssinia J Sci Technol 3(1):1–12
Adang LK, Isah Z (2016) Prevalence of Eimeria species in local breed chickens in Gombe metropolis, Gombe State, Nigeria. Int J Biol Chem Sci 10(6):2667–2676
Adhikari A, Gupta R, Pant GR (2008) Prevalence and identification of coccidian parasite (Eimeria spp) in layer chicken of Ratnanagar Municipality, Chitwan District, Nepal. J Nat Hist Mus 23:45–50
Agishi G, Luga II, Rabo JS (2016) Prevalence of Coccidiosis and Eimeria species in layers and broilers at slaughter houses in Makurdi, Benue State. Int J Eng Sci 5(2):8–11
Akanbi OB, Taiwo VO, Ola-Fadunsin SD (2021) Immunisation of chickens with commercial anticoccidial vaccines IMMUCOX and LIVACOX showed varied protection against virulent local isolate of Eimeria tenella and Houghton strain. Bulg J Vet Med. https://doi.org/10.15547/bjvm.2021-0045
Akanbi OB, Ola-Fadunsin SD, Yahaya S, Kaye R, Shamaki R (2022) Parasites and parasitic diseases of laboratory animals in Plateau State Nigeria: the zoonotic implications. J Parasit Dis 46(1):56–63. https://doi.org/10.1007/s12639-021-01420-y
Al-Quraishy S, Abdel-Baki AS, Dkhil MA (2009) Eimeria tenella infection among broiler chicks Gallus domesticus in Riyadh city, Saudi Arabia. J King Saud Univ Sci 21:191–193
Awais MM, Akhtar M, Iqbal Z, Muhammad F, Anwar MI (2012) Seasonal prevalence of coccidiosis in industrial broiler chickens in Faisalabad, Punjab, Pakistan. Trop Anim Health Prod 44(2):323–328
Bachaya HA, Raza MA, Khan MN, Iqbal Z, Abbas RZ, Murtaza S, Badar N (2012) Predominance and detection of different Eimeria species causing coccidiosis in layer chickens. J Anim Plant Sci 22(3):597–600
Blake DP, Knox J, Dehaeck B, Huntington B, Rathinam T, Ravipati V, Ayoade S, Gilbert W, Adebambo AO, Jatau ID, Raman M, Parker D, Rushton J, Tomley FM (2020) Re-calculating the cost of coccidiosis in chickens. Vet Res 51(1):115. https://doi.org/10.1186/s13567-020-00837-2
Chapman HD (2014) Milestones in avian coccidiosis research: a review. Poult Sci 93:501–511. https://doi.org/10.3382/ps.2013-03634
Conway DP, McKenzie ME (2007) Poultry coccidiosis diagnostic and testing procedures, 3rd edn. Blackwell, Hoboken
FAO Stat (2015) Food and Agriculture Organization of the United Nations FAOstatdatabase
Grema HA, Suleiman A, Rabana JL, Geidam YA (2014) A six year (2005–2010) retrospective study of avian coccidiosis diagnosed in Gombe veterinary clinic, Nigeria. Sokoto J Vet Sci 12(2):8–13
Hamid PH, Kristianingrum YP, Wardhana AH, Prastowo S, da Silva LMR (2018) Chicken coccidiosis in Central Java, Indonesia: a recent update. Vet Med Int. https://doi.org/10.1155/2018/8515812
Jatau D, Sulaiman NH, Musa IW, Lawal AI, Okubanjo OO, Isah I, Magaji Y (2012) Prevalence of coccidia infection and preponderance Eimeria species in free range indigenous and intensively managed exotic chickens during hot-wet season, in Zaria, Nigeria. Asian J Poult Sci 6:79–88
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28:30–36
Lawal JR, Jajere SM, Mustapha M, Bello AM, Wakil Y, Geidam YA, Ibrahim UI, Gulani IA (2015) Prevalence of newcastle disease in Gombe, Northeastern Nigeria: a ten-year retrospective study (2004–2013). Br Microbiol Res J 6(6):367–375
