Abstract
The intracellular protozoan Toxoplasma gondii may cause congenital toxoplasmosis and serious brain damage in fetus. However, the underlying mechanism of neuropathogenesis in brain toxoplasmosis remains unclear. For this study, neural progenitor cells (NPCs) were obtained from embryo telencephalons (embryonic day 13) and induced to proliferation in the presence of growth factors (GFs). For gathering insights into the biological effects of resveratrol (RSV) on neurogenesis, this study aimed to investigate effects of RSV concentrations (0.1 to 100 μM) on proliferation, migration and differentiation of NPCs infected by T. gondii. T. gondii infection increased the presence of cells in Sub G1 phase, reducing the global frequency of undifferentiated cells in S and G2/M phases of cell cycle and reduced cell viability/mithochondrial activity of infected NPCs. Moreover T. gondii stimulated neural migration and gliogenesis during neutral differentation. However, the treatment with RSV stimulated cell proliferation, restored cellular viability of infected NPCs and exerted an inhibitory effect on gliogenesis of infected NPCs favorecing neuronal maturation during toxoplasmosis infection. Thus, we have successfully to demonstrated that RSV is promising as therapeutic for congenital toxoplasmosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii is a neurotropic intracellular parasite that infects any warm-blooded animals, capable of causing toxoplasmosis [1]. Congenital toxoplasmosis is regarded as the most serious outcome of T. gondii infection in worldwide and has still a high incidence in live births [2].
There is a close relationship between congenital toxoplasmosis and the appearance of neurodegenerative disorders that can lead to irreversible injuries of the fetus brain [3]. Congenitally infected children usually show clinical signs, such a of hydrocephalus and frequently low intelligence quotients [4, 5]. In pregnant women, T. gondii infection can cause abortion, stillbirth or brain abnormalities with detrimental consequences for the fetus [6].
Experimental evidence exists that in vitro inoculation with T. gondii induces apoptosis of neural stem cells (NSCs) reducing proliferation rates during cellular differentiation [7], and that the parasite is capable of infecting and encysting in both astrocytes and neurons, the major central nervous system (CNS) cell types implicated during a chronic infection [8].
Neurons, astrocytes and microglial cells in the CNS are highly sensitive to T. gondii infection, especially in toxoplasmic encephalitis [9], and during brain development. One of the most important steps during brain development is the generation of cellular diversity and the neural fate decision to form neurons or glial cells. In this study, we suggest novel functions for resveratrol (RSV), a natural compound with health benefits, promoting neurogenesis during toxoplasmosis infection.
Resveratrol (3,4′,5trihydroxystilbene) is a polyphenolic phytoalexin abundant in grapes, peanuts, mulberries and red wine [10]. This compound has protective effects against oxidative stress, immune response and celular damage [11, 12]. Furthermore, RSV protected neurons against degeneration in experimental models of ischemic stroke, Alzheimer, and Parkinson disease [13, 14]. RSV has been investigated for treatment of toxoplasmosis in mice experimentally infected with T. gondii revealing significant results such as decreases in brain damage and cyst numbers [15]. In view of the cited recent results, the present study is expected to provide interesting insights into the effect of RSV in cell fate determination during the process of neural differentiation [16].
Thus, to further elucidate the mechanisms of RSV on neuropathogenesis of congenital toxoplasmosis, this study aimed to investigate cellular and molecular mechanisms mediated by RSV on NPCs from embryos infected with T. gondii as restorative therapy recovering from the loss of NPCs and neurogenesis.
Experimental Procedures
Animals and Infection
For this study, ten Swiss female mice with a mean age of 60 days weighing 25 ± 5 g were kept in boxes containing five animals each, under a 12 h light/dark cycle with controlled temperature and humidity (25 °C, 70% respectively). All animal procedures were approved by the Ethics Committee on Animal of Federal University of Santa Maria (UFSM, protocol number 95090109/15). The animals went through an adaptation period of 10 days and were fed with commercial feed and water ad libitum. The animals were infected orally with T. gondii tachyzoites of the VEG strain (type III) orally. Twenty days post infection the female mice were mating with male mice.
Resveratrol
Resveratrol (C14H12O3; molecular weight 228.25 g/mol; purity >98, Sigma Aldrich, St. Louis, MO) was diluted in phosphate-buffered saline (PBS) and added to NPC cultures at concentrations from 0.1 to 100 μM prior to induction of differentiation.
Isolation and Culture of Neural Progenitor Cells (NPCs)
NPCs were isolated from the embryonic day13 (E13) telencephalons of embryonic mice according to the methodology described by Hutton and Pevny [17]. Embryonic telencephalons were dissected and incubated with 0.1% trypsin for 5 min at 37 °C an equal volume of fetal bovine serum (FBS) was added for the inactivation of trypsin [18]. The cells were further mechanically dissociated. The cells were plated at a density of 2 × 105 cells/mL in Dulbecco’s Modified Eagle’s medium (DMEM)/Ham’s F-12 culture medium supplemented with 2% of B-27 (Life Technologies, Carlsbad, CA), 20 ng/ml of epidermal growth factor (EGF) and fibroblast growth factor (FGF)-2 (both reagents from Sigma-Aldrich) and antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin) and cultured at 37 °C in a water-saturated atmosphere and 5% of CO2 during 5 days. Growth and sizes of neurosphere were measured using ImageJ software, and the results expressed as numbers and sizes of neurosphere, respectively.
