Abstract
Rat-tailed larvae of the syrphid species Palpada scutellaris (Fabricius, 1805) are documented causing an enteric human myiasis in Costa Rica. This is the first time that the genus Palpada is recorded as a human myiasis agent. We report a 68-year-old woman with intestinal pain and bloody diarrhea with several live Palpada larvae present in the stool. Using molecular techniques (DNA barcodes) and both electronic and optical microscopy to study the external morphology, the preimaginal stages of the fly were unambiguously identified. An identification key to all syrphid genera actually known as agents of human and animal myiases is provided for larvae, puparia, and adults. Moreover, a critical world review of more than 100 references of Syrphidae as myiasis agents is also given, with emphasis on the species with rat-tailed larvae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myiasis is the term proposed by Hope (1840) to define the presence of the larvae of flies (Insecta: Diptera) in the body of humans and other animals. However, the term was more precisely defined by Zumpt (1965) as the parasitic infestation of organs or tissues of humans and other live vertebrates by dipteran larvae which feed on a host’s necrotic or living tissue, liquid body substances, or ingested food. This definition is currently followed unanimously in all main reviews of this pathology (e.g., Noutsis and Millikan 1994; Hall and Wall 1995; Francesconi and Lupi 2012; Singh and Singh 2015; Bernhardt et al. 2019). If the host is human, myiasis may have medical and public health importance. Myiasis has a widespread incidence among domestic and wild animals all over the world with relatively high biological and economic importance, especially in tropical countries. The incidence of myiasis in humans may be correlated with the existing level of sanitation, the density of prevailing fly population, and the economic status of individuals (Singh and Singh 2015). Despite this, myiasis is diagnosed more frequently in temperate regions and is less correlated with the previously mentioned factors due to increasing travel to exotic destinations, as noted by Noutsis and Millikan (1994).
Myiases can be classified based on the behavior of the fly species involved and the nature of the parasitic relationship, i.e., they can be divided into obligatory and facultative myiases if the involved species can complete their development exclusively parasitizing live hosts, or if the species can develop on both living and dead organic matter, respectively. Four families of flies are responsible for most cases of myiasis, namely Muscidae, Oestridae, Sarcophagidae, and Calliphoridae. These are all calyptrate flies (Diptera, Cyclorrhapha, Schizophora, Calyptratae), a monophyletic group that shares a common evolutionary ancestor (Zhang et al. 2016). Members of the family Muscidae are not involved in obligatory myiases but the other three families include both obligatory and facultative myiasis species, and their natural histories have been analyzed comparing them with their phylogenetic relationships (Stevens and Wallman 2006; Stevens et al. 2006). A third type of myiasis named accidental myiasis or pseudomyiasis (Patton 1921; James 1947; Zumpt 1963) has been documented from a variety of fly families, many not related to calyptrates. Accidental myiasis occurs when the larvae of a normally free-living species are swallowed with contaminated food, passing through the alimentary canal where they may cause pathological reactions (Zumpt 1965; Leclercq 1969).
Syrphidae is a speciose family of true flies with more than 6200 valid species (Thompson 2013), absent only from some remote islands and Antarctica (Thompson and Rotheray 1998). Commonly known as flower flies or hover flies, syrphids are favorites among citizen scientists and nature lovers. Adults play an important role as potential pollinators (Larson et al. 2001; Inouye et al. 2015) as they feed on pollen and nectar and are frequent flower visitors. The larvae of these flies present a large array of natural histories and feeding modes, including phytophagy, saprophagy, mycophagy, and predation (Rotheray and Gilbert 2011), but also some more specialized trophic strategies (e.g., Pérez-Lachaud et al. 2014; Fleischmann et al. 2016). The family is currently divided into four subfamilies (Mengual et al. 2015), the largest of which (Eristalinae) has the highest number of larval feeding modes. Rat-tailed maggots are members of the tribe Eristalini that form part of this subfamily and mostly include species with saprophagous larvae in decaying liquid or semisolid matter (Rotheray and Gilbert 2011). These larvae have a characteristic elongated anal segment with the posterior respiratory process at the tip, which allows them to breathe air while submerged in ponds and mud with accumulations of decaying vegetation or from farmyard manure or silage (Rotheray 1993). Some species with rat-tailed maggots are fairly tolerant of pollution and water bodies with high organic content and low oxygen concentration. The adults are commonly known as drone flies because they mimic bees and play an important role as pollinators of both natural and hand-managed ecosystems, making them economically important as the decline in the numbers of insect pollinators has significant environmental and economic consequences (Golding et al. 2001; Ratnieks and Carreck 2010; Potts et al. 2010).
