Abstract
The central nervous system of the intermediate host plays a central role in lifelong persistence of Toxoplasma gondii as well as the pathogenesis of congenital toxoplasmosis and reactivated infection in immunocompromised individuals. The purinergic system has been implicated in a wide range of immunological pathways for controlling intracellular responses to pathogens, including T. gondii. In the present study, we investigated the effect of resveratrol (RSV) on ectonucleotidases, adenosine deaminase (ADA), and purinergic receptors during chronic infection by T. gondii. For this study, Swiss mice were divided into control (CTL), resveratrol (RSV), infected (INF), and INF+RSV groups. The animals were orally infected with the VEG strain and treated with RSV (100 mg/kg, orally). Ectonucleotidase activities, P2X7, P2Y1, A1, and A2A purinergic receptor density, ROS, and thiobarbituric acid reactive substances levels were measured in the cerebral cortex of mice. T. gondii infection increased NTPDase and reduced ADA activities. Treatment with RSV also affected enzymes hydrolysing extracellular nucleotides and nucleosides. Finally, RSV affected P1 and P2 purinergic receptor expression during T. gondii infection. Overall, RSV-mediated beneficial changes in purinergic signalling and oxidative stress, possibly improving cerebral cortex homeostasis in T. gondii infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasmosis is an anthropozoonosis of warm-blooded hosts, caused by the intracellular protozoan Toxoplasma gondii. It affects an enormous proportion of the world’s population (Tenter et al. 2000; Hill & Dubey, 2006). T. gondii traffics to the central nervous system (CNS), where causes neurotoxoplasmosis (McAuley, 2014). Intracerebral proliferation of T. gondii in immunocompromised individuals easily disseminates in neurons, and astrocytes can lead to Toxoplasma encephalitis, characterised by high morbidity and mortality rates (Cook et al. 2015).
Dissemination of T. gondii throughout the host CNS is thought to be mediated by infected immune cells, including phagocytic brain macrophages, which efficiently restrain parasite growth and act as important inhibitors of T. gondii spread throughout the CNS (Hitziger et al. 2005; Mendez and Koshy, 2017). However, the parasite can manipulate host resistance mechanisms at several points along inflammatory pathways, including purinergic and oxidative systems (Petit-Jentreau et al., 2017). This delicate balance between host and parasite survival promotes modifications of extracellular nucleotide and nucleoside levels and causes excessive tissue damage, leading to host cell apoptosis (Chaudhary et al. 2004).
Purinergic signalling enzymes and receptors participate in many vital functions in the CNS, including neurotransmission, synaptic plasticity, neuromodulation, and regulation of inflammatory responses (Burnstock & Boeynaems, 2014). The P2X7 receptor recently emerged as an important component of the innate immune response against infectious diseases including T. gondii infection. Several studies have implicated the role of the purine nucleotide ATP and the P2X7 receptor in the immune response and clearance of T. gondii (Lees et al., 2010; Corrêa et al., 2010). ATP acts as an important extracellular messenger produced as a result of cellular damage (Gallucci & Matzinger, 2001).
ATP binding to the P2X7 receptor initiates a cascade of mechanisms in order to remove the pathogen by fusion of phagosomes, production of reactive oxygen species (ROS), and modulation of host cell apoptosis during T. gondii infection (Corrêa et al., 2010).
Similarly, the stimulation of adenosine receptors by extracellular adenosine plays an important role as a homeostatic modulator in the CNS (Cunha, 2001). Several studies have highlighted the fact that CD73 enzyme knockout mice that lack the ability to generate extracellular adenosine are protected from T. gondii chronic infection; infected animals are characterised by significantly fewer parasite cysts (Mahamed et al., 2012, 2015). A1 and A2A receptors are widely expressed in the CNS; their activation may be an important stop signal to prevent excessive stimulation of inflammation, avoiding excessive cellular damage in the pathogenesis of infectious diseases such as toxoplasmosis (Thiel et al., 2003). Some studies conducted by our research group have reported the influence of T. gondii infection on purine enzymes and receptors in cultures of infected neural precursor cells (Bottari et al., 2018a, b). In the present study, we determined, for the first time, the regulatory effect of RSV on the purinergic system during in vivo chronic infections by T. gondii.