Cell Cycle and Cellular Viability of NPCs
Proliferation of uninfected and infected undifferentiated NPCs treated with resveratrol (0.1 to 10 μm) were evaluated by cell cycle measurements using propidium iodide (PI) as described previously [18]. After a 24-h treatment, cells were resuspended in PBS, fixed with 70% ethanol, labeled with PI (0.05 mg/ml), incubated at room temperature in the dark for 30 min, and filtered through 41-μm spectra/mesh nylon filters (Spectrum, Rancho Dominguez, CA). DNA content was then analyzed using flow cytometer (BD FACSCalibur; BD biosciences). Obtained data were analyzed using the FlowJo V10 software (Ashland, OR) and expressed as counted cells (% of control).
After the indicated treatments, cellular viability of NPCs was analyzed by the 3–4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) method [19]. NPCs were incubated with 5 mg/mL of MTT for additional 2 h at 37 °C under 5% CO2 and 95% air. Dimethylsulfoxide was used to extract MTT formazan, and absorbances of each well at 490 nm were read in an automatic microplate reader. Results were expressed as percentages of control groups.
Neurosphere Differentiation
For neural differentiation, neurospheres were plated into adherent poly-L-lysine- and laminin coated cell culture dishes and cultured without growth factors (EGF and FGF2) in the absence or presence of RSV (0.1 to 10 μM). Migration rates were determined on the seventh days of differentiation as the distance migration of the foremost cells to the neurosphere analyzed by differential interference contrast microscopy [20, 21]. The radial migration was mensured using ImageJ software, and data were reported as percentage of migration compared to untreated controls.
Immunocytochemistry
For immunocytochemistry analysis neurospheres were fixed in 4% of paraformaldehyde (PFA) for 20 min at 37 °C, and and permeabilized for 20 min with PBS plus 3% FBS and 0.1% Triton X-100 [22]. After 2 h of incubation with rabbit anti-glial fibrillary acidic protein (GFAP) antibodies (1:500; DAKO Systems, Carpinteria, CA), and mouse anti-β3-tubulin antibodies (1:500; Sigma-Aldrich, St. Louis, MO) and counterstained with 40,6-diamidino-2-phenylindole, dilactate (DAPI) solution (0.3 μg/mL; Sigma- Aldrich). In the same solution, NPCs were washed with PBS and incubated for 1 h at room temperature (RT) with secondary Alexa Fluor 488 or 555 (1:1000; Life Technologies) antibodies. Coverslips were mounted, and slides were analyzed under a fluorescence microscope (Axiovert 200, Zeiss, Jena, Germany).
Flow Cytometry Analysis
Flow cytometry experiments were performed as described previously by researchers [21, 23]. Differentiated cells were dissociated and fixed for 20 min in ice-cold 4% PFA in PBS, washed with PBS supplemented with 2% FBS, and incubated for 30 min with primary anti-Nestin (1:500; Sigma-Aldrich), anti-GFAP (1:500; DAKO Systems, Carpinteria, CA), anti-β3-tubulin (1:500; Sigma- Aldrich), or rabbit anti-microtubule associated protein-2 (MAPs2, 1:500; Sigma-Aldrich) antibodies. Following a washing step with PBS, cells were incubated with Alexa Fluor 488- or 555-conjugated secondary antibodies (1:500) (Life Technologies) and analyzed by flow cytometer (BD Accuri). Thirty-thousand events were acquired per samples. Forward and side light-scatter signals were used to exclude dead cells and debris. Data were analyzed using theFlowJo V10 software (FlowJo, Ashland, OR).
DNA Extraction and Reaction of Polymerase Chain (PCR) Detection
Adult brain and telencephalon of embryos from infected mice samples frozen at −80 °C were processed following the protocol described by Sambrook et al. [24] with modifications. The tissue was incubated with 20 μL of proteinase K (20 mg/mL), overnight at 37 °C. After this period, 500 μL of phenol-chloroform (1:1) was added and mixed, followed by centrifugation at 12.000 g for 10 min. The aqueous phase was transferred to the same volume of absolute isopropanol. The mixture was precipitated overnight, and centrifuged at 12.000 g for 30 min. The pellet was resuspended in 1 mL of cold ethanol 70%, followed by centrifugation at 12.000 g for 10 min. And then resuspended in 30 μL de TE (10 mMTris-HCl pH 8.0; 1 mM EDTA pH 8.0) and incubated at 56 °C for 10 min, followed by a further centrifugation for final storage at −20 °C.