The common drone fly, Eristalis (Eristalis) tenax (Linnaeus, 1758), an extraordinary mimic of the honeybee Apis mellifera Linnaeus, 1758 (Atkins 1948), is probably the best studied representative of the eristaline flies and, with human assistance, is now a cosmopolitan species (Thompson 2013). Because of its importance as pollinator and its potential economic usefulness for the biodegradation of organic animal waste, its genetic and phenotypic diversity have been studied in both wild and captive populations (Francuski et al. 2011, 2014). On the other hand, rat-tailed larvae have been reported in several forensic case reports (Lee 1994; Archer and Ranson 2005), and indeed, larvae of E. tenax and related species have a relevant role in forensic entomology, typically in cases where the corpse is found in fresh aquatic environments (Salleh et al. 2007; Lindgren et al. 2015; Heo et al. 2019). Additionally, the larval ability to survive in aquatic habitats with high content of organic matter and rich in microorganisms such as drains, sewage pools, or manure storage pits prompted the study of the immune-inducible transcriptome of the common drone fly in order to find genes related to septic injury (Altincicek and Vilcinskas 2007).
The medical importance of Syrphidae is almost null for most species. However, larvae of E. tenax have been reported in cases of nuisance as urban and farm pests due to high concentrations that occur when prepupal larvae seek suitable pupation sites (Gil Collado 1961; Wilson et al. 2009). They can become health and sanitary issues as they have been associated with viruses and amoebae that are present in freshwater (Boughalmi et al. 2013), and porcine pathogenic intracellular bacteria (McOrist et al. 2011) and imagoes of this species may act as potential mechanical vectors of pathogens causing mycobacterial infections in cattle farms (Fischer et al. 2005, 2006; Boughalmi et al. 2013).
Despite this, there are scattered in the literature many references of drone flies as accidental myiasis agents in humans and livestock around the world, although Mathison and Pritt (2014) stated that there is no evidence of saprophagous syrphid larvae causing clinical disease in humans. Most of these cases are considered as intestinal myiasis that were probably caused by the drinking of putrid water-containing eggs or small larvae, or by ingestion of contaminated food (Rotheray and Gilbert 2011). Clinical presentation is varied, and although it may be asymptomatic, some patients experience abdominal pain, nausea, and vomiting (Aguilera et al. 1999; Derraik et al. 2010). Other authors proposed an alternative hypothesis, called “rectal myiasis” (Zumpt 1963; see also Graham-Smith 1913). In this hypothesis, flies, attracted to feces, may deposit their eggs or larvae near or into the anus and the larvae then penetrate further into the rectum. Although more than 200 species with rat-tailed maggots have been described and the preimaginal stages of most of them remain undescribed (Pérez-Bañón et al. 2003a, 2013; Campoy et al. 2017), the common drone fly is cited in virtually all reported cases of myiasis occasioned by flower flies around the world.
In the present study, the first human myiasis caused by an eristaline species of the genus Palpada Macquart, 1834 is reported. In order to identify the species of this genus, DNA barcoding techniques (Hebert et al. 2003a, 2003b) as well as morphological inspection of a pre-adult were carried out. Along with this new report, a critical world review of myiasis occasioned by syrphid species, with special reference to the New World, is given after an exhaustive literature research, as well as the first identification key to syrphid genera cited as myiasis agents for third instar larvae, puparia, and adults.
Material and methods
Case report
The patient, a 68-year-old woman, sought consultation on May 17th, 2015 for bloody diarrhea and strong spasmodic abdominal pain at the colon level in the Centro de Atención Primaria Estatal (Barrio San José, Alajuela, Costa Rica). She did not have any other abdominal or general symptoms, and her physical examination was normal. The complementary studies, including blood analysis, abdominal X-ray films, and abdominal ultrasonography, also were normal. No specific treatment was given to the patient, but she returned on July 3rd, 2015 with the same symptoms and a sample of mucus-bloody stool. In this sample, two larval forms were found with a rat-tailed morphology, compatible with the larval morphology of eristaline species. The patient was treated with an anthelmintic and the sample was sent to the Santa Lucia Laboratory (Heredia, Costa Rica) for microscopic analysis, and later referred to the Universidad de Costa Rica for morphological study. The first morphological studies confirm that the larval morphology is compatible with eristaline flies. During this time, one of the larvae pupated before being transferred to pure ethyl alcohol. Later, the immatures were sent to the Universidad de Alicante for their identification using electronic and optical microscopy. Finally, the pupa with a pre-adult was sent to the Zoologisches Forschungsmuseum Alexander Koenig (ZFMK) for molecular study.