Identification of metabolic differences in infected host cells and comprehension of molecular mechanisms underlying T. gondii infection has become relevant to define new targets for pharmacological agents, leading to more effective therapeutic approaches for neurotoxoplasmosis. In this study, we investigated the effects of resveratrol (RSV), a non-flavonoid polyphenol, naturally present in a number of dietary sources including red grapes, berries (e.g. cranberries, bilberries, blueberries), peanuts, and red wine (Frémont, 2000). A study showed that RSV has pleiotropic activities, including antioxidant and anti-inflammatory effects, as well as antiapoptotic actions, supporting the idea that this is one of the most promising compounds for the treatment of neurological conditions and diseases (Baur and Sinclair, 2006).
A prior study conducted by our research group showed that RSV improved behavioural alterations and attenuated tissue inflammatory processes in the brains of mice infected by T. gondii (Bottari et al. 2016). RSV counteracted the effects of T. gondii on enzymes hydrolysing extracellular nucleotides and nucleosides in an in vitro study using neural progenitor cells (Bottari et al., 2018a, b). Against this background, in the present study, we determine whether treatment with RSV would affect purinergic signalling and reduce oxidative damage in the cerebral cortex of mice infected with T. gondii in order to preserve CNS homeostasis.
Material and methods
Animals and infection
For this study, we maintained 40 Swiss female mice with a mean age of 60 days, weighing 25 ± 5 g, in boxes of five animals each, under a 12-h light/dark cycle with controlled temperature and humidity (25 °C, 70%, respectively). The animals went through an adaptation period of 10 days and were fed with commercial food and water ad libitum. All animal procedures were approved by Ethics Committee on the Use of Animals from Federal University of Santa Maria (protocol number: 95090109/15).
The T. gondii VEG strain-type III was kept in the laboratory using mice as hosts, performing a constant passage from one animal to another in order to maintain virulence. Animals were orally infected with 100 μL of cerebral homogenate containing 30 parasitic cysts of T. gondii. For verification of infection, one of the animals of this group was euthanised, and brain homogenate containing tissue cysts was used for inoculum.
RSV treatment
RSV (C14H12O3; molecular weight 228.25 g/mol; purity of > 98%) purchased from Sigma-Aldrich (St. Louis, MO, USA) was diluted in 0.1% dimethylsulfoxide (DMSO) and water to a final concentration of 100 mg/kg. RSV was orally administered for 10 days to adult mice, as previously described by Bottari et al. (2016).
Experimental design
Mice were randomly divided into four experimental groups (n = 10): control (CTL), resveratrol (RSV), infected (INF), and INF treated with RSV (INF+RSV). Twenty days post-infection, animals were further treated with RSV over the subsequent 10 days. After 30 days, the animals were anaesthetised under isoflurane before cardiac puncture and were euthanised. The brains were removed and the cortexes were isolated for subsequent analyses.
Brain tissue preparation
The brains were removed and separated into cerebral cortex sections. Total cerebral cortexes were weighed and allocated into test tubes. Hemispheres were homogenised at a 1:10 volume ratio in 10 mM Tris–HCl, pH 7.2, buffer. All these procedures were performed at 4 °C.
Enzymatic assays
E-NTPDase and E-5′-nucleotidase (5′-NT) enzymatic activities of cerebral cortex were determined using the methods described by Schetinger et al. (2000) and Heymann et al. (1984), respectively. Enzymatic preparations (20 μL; 8–12 μg of protein) were added to the system for E-NTPDase or 5′-nucleotidaseand pre-incubated at 37 °C for 10 min. The reactions were initiated by the addition of substrate (ATP, ADP, or AMP). Enzyme activities are reported as nmol Pi released/min/mg protein.
ADA activity of cerebral cortex was determined according to Guisti & Galanti (1984). Brain samples (50 μL of homogenates) were added to 21 mM/L of adenosine pH 6.5 and incubated for 60 min at 37 °C. The results were expressed as U ADO/mg protein.