PCR reactions were carried out in order to determine the presence of T. gondii DNA using the gene target B1, the forward primer: 5’AGCGTCTCTCTTCAAGCAGCGTA-3′ and reverse primer: 5’TCCGCAGCGACTTCTATCTCTGT-3’for amplification of a 300 bp fragment as described [25]. The reaction were performed at a final volume of 25 μL containing 50 ng of DNA template, 6 μM of each primers, 100 μM of dNTPs (Life Technologies), 2 mM MgCl2, 2.5 U Taq DNA polymerase (Life Technologies) and 2.5 μL of enzyme buffer. PCR products were subjected to electrophoresis and visualized by staining with 2% ethidium bromide.
Statistical Analysis
Results are expressed as mean values ± standard errors of mean (SEM) representative for at least three independent experiments. Statistical analysis was assessed by two-way analysis of variance (ANOVA) followed by the Tukey post-test using GraphPad Prism (Version 5.0) software. Values of p < 0.05 were considered as statistically significant.
Results
Congenital Transmission of T. gondii
Qualitative DNA analysis was performed in brain of adult mice (n = 5) and telencephalon of embryos from infected mice (n = 5) in order to confirm congenital transmission of T. gondii by polymerase chain reaction (PCR). A specific band (300 bp) was observed in adult brain and embryos indicating congenital transmission, as confirmed with a positive control (cistogenic strain VEG) (Fig. 1).
Maternal-fetal transmission of T. gondii consistent with congenital toxoplasmosis. 1: Specific band (± 300 bp, indicated by the arrow) of T. gondii DNA detected by qualitative PCR in the brain of infected female mice. 2: T. gondii DNA in telencephalon of embryos (E13). 3: Negative control. Agarose gel (1.5%) stained with ethidium bromide showing specific amplicons M: molecular weight marker 1000 bp
Stimulatory Effects of Resveratrol during Neurosphere Growing
NPCs isolated from telencephalon embryonic day-13 (ED-13) gave clonal expansion to neurospheres in serum free neurobasal medium supplemented with growth factors. We first studied RSV concentration dependence effects modifies the growing of neurospheres of uninfected and infected NPC cultures (Fig. 2A). Lower concentrations of RSV (1 μM and 10 μM) led to an increase in the number of neurospheres formed by uninfected NPCs after 5 days of exposure, whereas higher doses (100 μM) inhibited neurosphere growth (Fig. 2B).
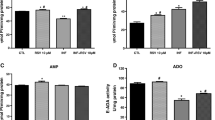
Effects of resveratrol on numbers and size of neurospheres. A: Uninfected and infected NPCs proliferate as neurospheres after 5 days of growing in presence or absence of RSV (0.1 to 100 μM). Scale bar: 100 μm. B: RSV treatment increased the numbers of neurosphere in uninfected NPCs. Neurosphere counts were performed using flow cytometry. C: Sizes of uninfected and infected neurosphere after treatment with RSV (1 and 10 μM). The sizes were determined with the ImageJ software. Data are expressed as mean values ± SEM using Two-Way ANOVA with post-hoc Tukey tests within the GraphPad Prism software; significant differences p < 0.05. (*Uninfected vs experimental group) (# Infected vs experimental groups)
To confirm the effects of RSV and parasite influence during neurosphere formation, sizes of neurospheres were analyzed with ImageJ software (Fig. 2C). RSV (10 μM) significantly increased sizes of uninfected neurospheres when compared to controls (Fig. 2D). Surprisingly, T. gondii infection also increased sizes of neurospheres when compared to control (p < 0.05). RSV at 1 μM and 10 μM concentration enhanced proliferation in a dose-dependent manner (Fig. 2D).
Resveratrol Increased Proliferation and Cellular Viability of Infected NPCs
Cell cycle measurements were conducted for further studying effects on proliferation (Fig. 3A). After 5 days of culture, T. gondii increased Sub G1 phase and reduced S and G2/M phases of the cell cycle in comparison to control (p < 0.05) (Fig. 3A). The treatment with RSV increased S and G2 phases of cell cycle in infected NPCs when compared to infect NPCs.
Resveratrol-induced increases on proliferation and viability of infected NPCs. A: Flow cytometry analysis of cells in Sub G1, G0/G1, S, G2/M phases of cell cycle of uninfected and infected treated with RSV (1 and 10 μM). B: Percentages of cellular viability/mithochondrial activity in relation to control cells in the absence and presence of RSV (1 and 10 μM). Data represent mean values ± SEM of three independent experiments using Two-Way ANOVA with the post-hoc Tukey test. *p < 0.05 (* Control vs infected groups) (#Infected vs experimental group)
NPC viability/mithochondrial activity is shown in Fig. 3B. T. gondii decrease NPC viability of neurospheres when compared to uninfected (p < 0.05). Treatment with RSV at 1 and 10 μM concentration significantly prevented cellular death of uninfected.