Morphological studies
In order to identify the pupa and larvae, numerous larvae and pupae of syrphid species cited as accidental myiasis agents were studied from collections. Most of the material examined was collected in natural conditions and preserved in 70% ethyl alcohol. In all cases, larvae and pupae were unambiguously identified because larvae were captive-reared. For preparation of the identification key to third instar larvae, puparia, and adults of syrphid genera causing myiasis, voucher specimens in the entomological collections of Universidad de Alicante (CEUA) and ZFMK were studied.
Terminology for adult morphology follows Thompson (1999) and Thompson et al. (2010). Terminology used in the identification key of the immature stages follows Hartley (1961) and Rotheray (1993). Because many of the preimaginal stages of Central and South American flower flies remain undescribed, a diagnostic key to genera with potential role as myiasis agent in the New World was developed. All the syrphid genera reported in the world literature as potential myiasis agents were included in the identification key, including species with rat-tailed maggots but also other saprophagous species without this characteristic morphology. There is a need for medical professionals to have a reliable identification key as shown by Rojas Soto et al. (2017), who used an identification key more than 50 years old (OPS, Organización Panamericana de la Salud 1962) where all rat-tailed larvae were assigned to the genus Eristalis Latreille, 1804.
Illustrations and measurements (mean ± standard error) were made on preserved material using a binocular microscope with an eyepiece micrometer and FSA 25 PE drawing tube. The photographs were taken with a scanning electron microscope (SEM) operated at 20 kV.
Molecular studies
A pre-adult from inside a puparium was used to obtain a DNA barcode in order to identify the species. The DNA barcoding method described by Hebert et al. (2003a, 2003b) is a DNA sequence-based approach for the accurate identification of specimens and for species discovery. In most animal groups, the standard is a 658-base pair (bp) fragment of the mitochondrial cytochrome c oxidase subunit I (COI) gene.
Whole-body extractions were carried out using the NucleoSpin Tissue DNA Extraction kit (Machery-Nagel, Düren, Germany) following the manufacturer’s instructions; samples were resuspended in 100 μl ultra-pure water. Remnants of specimens were preserved and labeled as DNA voucher specimens for the purpose of morphological studies and deposited at the ZFMK (ZFMK-DIP-00017477). PCR amplification protocols for mitochondrial COI gene were the same as described in Mengual et al. (2008, 2012) and Rozo-Lopez and Mengual (2015). The COI fragment was amplified using the forward primer LCO1490 (5’-GGTCAACAAATCATAAAGATATTGG-3’) and the reverse primer C1N2191 (alias Nancy) (5’CCCGGTAAAATTAAAATATAAACTTC-3’) (Simon et al. 1994). PCR products were cleaned using the commercially available QIAquick PCR Purification Kit (QIAgen®). Bidirectional sequencing reactions were carried out by Macrogen© Inc. Chromatograms were edited in Geneious 7.0.6 (Biomatters© Ltd). New sequence was submitted to GenBank (accession number KU216217).
For identification, primer sequences were removed from the edited contig and the sequence was trimmed to 658 bp for comparison in the Barcoding of Life Database Systems database (BOLD; http://www.boldsystems.org/). BOLD uses a tree-based approach to identify sequence queries (Ratnasingham and Hebert 2007), where a distance matrix based on nucleotide sequences is generated and subjected to a tree building operation using the neighbor-joining (NJ) algorithm (Saitou and Nei 1987; Howe et al. 2002). This NJ tree represents a summary of divergence percentages, and itself is taken to represent both a statement of proximity of a given specimen to other (named) biological entities and a graphical tool with which to delimit species boundaries (Goldstein and DeSalle 2011).
Results
Species identification
Morphological inspection of the pre-adult extracted from puparium revealed that it belongs to a male of the genus Palpada. Species identification based on morphological characters was not possible due to underdevelopment of the individual.
The obtained COI sequence from the pre-adult was checked against the BOLD database, and the BLAST-ID reported a 100% sequence similarity with the COI haplotypes of four Palpada scutellaris (Fabricius, 1805) (Process IDs: ASIND2348-12, ASIND2498-12, GMACF009-15, SRCNC068-16).