Western blot
Samples of the cerebral cortex were homogenised in ice-cold radioimmunoprecipitation assay buffer (RIPA buffer) with 1 mM protease and phosphatase inhibitors and centrifuged at 10.000 g at 4 °C for 10 min. Protein concentrations were determined using the BCA Protein Assay Kit (Sigma-Aldrich). Diluted samples were separated using sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Amersham Biosciences, UK). After blocking, membrane samples were incubated overnight at 4 °C with primary antibodies purchased from Santa Cruz Biotechnology directed against the P2X7 receptor (dilution 1:500), the P2Y1 receptor (dilution 1:500), the adenosine A1 receptor (dilution 1:500, Santa Cruz Biotechnology, CA, USA), or the A2A (1:800); membranes were incubated with anti-rabbit or anti-mouse secondary antibodies (dilution 1:10.000, Santa Cruz Biotechnology) for 90 min at room temperature. The membranes were incubated with an enhanced chemifluorescent substrate (Amersham Biosciences) and analysed with an Amersham Imager 600. The membranes were re-probed and tested for β-actin immunoreactivity as a control for protein concentration as described by Rebola et al. (2003).
Reactive species and thiobarbituric acid reactive substance measurements
Intracellular reactive oxygen species were measured by 2′-7′-dichlorofluorescein (DCF) levels by Myhre et al. (2003). DCF levels were determined using a standard curve of DCF, and the results were expressed as log U DCF/mg protein.
The lipid peroxidation was determined by thiobarbituric acid reactive substance (TBARS) levels according to Ohkawa et al. (1979) as an end-product of lipid peroxidation by reaction with thiobarbituric acid (TBA). The results of TBARS levels were expressed as nmol MDA/mg protein.
Protein determination
Protein levels in SDS-PAGE were measured by Coomassie blue method as previously described by Bradford (1976) using bovine albumin serum as standard.
Quantitative cysts distribution and histopathology analysis in cerebral cortex
The distributions of T. gondii cysts and histopathological lesions in the brains of infected mice were analysed using standard histological techniques. Three cerebral cortex each group were fixed in formaldehyde (10% in PBS) at pH 7.2 for subsequent dehydration and paraffin embedding. Slices of 5 μm were prepared and stained with haematoxylin-eosin (H&E). The images were obtained by digital camera and light microscope images. The cyst number was counted twice in 10 μL of the homogenate cortex using light microscopy.
Statistical analysis
Results were expressed as mean values ± standard error of the mean (SEM). Statistical analysis was assessed by two-way analysis of variance (ANOVA) followed by the Tukey’s post hoc test using the GraphPad Prism (Version 5.0) software. Differences between mean values were considered statistically significant at *p < 0.05 from the control group or #p < 0.05 from the infected group.
Results
RSV modulates ectonucleotidase and ADA enzyme activities in the cerebral cortex of mice infected by T. gondii
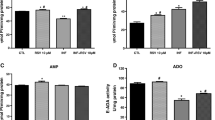
The results obtained for E-NTPDase, E-5′-NT, and ADA activities are shown in Fig. 1. ATP, ADP (Fig. 1a, b) hydrolysis by NTPDase, and AMP (Fig. 1c) hydrolysis by 5′-NT were significantly increased (50%, 2%, and 48%, respectively) in the INF group compared with the CTL group. Treatment with RSV significantly decreased ATP (20%) and AMP (10%) hydrolysis by NTPDase and 5′-NT, respectively, in the INF+RSV group compared with the INF group, possibly acting as an anti-inflammatory molecule on these enzymes.
Effects of RSV on nucleoside triphosphate diphosphohydrolase (NTPDase), 5′-nucleotidase (5′-NT), and adenosine deaminase (ADA) activities in the cerebral cortex of mice infected by T. gondii. a ATP hydrolysis. b ADP hydrolysis. c AMP hydrolysis. d Adenosine as a substrate. Data represent mean values ± SEM (n = 10 animals per group) analysed by two-way ANOVA with Tukey’s post hoc test. *p < 0.05 (*significant differences from the control group; #significant differences from the infected group)
Infected mice by T. gondii presented a reduction in ADA activity (62%) when compared with the control group. RSV treatment was able to increase ADA activity (8%) in the cerebral cortex of infected mice when compared with the INF group, possibly due to RSV neuroprotector effects (Fig. 1d).