Resveratrol Promoted Migration of Infected NPCs during Brain Development
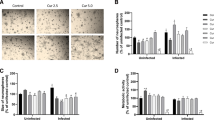
NPCs present a radial migration pattern. Figure 4 shows representative images of uninfected and infected differentiated neurospheres, cultured in the presence or absence of resveratrol (0.1-10 μM). The region enclosed between the dotted lines comprises 95% of migrating cells. T. gondii stimulated migration of infected NPCs after 7 days of differentiation when compared to control experiments (Fig. 4A). Treatment with RSV restored radial migration in infected NPCs similar to that observed with control cells (p < 0.05) (Fig. 4B).
Resveratrol-induced decrease in radial migration of infected NPCs. A: Phase contrast images representing radial migration pattern after 7 days of neural differentiation in the presence or absence of RSV in uninfected and infected NPCs. The region comprised by the dotted lines corresponds to approximately 95% of migrating cells. B: Quantification of migration of uninfected and infected NPCs treated with RSV (0.1 to 10 μM). The quantification was done using ImageJ software and expressed as percentage. Significant results p < 0.05, using Two-Way ANOVA with the post-hoc Tukey test. (*Uninfected vs experimental group) (# Infected vs experimental groups)
T. gondii Infection Stimulated Gliogenesis in Infected NPC
For assaying the progress of neural differentation, marker expression for NPCs, glial cells and neurons were studied by immunocytochemistry (Fig. 5). The images indicate an increase in GFAP-immunostaining of infected NPCs compared to control cells (Fig. 5A). Sample immunostaining for β3-tubulin demonstrated that neuronal differentation was not affected by T. gondii infection or RSV treatment; in other words, there were not any effects on neurogenesis (Fig. 5B). However, during T. gondii infection, frequencies of GFAP+ cells were augmented when compared to uninfected control cells (p < 0.05).
Neural progenitor differentiation induced by T. gondii. A: GFAP expression in differentiated neurospheres from uninfected and infected NPCs treated with RSV (0.1 to 10 μM). B: β3-tubulin expression by in uninfected and infected NPCs treated with RSV (0.1 to 10 μM). C: Typical immunofluorescence merged images of neurospheres on day 7 of differentiation, showing glial GFAP) and neuronal β3-tubulin expression
Resveratrol Enhanced Neural Maturation of Infected NPC
Analysis by flow cytometry confirmed the differences in the frequency of nestin (NPCs marker), anti-GFAP (glial marker), cells expressions anti- β3-tubulin (immature neurons marker) and MAP2 (mature neurons marker) (Fig. 6). An increase of NPC positive cells was observed in infected NPCs when compared to controls (Fig. 6A). RSV (0.1 to 10 μM) increased the percentage of nestin+cells. While RSV (1 and 10 μM) increased the number of infected cells expressing nestin NPCs stimulated proliferation of NPCs during T. gondii infection.
Resveratrol restored neurogliogenesis during T. gondii infection. Flow cytometry analysis using specific antibodies conjugated with Alexa Fluor (AF) 488 or AF 555 fluorophores in uninfected and infected NPCs treated with different concentrations of RSV (1 and 10 μM). Percentages of A: NPCs (Nestin marker). B: Glial cell (GFAP+ cells). C: immature neurons (β3-tubulin+ cells). D: Percentages of mature neurons (MAP2). The data represents mean values ±SEM of three independent experiments. Significant results *p < 0.05, using ANOVA-two way by GraphPad Prism. (*Uninfected vs experimental group) (# Infected vs experimental groups)
GFAP marker analyzed the percentage of glial cells. The T. gondii increase GFAP+ cells when compared to control (p < 0.05) (Fig. 6B). The exposition of RSV (0.1 to 10 μM) significantly increase GFAP+ cells in per se effect when compared to control. However, 1 μM RSV was able to restore glial expression in infected NPCs (Fig. 6B). No significant differences were observed on regarding expression of the neural marker β3-tubulin between groups (p > 0.05) (Fig. 6C). In addition, immunocytochemistry analysis suggested an increase in gliogenesis (GFAP+ cells) over neurogenesis (β3-tubulin− cells).
As T. gondii and RSV have not any influence on neuronal fate determination, neuronal maturation was further investigated by anti-MAP-2 immunostaining.. The results showed a decrease in the number of MAP2+ cells following T. gondii infection when compared to control (Fig. 6D). The lower doses of RSV (0.1 μM and1μM) increased the percentage of MAP2+ cells. The treatment with RSV (0.1 to 10 μM) stimulated neuronal maturation in infected NPCs. These results indicate that the concentration used in this study promotes neural maturation during T. gondii infection of NPCs.
Discussion
CNS plays a central role in the lifelong persistence of T. gondii as well as in the pathogenesis of congenital toxoplasmosis. This study investigated the effects of resveratrol on modulation of neurogliogenesis in infected NPCs. Our first experimental strategy was to mimic an infection of the female aiming T. gondii vertical transmission through ingestion of cysts of the VEG cistogenic strain. The PCR analysis showed presence of T. gondii DNA in brain of female adult mice and telencephalon of infected embryos confirming vertical transmission (Fig. 1). These data support the severity of toxoplasmosis infection and are in accordance with previous results [26], which indicated a high congenital transmission rate (90%) in the mouse model.