Lawal JR, Jajere SM, Ibrahim UI, Geidam YA, Gulani IA, Musa G, Ibekwe BU (2016) Prevalence of coccidiosis among village and exotic breed of chickens in Maiduguri, Nigeria. Vet World 9(6):653–659
Mshelia IT, Atsanda NN, Bitrus AA, Mohammed AB, Fika II, Balami SB, Jauro S, Malgwi SA (2016) Retrospective study of selected endemic viral diseases of poultry diagnosed in Maiduguri North-Eastern Nigeria. J Anim Health Prod 4(2):60–64
National Bureau of Statistics (NBS) (2016) Annual abstract of statistics. Federal Republic of Nigeria
Ola-Fadunsin SD (2017) Investigations on the occurrence and associated risk factors of avian coccidiosis in Osun State, Southwestern Nigeria. J Parasitol Res. https://doi.org/10.1155/2017/9264191
Ola-Fadunsin SD, Ademola IO (2014) Anticoccidial effects of Morinda lucida acetone extracts on broiler chickens naturally infected with Eimeria species. Pharm Biol 52(3):330–334. https://doi.org/10.3109/13880209.2013.836545
Ola-Fadunsin SD, Ganiyu IA, Rabiu M, Hussain K, Sanda IM, Musa SA, Uwabujo PI, Furo NA (2019a) Gastrointestinal parasites of different avian species in Ilorin, North Central Nigeria. J Adv Vet Anim Res 6(1):108–116. https://doi.org/10.5455/javar.2019.f320
Ola-Fadunsin SD, Uwabujo PI, Sanda IM, Hussain K, Ganiyu IA, Rabiu M, Balogun RB (2019b) Cross-sectional study of Eimeria species of poultry in Kwara State, North-Central Nigeria. J Parasit Dis 43(1):87–95. https://doi.org/10.1007/s12639-018-1062-3
Prakashbabu BC, Thenmozhi V, Limon G, Kundu K, Kumar S, Garg R, Clark EL, Srinivasa Rao ASR, Raj DG, Raman M, Banerjee PS, Tomley FM, Guitian J, Blake DP (2017) Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet Parasitol 233:62–72
Soulsby EJL (1982) Helminths, arthropods and protozoa of domestic animals, 7th edn. Bailliere Tindall, London
Soutter F, Werling D, Kim S, Pastor-Fernández I, Marugán-Hernández V, Tomley FM, Blake DP (2021) Impact of Eimeria tenella oocyst dose on parasite replication, lesion score and cytokine transcription in the caeca in three breeds of commercial layer chickens. Front Vet Sci 8:640041. https://doi.org/10.3389/fvets.2021.640041
Taylor MA, Coop RL, Wall RL (2007) Veterinary parasitology, 3rd edn. Black Well, Oxford
Taylor MA, Coop RL, Wall RL (2016) Veterinary Parasitology, 4th edn. Wiley Blackwell, West Sussex
Wondimu A, Mesfin E, Bayu Y (2019) Prevalence of poultry coccidiosis and associated risk factors in intensive farming system of Gondar town, Ethiopia. Vet Med Int. https://doi.org/10.1155/2019/5748690
Acknowledgements
The authors appreciate the technical assistance of Mr. Gyang Dung, Mr. Alesa, Mrs. Olabode and Mrs. Gyang of the Central Diagnostic Laboratory.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest among them.
Ethical approval
This study was approved by the Animal Welfare and Ethics Committee of the National Veterinary Research Institute, Vom, Nigeria.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akanbi, O.B., Ola-Fadunsin, S.D., Odita, C.I. et al. Eimeria infections among commercial laying chickens in Nigeria: the prevalence and clinico-histopathological changes. J Parasit Dis 46, 860–868 (2022). https://doi.org/10.1007/s12639-022-01509-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-022-01509-y