RSV-mediated reversal of changes in purine receptors in the cerebral cortex of mice infected by T. gondii
Considering the alterations of ectonucleotidases enzymes by T. gondii infection, P1 and P2 purinergic receptor subtype expression patterns were determined by Western blot (Fig. 2). The cerebral cortex showed a significant increase in P2X7 receptor expression in the INF group in comparison with the CTL group. RSV treatment decreased P2X7 receptor density in a per se effect, probably due to its anti-inflammatory property. In addition, RSV diminished P2X7 receptor expression in the infected treated groups (INF+RSV) when compared with controls (p < 0.05) (Fig. 2a).
Western blot analysis of P2X7 (a), P2Y1 (b), A1 (c), and A2A (d) receptors’ protein expression in samples of the cerebral cortex of mice treated with RSV and infected by T. gondii. β-actin was used as a loading control to normalise protein levels. Values are expressed as mean values ± S.E.M (n = 10 animals per group) analysed by two-way ANOVA with Tukey’s post hoc test. *p < 0.05 (*significant differences from the control group; #significant differences from the infected group)
T. gondii infection induced an increase in P2Y1 receptor expression in the cerebral cortex (p < 0.05) when compared with the CTL group (Fig. 2b). RSV significantly reduced of P2Y1 receptor expression levels in the infected treated (INF+RSV) groups when compared with INF and CT (control) groups (p < 0.05), demonstrating the influence of RSV on P2 receptors.
A1 receptor density was significantly increased in the INF group compared with the CTL group (Fig. 2c). On the other hand, infected animals treated with RSV (INF+RSV) increased A1 receptor expression in the cerebral cortex when compared with the INF group, suggesting that RSV could trigger a protector mechanism against T. gondii infection. A2A receptor density showed a decrease in the INF group in comparison with the CTL group (Fig. 2d). Instead, infected animals treated with RSV (INF+RSV) decreased A2A receptor density when compared with the INF group, as a possible mechanism to attenuate neuroinflammation.
RSV attenuates lipid peroxidation and reactive species triggered by T. gondii infection in the cerebral cortex
Once T. gondii crosses the blood-brain barrier, the parasite invades the host cell and triggers an inflammatory response in the brain. Thus, we hypothesised that the presence of T. gondii cysts in the cerebral cortex establishes an oxidative process leading to the apoptosis of the host cell and the release of high ATP levels. In this sense, ROS and TBARS were determined (Fig. 3). Production of RS was significantly increased in the INF group compared with the control (p < 0.05). However, infected animals treated with RSV (INF+RSV) demonstrated decreased RS production compared with the control group (Fig. 3a).
RSV treatment decreases lipidic peroxidation and reactive species in the cerebral cortex induced by T. gondii in mice. a Intracellular reactive species production by 2′-7′-dichlorofluorescein levels in the cerebral cortex of T. gondii-infected mice treated with RSV. b Lipid peroxidation measured by thiobarbituric acid reactive substances in the cerebral cortex of T. gondii-infected mice treated with RSV. The data represents mean values ± SEM (n = 10 animals per group). ANOVA followed by Tukey’s post hoc test. *p < 0.05 (*significant differences from the control group; #significant differences from the infected group)
Besides the formation of intracellular reactive species, T. gondii also induced membrane damage (Fig. 3b). TBARS levels were significantly increased in the INF group compared with the control group (p < 0.05). Infected animals treated with RSV showed decreased TBARS levels in their cerebral cortex compared with the INF group. Treatment with RSV per se had no influence on TBARS levels (p > 0.05).