T. gondii is capable of invading almost any nucleated cells [27]. Thus, NPCs are presumably one of these, and are crucial for the process of brain development and postnatal and adult neurogenesis. Considering that NPCs originate in the CNS, we hypothesize that T. gondii on the NPCs may play an important role in the determination of cell fate in the process of neural differentiation. Congenital toxoplasmosis, especially in the first trimester of pregnancy, may result in severe brain changes in the newborn. The first evidence observed in this study was a high spontaneus abortion rate in infected female mice. Three of eight infected animals underwent spontaneous abortion or decreases in embryo size and numbers (data not showed).
The parasite may promote or inhibit the cell apoptotic machinery, depending on the host cell type, infection stage as well as on its virulence and parasite load [28]. Our results suggest that T. gondii caused an expansion of neurosphere growth, but not an increase in neurosphere number. Our hypothesis is that the major host tissue cells could act as the bystanders in chronic infection, and cell aggregation may favor the secretion of cytokines by parasite-infected cells [29, 30].
Resveratrol has protective effects against many diseases, including toxoplasmosis [15]. In the present study, we evaluated the effects of RSV on growing, proliferation and survival of NPCs from uninfected and infected neurospheres. Among the tested concentrations, 1 and 10 μM of RSV were the most effective in stimulating NPC growth (Fig. 2A and 2B). On the other hand, the RSV inhibits neurosphere growing when administered in high dose (100 μM) in the culture (Fig. 2A and 2B); this clearly indicates that RSV at a high dose/concentration has a strong pro–apoptotic effect. According to literature, the RSV exerts biphasic effects on NPCs [31]; low concentrations (<10 μM) stimulated cell proliferation, whereas high concentrations (>20 μM) exhibited inhibitory effects. Our findings are consistent with evidence that RSV can increase numbers and sizes of neurospheres; consistent with the possibility that RSV crosses the blood-brain barrier and acts directly on proliferation of cells [16].
To confirm the effect of RSV on infected NPCs, cell cycle phases were analyzed. T. gondii decreased S and G2/M phases of cycle (Fig. 3A). During neurogenesis a heterogeneous population of NPCs, neuroblasts and glial cells are proliferating and dividing. Thus, when there are change during embryonic development and/or neural tube formation, the cell cycle extends. The treatment with RSV changes in cell cycle distribution of uninfected neurospheres and resulting in symetric proliferative division with a rapid expansion NPCs. On the other hand, infected NPCs arrested in G0/G1 phase shows that neurogenesis may be influenced by the treatment (Fig. 3A). RSV operated as stimulus for infected NPCs during S and G2/M phases, probably improving DNA sinthesis and acting on the checkpoint in the final step of G2, to cease the cycle if the DNA is damaged or if DNA replication has been incomplete. Previous results have reported that RSV arrests the cell division cycle at S/G2 phase transition [32] as observed here.
The cellular viability of undifferentiated NPCs are strongly affected by T. gondii (Fig. 3B). The data shows an appoptotic effect of parasite in infected NPCs. Our results are in accordance with other studies [33], which showed that the proliferation level was lower in infection groups than the controls, as determined by the MTT method. Accordingly, T. gondii induced apoptosis of NSCs through the ERS signal pathway via activation of CHOP, caspase-12 and JNK [7], which may constitute a potential molecular mechanism responsible for the cognitive disturbance in neurological disorders caused by T. gondii. The treatment with RSV restored cellular viability of infected NPCs. This was probably due to reducing the production of prostaglandins and the formation of ROS evidenced in lipopolysaccharide (LPS)-activated microglial cells [12]. Moreover, RSV was reported to suppress the activity of T and B-cells and macrophages [34].
The transition from neuroepithelial cells to the radial glands during embryonic development is being associated with the cell cycle. To gain insights into the biological effects of RSV on neurogenesis, we examined its influence on neural migration. Our data reveal that RSV (0.1 to 10 μM) enhanced neural migration of uninfected NPCs (Fig. 4A). However, T. gondii infection seems to led to aberrant neural migration (Fig. 4B). These results suggest that alteration of migration may also influence neurogenesis and gliogenesis networks.
In T. gondii infection, NPCs migrate from the base during the G1 phase of the cell cycle, reach the apex during the S phase (low extension phase) and return to the base of the neural tube during the G2 phase of mitosis (low extension). However, the treatment with RSV (0.1–10 μM) decreased neural migration in infected NPCs, playing a key role in the neural differentiation of cells. Specifically, a reduction in accuracy of spindle positioning relative to the apical-basal axis of neuroepithelial cells may be sufficient to result in cleavages other than the symmetric pattern. These interkinetic motions and symmetric divisions characterize the early stages of central nervous system expansion, increasing the number of cells [35], as observed here.