Histopathological impacts of RSV in brain of infected mice
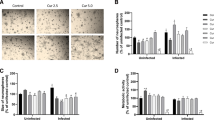
In order to provide parasite infection and verify brain alterations induced by T. gondii cysts, number and cortex histopathology were performed (Fig. 4). The data shows presence of T. gondii cysts in cortex of infected mice (7 ± 1.38) (Fig. 4a). Moderate inflammatory infiltrates composed of lymphocytes, plasma cells, epithelioid macrophages, and neutrophils can be observed (Fig. 4c) in infected animals. However, RSV treatment reduces inflammatory infiltrates (Fig. 4d) but not cyst number (5 ± 1.94) (Fig. 4f) in infected animals. No significant differences were observed in cyst number quantification between groups.
Histopathological impacts of RSV in brain of infected mice. a Brain histopathology of T. gondii-infected mice showing parasitic cysts (arrow). b Brain histopathology of T. gondii-infected and treated with RSV mice. c Moderate inflammatory infiltrates (*) composed of lymphocytes, plasma cells, epithelioid macrophages, infiltration of macrophages, and neutrophils. d Light lymphoplasmacytic inflammatory infiltrate (*) consisting predominantly of lymphocytes and plasma cells, as well as some macrophages and presence of parasitic cysts of T. gondii (arrow). e Parasitic cysts measuring about 30 μm diameter and filled with bradyzoites. f Quantitative cyst distribution (number cysts/μl homogenate) in cortex of mice infected by T. gondii. The data represents mean values ± SEM (n = 5 animals per group) analysed by Student’s t test (no difference between groups; p > 0.05)
Discussion
Different mechanisms have been developed by host cells to limit the immune response during chronic toxoplasmosis disease and to eliminate the parasite. In this context, the purinergic system is a common route of cell-cell communication involved in many neuronal and non-neuronal mechanisms and in short- and long-term events, including immune responses, inflammation, and cell death that can contribute to parasite elimination (Burnstock and Boeynaems, 2014). In the present study, we investigated for the first time whether RSV modulates purinergic signalling during in vivo T. gondii chronic infection and could ameliorates cerebral oxidative stress and inflammatory process.
We found that NTPDase and 5′-NT enzyme activities in the cortex were affected by RSV treatment during T. gondii infection. NTPDase and 5′-NT activities of the cerebral cortex in infected and treated mice were reduced when compared with infected and untreated mice group (Fig. 1a, c) suggesting that RSV exerts effects on controlling ATP and ADP degradation, respectively. During T. gondii infection, the release of ATP by host cells constitutes a danger signal alerting the immune system about abnormal cell death (Di Virgilio et al. 2009). As observed in this study, the increase in NTPDase activity in infected mice seems to be necessary to hydrolyse high extracellular ATP levels.
In addition, adenosine deaminase (ADA) activity was significantly reduced in infected animals when compared with control mice (Fig. 1d). T. gondii uses host extracellular adenosine to persist in the CNS as an encapsulated form, as already reported by Krug et al. (1989), also suggesting that adenosine production could be significantly diminished during T. gondii infection. However, ADA activity augmented following RSV treatment of T. gondii-infected mice. These results suggest a subtle neuromodulator effect of RSV on adenosine levels in the brain through ADA activity during T. gondii infection.
In pathological conditions, adenosine plays a protective role by modulating the release of neurotransmitters and also acting as an endogenous regulator of innate immunity and in the defence of the host against excessive tissue damage associated with inflammation. In this line, adenosine is considered a signalling molecule also associated with cellular damage, but with antagonistic actions related to ATP (Cunha 2001; Haskó & Cronstein, 2004).
Once in the extracellular environment, nucleosides and nucleotides activate two families of purinergic receptors, named P1 and P2 receptors, respectively, which are located on the surface of immune resident cells in the CNS (Burnstock & Boeynaems, 2014). Our results showed that T. gondii infection stimulated both P2X7 and P2Y1 receptor expression in the cerebral cortex (Fig. 2a, b). Thus, extracellular ATP, liberated by cells during apoptosis, activates the P2X7 receptor and also upregulates its expression. The discrepancy is potentially due to differences in cells that may express different levels of P2X7. Similarly, ATP and ADP nucleotides binding to the P2Y1 receptor increase its cellular expression. Studies have demonstrated that the upregulation of the P2X7 receptor during T. gondii infection triggers inflammasome formation, culminating in mature IL1-β release, also known to participate in pro-inflammatory events (Qu et al. 2007). Furthermore, continued activation of P2X7 receptors by ATP during chronic infection has been proposed as a mechanism for the elimination of T. gondii tachyzoites from infected macrophages (Côrrea et al. 2010).