The progress of neural differentiation is closely related to cell migration and neuron-glia interactions [36]. Progresses in neural and glial differentiation of NPCs were evaluated using anti-GFAP (Fig. 5A) and anti-β3-tubulin (Fig. 5B) antibodies in immunofluorescence studies. The results revealed that T. gondii favored gliogenesis over neurogenesis in neurotoxoplasmosis.
Quantification of nestin (NPC+), GFAP+ cells and β3-tubulin+cells by flow cytometry showed that T. gondii significantly increased the frequencies of nestin+ and GFAP+ cells when compared to controls assay (Fig. 6A, B). These results indicate, for the first time, an important role of e T. gondii in indirectly increasing NPC proliferation and glial fate determination.
Neurospheres were exposed to different concentrations of RSV (0.1 - 100 μ M), the results indicate that intermediary concentrations in the number of RSV (1 and 10 μM) exposure resulted in an increase of NPC compared to uninfected NPCs. The results are consistent with literature [16], which show differential actions of RSV on proliferation of neural progenitor cells and hippocampal neurogenesis.
In the range of tested concentrations, 1 μM RSV seemed to be most effective in reducing glial cell production induced by T. gondii. Astrocytes may contribute to neuroinflammation, such us microgrial cells which are considered immune cells of the CNS. When stimulated, these cells react and produce inflammatory mediators. These molecules contribute to the dysfunction of the neural network in the CNS. Evidence supporting the involvement of inflammatory mediators in neurodegenerationis well documented with microglia playing a key role [28, 30]. Thus, the development of compounds modulating microglial activation has been suggested as one of the potential strategies for the treatment or prevention of neurodegenerative diseases.
T. gondii displayed a differential invasive and developmental preference in neurogliogenesis during the course of infection [8]. Several studies have highlighted the ability of the parasite to infect a large number of glia cells during the acute phase, in contrast to the low numbers of infected neurons [37, 38]. However, studies showed that T. gondii infects and encysts in both astrocytes and neurons [39, 40].
In addition, we also carried out immunostaining studies to investigate the frequency of β3-tubulin+ cells in neurospheres following the exposure to RSV. Quantitative analysis of anti-β3-tubulin expression by flow cytometry did not reveal any differences of expression in uninfected and infected NPCs exposed to RSV (Fig. 6C).
While here no significant differences were observed in percentages of immature neurons (β3-tubulin+ cells), a reduction in the frequency of MAP2+cells (mature neurons) occurred in differentiated cultures of infected NPCs (Fig. 5D). MAP-2 is a neuron-specific cytoskeletal protein that is used as a marker of neuronal phenotypes. Expression is weak in neuronal precursors, but becomes pronounced later (approximately one day after expression of neuron-specific tubulin isoform β3) [41]. Our findings are in accordance with the work of Creuzet et al. [37], in which quantitative in vitro analyses with a RH strain of T. gondii revealed that approximately 10% of neurons and astrocytes were infected, while 30% of the microglial cells harbored intracellular parasites [42]. One possibility is that both astrocytes and neurons are infected in vivo, but only infected astrocytes or parasites kill less mature neurons suffer in their viability from parasite infection.
Neural replication occurs only in distinct regions of the brain and in limited amounts [43]. While microglia and infiltrating macrophages are essential for controlling T. gondii infection in the brain, neurons and astrocytes are the parenchymal cells that have been most implicated in playing a role in CNS toxoplasmosis. Consequently, for the correct adjustment of cells and cell differentiation in the embryonic stage of the CNS, sufficient number of cells predominate in the early stages. this implies that there is a greater probability in generating differentiated cells that will favor the production of astrocytes or neurons. On the other hand, RSV contributed to neural differentiation of NPCs, favoring their proliferation, migration, gliogenesis and neural maturation.
Our study provides some initial clues regarding the potential effects of RSV during neurogliogenesis in infected NPCs. Our data suggest that the RSV-induced effects on infected NPCs is mediated by a decrease glial differentiation during chronic infection by T. gondii. Neural proliferation was severely affected by the parasite during brain development, resulting in decreased NPC proliferation and neural maturation. NPCs proliferation and neural maturation. On the other hand, the RSV promoted an increase in infected NPCs and restored neural determination during infection. Thus, we suggest the potential of RSV as a restorative therapy against toxoplasmosis brain disorders. Future studies of these striking form of effects on NPC plasticity will not only contribute to our understanding of the mechanisms and functional significance of neurogenesis in infected embryonic mammalian brain, but may also lead to novel strategies for therapy of neurological disease induced by T. gondii.