Treatment with RSV regulated P2X7 and P2Y1 receptor expression in the cerebral cortex of infected mice (Fig. 2a, b). The effect of RSV on P2X7 receptors was shown by our research group in infected neural precursor cells (Bottari et al., 2018a, b). Together, our data suggest that increased expression of P2X7 and P2Y1 receptors during T. gondii infection was reverted by RSV treatment due to the anti-inflammatory property of this molecule as already reported by Huang et al. (2014).
T. gondii infection increased A1 but not A2A receptor density in the cortex of infected mice (Fig. 2c, d). In addition, RSV treatment significantly up- and downregulated A1 and A2A receptor densities, respectively, in the cortexes of infected mice. A1 and A2A adenosine subtypes of receptors are highly expressed in the brain cortex and play important roles in the regulation of many brain functions in the CNS. Most notably A1 receptors are associated with neuromodulator effects, and A2A receptors are involved in neuroinflammatory and brain injury processes (Cunha, 2001). Our results reveal that RSV modulates adenosine signalling through A1 receptor-mediated neuroprotector action during T. gondii infection (Fig. 2c). On the other hand, results indicate that RSV acts as an antagonist of the A2A receptors, preventing neuroinflammation during T. gondii infection (Fig. 2d).
To strengthen the hypothesis of whether RSV reduces oxidative damage in the cerebral cortex and attenuates cellular damage, oxidative parameters were also evaluated. Here, we showed that the intracellular parasite T. gondii increases RS and TBARS levels’ production in the cerebral cortex during chronic infection when compared with the control group (Fig. 3a, b) inducing oxidative stress. Previous studies revealed that T. gondii infection can lead to oxidative stress as a defence mechanism of the host cell to avoid parasite survival and replication (Coutinho-Silva et al., 2009). However, RSV treatment proved to be a potent antioxidant reducing the RS and TBARS production in infected mice, avoiding lipid peroxidation and reactive species release during CNS infection by T. gondii.
Finally, during the chronic stages of infection, encysted parasites are found in the brain (Fig. 4). A recent study by Chen et al. (2019) showed direct and indirect inhibitory effects of RSV against T. gondii tachyzoites in vitro showing a clear image of the effect of this compound on the parasitic load. Treatment with RSV was not able to reduce parasite cysts but it attenuated inflammatory processes in the cerebral cortex, probably due to anti-inflammatory effects and modulation of purinergic signalling.
Conclusion
We demonstrated the potential effects of RSV on purinergic signalling, oxidative, and inflammatory status during chronic infection by T. gondii. We found that RSV affected enzymes hydrolysing extracellular nucleotides and nucleosides and positively regulated purinergic receptors as a mechanism to eliminate the parasite and avoid oxidative damage in the cerebral cortex of infected mice. We also found an anti-inflammatory and neuroprotector mechanism of RSV through adenosine signalling by regulating A1 and A2A receptors expression during T. gondii infection. Taken together, our findings suggest that targeting purinergic signalling through RSV modulation could be a novel therapeutic strategy in the treatment of neurotoxoplasmosis and may counterbalance the negative alterations triggered by T. gondii in the CNS.
References
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5(6):493–506. https://doi.org/10.1038/nrd2060
Bottari NB, Baldissera MD, Tonin AA, Rech VC, Alves CB, D’Avila F, Thomé GR, Guarda NS, Moresco RN, Camillo G, Vogel FF, Luchese C, Schetinger MRC, Morsch VM, Tochetto C, Fighera R, Nishihira VSK, da Silva AS (2016) Synergistic effects of resveratrol (free and inclusion complex) and sulfamethoxazole-trimethropim treatment on pathology, oxidant/antioxidant status and behavior of mice infected with Toxoplasma gondii. Microb Pathog 95:166–174. https://doi.org/10.1016/j.micpath.2016.04.002
Bottari NB, Pillat MM, Schetinger MRC, Reichert KP, Machado V, Assmann CE, Ulrich H, Dutra A, Morsch VM, Vidal T, da Cruz IBM, Melazzo C, da Silva AS (2018a) Resveratrol-mediated reversal of changes in purinergic signaling and immune response induced by Toxoplasma gondii infection of neural progenitor cells. Purinergic Signalling 15(1):77–84. https://doi.org/10.1007/s11302-018-96343
Bottari NB, Schetinger MRC, Pillat MM, Palma TV, Ulrich H, Alves MS, Morsch VM, Melazzo C, de Barros LD, Garcia JL, da Silva AS (2018b) Resveratrol as a therapy to restore neurogliogenesis of neural progenitor cells infected by Toxoplasma gondii. Mol Neurobiol 56(4):2328–2338. https://doi.org/10.1007/s12035-018-1180-z
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Burnstock G, Boeynaems JM (2014) Purinergic signalling and immune cells. Purinergic Signal 10(4):529–564. https://doi.org/10.1007/s11302-014-9427-2
Chaudhary K, Darling JA, Fohl LM, Sullivan WJ Jr, Donald RGK, Pfefferkorn ER, Ullman B, Roos DS (2004) Purine salvage pathways in the apicomplexan parasite Toxoplasma gondii. J Biol Chem 279(30):31221–31227. https://doi.org/10.1074/jbc.M404232200
Chen QW, Dong K, Qin HX, Yang YK, He JL, Li J, Zheng ZW, Chen DL, Chen JP (2019) The direct and indirect inhibition effects of resveratrol against Toxoplasma gondii tachyzoites in vitro. Antimicrob Agents Chemother 63(3):e01233–e01218. https://doi.org/10.1128/AAC.01233-18
Cook TB, Brenner LA, Cloninger CR, Langenberg P, Igbide A, Giegling I, Hartmann AM, Konte B, Friedl M, Brundin L, Groer MW, Can A, Rujescu D, Postolache TT (2015) “Latent” infection with Toxoplasma gondii: association with trait aggression and impulsivity in healthy adults. J Psychiatr Res 60:87–94. https://doi.org/10.1016/j.jpsychires.2014.09.019
Corrêa G, Marques da Silva C, de Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R (2010) Activation of the P2X7 receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect 12(6):497–504. https://doi.org/10.1016/j.micinf.2010.03.004
Coutinho-Silva R, Corrêa G, Sater AA, Ojcius DM (2009) The P2X7 receptor and intracellular pathogens: a continuing struggle. Purinergic Signal 5(2):197–204. https://doi.org/10.1007/s11302-009-9130-x
Cunha RA (2001) Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38(2):107–125. https://doi.org/10.1016/S0197-0186(00)00034-6
Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP (2009) Purinergic signalling in inflammation of the central nervous system. Trends Neurosci 32(2):79–87. https://doi.org/10.1016/j.tins.2008.11.003
Frémont L (2000) Biological effects of resveratrol. Life Sci 66(8):663–673. https://doi.org/10.1016/S0024-3205(99)00410-5
Gallucci S, Matzinger P (2001) Danger signals: SOS to the immune system. Curr Opin Immunol 13(1):114–119. https://doi.org/10.1016/S0952-7915(00)00191-6
Guisti G, Galanti B (1984) Colorimetric method. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Weinheim, Verlag Chemie, pp 315–323
Haskó G, Cronstein BN (2004) Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25(1):33–39. https://doi.org/10.1016/j.it.2003.11.003
Heymann D, Reddington M, Kreutzberg GW (1984) Subcellular localization of 5′-nucleotidase in rat brain. J Neurochem 43(4):971–978. https://doi.org/10.1111/j.1471-4159.1984.tb12832.x
Hill DE, Dubey JP (2006) Toxoplasma gondii. In: Ortega Y, Sterling C (eds) Foodborne parasites. Food Microbiology and Food Safety, Springer US