References
Dubey JP, Lago EG, Gennari SM et al (2012) Toxoplasmosis in humans and animals in Brazil: High prevalence, high burden of disease, and epidemiology. Parasitology 139:1375–1424
Vauloup-Fellous C, Bouthry E (2015) Diagnosis of maternofetal infections. Presse Med 44:621–630. https://doi.org/10.1016/j.lpm.2015.04.016
Vargas-Villavicencio JA, Besné-Mérida A, Correa D (2016) Vertical transmission and fetal damage in animal models of congenital toxoplasmosis: A systematic review. Vet Parasitol 223:195–204
Elsheikha HM (2008) Congenital toxoplasmosis: Priorities for further health promotion action. Public Health 122:335–353. https://doi.org/10.1016/j.puhe.2007.08.009
Hampton MMM (2015) Congenital Toxoplasmosis: A Review. Neonatal Network:NN; New York 34:274–278
McAuley JB (2014) Congenital toxoplasmosis. J Pediatric Infect Dis Soc 3:30–35. https://doi.org/10.1093/jpids/piu077
Wang T, Zhou J, Gan X et al (2014) Toxoplasma gondii induce apoptosis of neural stem cells via endoplasmic reticulum stress pathway. Parasitology 141:988–995. https://doi.org/10.1017/S0031182014000183
Mendez OA, Koshy AA (2017) Toxoplasma gondii: Entry, association, and physiological influence on the central nervous system. PLoS Pathog 13:e1006351
Koshy AA, Dietrich HK, Christian DA, Melehani JH, Shastri AJ, Hunter CA, Boothroyd JC (2012) Toxoplasma co-opts host cells it does not invade. PLoS Pathog 8(18):e1002825. https://doi.org/10.1371/journal.ppat.1002825
Pangeni R, Sahni JK, Ali J, Sharma S, Baboota S (2014) Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin Drug Deliv 11:1285–1298. https://doi.org/10.1517/17425247.2014.919253
Gülçin I (2010) Antioxidant properties of resveratrol: A structure-activity insight. Innov Food Sci Emerg Technol 11:210–218. https://doi.org/10.1016/j.ifset.2009.07.002
Bastianetto S, Ménard C, Quirion R (2015) Neuroprotective action of resveratrol. Biochim Biophys Acta - Mol Basis Dis 1852:1195–1201
Sun AY, Wang Q, Simonyi A, Sun GY (2010) Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol 41:375–383
Pasinetti GM a, Wang J, Ho L et al (2015) Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim Biophys Acta 1852:1202–1208. https://doi.org/10.1016/j.bbadis.2014.10.006
Bottari NB, Baldissera MD, Tonin AA, Rech VC, Nishihira VSK, Thomé GR, Schetinger MRC, Morsch VM et al (2015) Sulfamethoxazole-trimethoprim associated with resveratrol for the treatment of toxoplasmosis in mice: Influence on the activity of enzymes involved in brain neurotransmission. Microb Pathog 79:17–23. https://doi.org/10.1016/j.micpath.2015.01.001
Kumar V, Pandey A, Jahan S, Shukla RK, Kumar D, Srivastava A, Singh S, Rajpurohit CS et al (2016) Differential responses of trans-resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci Rep 6. https://doi.org/10.1038/srep28142
Hutton SR, Pevny LH (2008) Isolation, culture, and differentiation of progenitor cells from the central nervous system. Cold Spring Harb Protoc 3:1–5. https://doi.org/10.1101/pdb.prot5077
William-Faltaos S, Rouillard D, Lechat P, Bastian G (2006) Cell cycle arrest and apoptosis induced by oxaliplatin (L-OHP) on four human cancer cell lines. Anticancer Res 26:2093–2099
William-Faltaos S, Rouillard D, Lechat P, Bastian G (2006) Cell cycle arrest and apoptosis induced by oxaliplatin (L-OHP) on four human cancer cell lines. Anticancer Res 26:2093–2099
Trujillo CA, Negraes PD, Schwindt TT, Lameu C, Carromeu C, Muotri AR, Pesquero JB, Cerqueira DM et al (2012) Kinin-B2 receptor activity determines the differentiation fate of neural stem cells. J Biol Chem 287:44046–44061. https://doi.org/10.1074/jbc.M112.407197
Pillat MM, Cheffer A, de Andrade CM, Morsch VM, Schetinger MRC, Ulrich H (2015) Bradykinin-induced inhibition of proliferation rate during neurosphere differentiation: Consequence or cause of neuronal enrichment? Cytom Part A 87:929–935. https://doi.org/10.1002/cyto.a.22705
Negraes PD, Schwindt TT, Trujillo CA, Ulrich H (2012) Neural differentiation of P19 carcinoma cells and primary neurospheres: Cell morphology, proliferation, viability. and functionality Curr Protoc Stem Cell Biol 1. https://doi.org/10.1002/9780470151808.sc02d09s20
McLaren FH, Svendsen CN, Van der Meide P, Joly E (2001) Analysis of neural stem cells by flow cytometry: Cellular differentiation modifies patterns of MHC expression. J Neuroimmunol 112:35–46. https://doi.org/10.1016/j.brainresbull.2008.08.019
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual. Cold Spring Harbor laboratory press
Yai LEO (2000) Avaliação da infecção experimental por Toxoplasma gondii em suínos pelas provas de bioensaio em camundongos e reação em cadeia de polimerase. Dissertação (Mestrado em Epidemiologia e Aplicação as Zoonoses). Faculdade de Med. Veterinaria e Zootecnia.
Wang T, Liu M, Gao XJ, Zhao ZJ, Chen XG, Lun ZR (2011) Toxoplasma gondii: The effects of infection at different stages of pregnancy on the offspring of mice. Exp Parasitol 127:107–112. https://doi.org/10.1016/j.exppara.2010.07.003
Dubey JP (2009) Toxoplasmosis of animals and humans. Lancet 363:1965–1976. https://doi.org/10.1017/S0031182000078914
Contreras-Ochoa CO, Lagunas-Martínez A, Belkind-Gerson J, Díaz-Chávez J, Correa D (2013) Toxoplasma gondii invasion and replication within neonate mouse astrocytes and changes in apoptosis related molecules. Exp Parasitol 134:256–265. https://doi.org/10.1016/j.exppara.2013.03.010
Mordue DG, Monroy F, La Regina M et al (2001) Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol 167:4574–4584. https://doi.org/10.4049/jimmunol.167.8.4574
Nishikawa Y, Kawase O, Vielemeyer O et al (2007) Toxoplasma gondii infection induces apoptosis in noninfected macrophages: Role of nitric oxide and other soluble factors. Parasite Immunol 29:375–385. https://doi.org/10.1111/j.1365-3024.2007.00956.x
Leong C-W, Wong CH, Lao S-C, Leong EC, Lao IF, Law PTW, Fung KP, Tsang KS et al (2007) Effect of resveratrol on proliferation and differentiation of embryonic cardiomyoblasts. Biochem Biophys Res Commun 360:173–180. https://doi.org/10.1016/j.bbrc.2007.06.025
Della Ragione F, Cucciolla V, Borriello A et al (1998) Resveratrol arrests the cell division cycle at S/G2 phase transition. Biochem Biophys Res Commun 250:53–58. https://doi.org/10.1006/bbrc.1998.9263
Sun AY, Wang Q, Simonyi A, Sun GY (2010) Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol 41:375–383
Kim YA, Kim G-Y, Park K-Y, Choi YH (2007) Resveratrol inhibits nitric oxide and prostaglandin E 2 production by lipopolysaccharide-activated C6 microglia. J Med Food 10:218–224. https://doi.org/10.1089/jmf.2006.143
Mora-Bermúdez F, Huttner WB (2015) Novel insights into mammalian embryonic neural stem cell division: Focus on microtubules. Mol Biol Cell 26:4302–4306. https://doi.org/10.1091/mbc.E15-03-0152
Gage FH, Temple S (2013) Neural stem cells: Generating and regenerating the brain. Neuron 80:588–601
Creuzet C, Robert F, Roisin MP, van Tan H, Benes C, Dupouy-Camet J, Fagard R (1998) Neurons in primary culture are less efficiently infected by toxoplasma gondii than glial cells. Parasitol Res 84:25–30. https://doi.org/10.1007/s004360050351
Cabral CM, Tuladhar S, Dietrich HK, Nguyen E, MacDonald WR, Trivedi T, Devineni A, Koshy AA (2016) Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog 12:e1005447. https://doi.org/10.1371/journal.ppat.1005447
Fischer HG, Nitzgen B, Reichmann G, Groß U, Hadding U (1997) Host cells of Toxoplasma gondii encystation in infected primary culture from mouse brain. Parasitol Res 83:637–641. https://doi.org/10.1007/s004360050311
Parlog A, Schlüter D, Dunay IR (2015) Toxoplasma gondii -induced neuronal alterations. Parasite Immunol 37:159–170. https://doi.org/10.1111/pim.12157
Izant JG, McIntosh JR (1980) Microtubule-associated proteins: A monoclonal antibody to MAP2 binds to differentiated neurons. Proc Natl Acad Sci U S A 77:4741–4745. https://doi.org/10.1073/pnas.77.8.4741
Lüder CGK, Giraldo-Velásquez M, Sendtner M, Gross U (1999) Toxoplasma gondii in primary rat CNS cells: Differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Exp Parasitol 93:23–32. https://doi.org/10.1006/expr.1999.4421
Berenreiterová M, Flegr J, Kuběna AA, Němec P (2011) The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: Implications for the behavioral manipulation hypothesis. PLoS One 6:e28925. https://doi.org/10.1371/journal.pone.0028925
Funding
This work was supported by the Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Project N°. 304,328/2015–4), Brazil. MPP acknowledges postdoctoral fellowship support by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Project N°. 2015/19478–3). H.U. acknowledges grant support from FAPESP for investigating mechanisms of neurogenesis (Project N°. 2012/50880–4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bottari, N.B., Schetinger, M.R.C., Pillat, M.M. et al. Resveratrol as a Therapy to Restore Neurogliogenesis of Neural Progenitor Cells Infected by Toxoplasma gondii. Mol Neurobiol 56, 2328–2338 (2019). https://doi.org/10.1007/s12035-018-1180-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1180-z