Hitziger N, Dellacasa I, Albiger B, Barragan A (2005) Dissemination of Toxoplasma gondii to immunoprivileged organs and role of Toll/interleukin-1 receptor signalling for host resistance assessed by in vivo bioluminescence imaging. Cell Microbiol 7(6):837–848. https://doi.org/10.1111/j.1462-5822.2005.00517.x
Huang TT, Lai HC, Chen YB, Chen LG, Wu YH, Ko YF, Lu CC, Chang CJ, Wu CY, Martel J, Ojcius DM, Chong KY, Young JD (2014) cis-Resveratrol produces anti-inflammatory effects by inhibiting canonical and non-canonical inflammasomes in macrophages. Innate Immun 20(7):735–750. https://doi.org/10.1177/1753425913507096
Krug EC, Marr JJ, Berens RL (1989) Purine metabolism in Toxoplasma gondii. J Biol Chem 264:10601–10607
Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, Mui EJ, Witola WH, Coyne JJ, Hargrave AC, Jamieson SE, Blackwell JM, Wiley JS, Smith NC (2010) P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol 184(12):7040–7046. https://doi.org/10.4049/jimmunol.1000012
Mahamed DA, Mills JH, Egan CE, Denkers EY, Bynoe MS (2012) CD73-generated adenosine facilitates Toxoplasma gondii differentiation to long-lived tissue cysts in the central nervous system. Proc Natl Acad Sci U S A 109(40):16312–16317. https://doi.org/10.1073/pnas.1205589109
Mahamed DA, Toussaint LE, Bynoe MS (2015) CD73-generated adenosine is critical for immune regulation during Toxoplasma gondii infection. Infect Immun 83(2):721–729. https://doi.org/10.1128/iai.02536-14
McAuley JB (2014) Congenital toxoplasmosis. J Pediatric Infect Dis Soc 3(1):S30–S35. https://doi.org/10.1093/jpids/piu077
Mendez OA, Koshy AA (2017) Toxoplasma gondii: entry, association, and physiological influence on the central nervous system. PLoS Pathog 13(7):e1006351. https://doi.org/10.1371/journal.ppat.1006351
Myhre O, Andersen JM, Aarnes H, Fonnum F (2003) Evaluation of the probes 2′,7′- dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65(10):1575–1582. https://doi.org/10.1016/S0006-2952(03)00083-2
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Petit-Jentreau L, Tailleux L, Coombes JL (2017) Purinergic signaling: a common path in the macrophage response against mycobacterium tuberculosis and Toxoplasma gondii. Front Cell Infect Microbiol 7:347. https://doi.org/10.3389/fcimb.2017.00347
Qu Y, Franchi L, Nunez G, Dubyak GR (2007) Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 179(3):1913–1925. https://doi.org/10.4049/jimmunol.179.3.1913
Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA (2003) Subcellular localization of adenosine A1 receptors in nerve terminals and synapses of the rat hippocampus. Brain Res 987(1):49–58. https://doi.org/10.1016/S0006-8993(03)03247-5
Schetinger MR, Porto NM, Moretto MB et al (2000) New benzodiazepines alter acetylcholinesterase and ATPDase activities. Neurochem Res 25(7):949–955. https://doi.org/10.1023/A:1007500424392
Tenter AMT, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30(12–13):1217–1258. https://doi.org/10.1016/S0020-7519(00)00124-7
Thiel M, Caldwell CC, Sitkovsky MV (2003) The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microbes Infect 5(6):515–526. https://doi.org/10.1016/S1286-4579(03)00068-6
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PROEX, Process No. 88887.186030/2018-00) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Project No. 304,328/2015–4). HU acknowledges grant support by the São Paulo Research Foundation (Project No. 2018/07366-4).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Section Editor: Dana Mordue
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bottari, N.B., Reichert, K.P., Fracasso, M. et al. Neuroprotective role of resveratrol mediated by purinergic signalling in cerebral cortex of mice infected by Toxoplasma gondii. Parasitol Res 119, 2897–2905 (2020). https://doi.org/10.1007/s00436-020-06795-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06795-0