Identification keys to Syrphidae genera cited as myiasis agents in the New World
Based on the literature and on the present study, four flower fly genera are included in the identification keys to myiasis agents in the Neotropical region (i.e., Central America, the Caribbean, and South America), namely Eristalis, Eristalinus Rondani, 1845, Ornidia Le Peletier & Audinet-Serville, 1828, and Palpada. The genus Helophilus Meigen, 1822 does not occur in Central or South America (Thompson 1999; Thompson et al. 2010), but 11 species occur in the Nearctic Region (Skevington et al. 2019). Thus, identification keys here given are also useful to identify all syrphid genera with species related with myiasis around the world. Nonetheless, caution should be taken if a new genus is reported in the future as more genera might be involved in accidental myiasis in the New World due to the unknown larval biology of many groups and the important diversity of species of this region (see Thompson 1997; Nunes Morales and Marinoni 2009).
Three different identification keys were developed during this study for third larval instar, puparia, and adults of these five genera. Identification keys exist for larvae of myiasis-causing fly species (e.g., Gil Collado 1956), to families, genera, and even species of Diptera based on adult morphology (James 1947; Zumpt 1965; Hall and Smith 1993), but there is no existing key dealing with all flower fly species that may become accidental myiasis agents. Previously, Dušek (1971) presented larval keys to known synanthropic eristaline species of the Palearctic region. van Doesburg (1962) illustrated but did not describe the puparium of P. scutellaris, and more recently, Pérez-Bañón et al. (2003b) provided a key for third instar larvae of all the Neotropical genera with long-tailed larva (see also Thompson et al. (2010) for a key of syrphid genera of Central America). The two subgenera of Eristalis, i.e., Eristalis and Eoseristalis Kanervo, 1938, were keyed out separately based on adult characters to facilitate the identification of Eristalis (Eristalis) tenax.
Identification key to myiasis-causing flower fly genera based on the third larval stage
-
1.
Short-tailed larvae (not rat-tailed maggots), anal segment not extended (Fig. 1c). Anal segment with three pairs of well-developed and about equally long lappets. Spiracular plate with central scars separated and three pairs of very convoluted spiracular openings (Fig. 2b)............. Ornidia
-
2.
Transverse row of spicules just in front of last pair of prolegs (Fig. 2c)............................................... Eristalinus
-
Transverse row of spicules in front of the last pair of prolegs not present (Fig. 2d), although a few scattered spicules may be present between the prolegs........................ 3
-
3.
Ventro-lateral surface of abdominal segments bearing two lines of long setae: first one with long and densely aggregated setae at level of sensilla 7–8, and second one with shorter and more scarce setae extended along the lateral margins of the prolegs (Fig. 3a).......................... Palpada
-
Ventro-lateral surface of abdominal segments without two lines of long setae (Fig. 3b)............................................. 4
-
4.
Last pair of prolegs with most of the large primary crochets facing toward the front of the body. Anal segment in the region just before the “tail” with three pairs of ventro-lateral fleshy projections (Fig. 1a)................... Helophilus
-
Last pair of prolegs with most of the large primary crochets facing toward the lateral margin of the body. Anal segment in the region before the narrow “tail” without fleshy projections (Fig. 1b)................................................ .........................Eristalis (including subgenus Eoseristalis)
Fig. 1 Lateral view of third instar larvae. a Long-tailed larva Helophilus sp. b Long-tailed larva Eristalis sp. c Short-tailed larva Ornidia sp. Scale for all is 1 cm. Figures modified from Sasaki and Mikami (2007) (a, b) and Rotheray et al. (2005) (c). Arrow indicates the presence of the pairs of fleshy projections or lappets (lp), the posterior respiratory process at the tip of the anal segment (prp), and the pairs of locomotory organs or prolegs (p)
Fig. 2 SEM photos of the spiracular plate (a, b) of the larval posterior respiratory process and detail of the ventral view of the last pair of prolegs (c, d). aEristalis sp. bOrnidia sp. cEristalinus sp. dEristalis sp. Arrow indicates the central scars (cs), the spiracular openings (so), and the presence of a transverse row of spicules (trs) in front of the last pair of prolegs (lpp)
-
Identification key to myiasis-causing flower fly genera based on puparia
-
1.
Short-tailed larvae, anal segment not extended (Fig. 1c). Spiracular plate with three pairs of very convoluted spiracular openings (Fig. 2b)..................................... Ornidia
-
2.
Anterior spiracles: facets arranged in more than one row on the clear area (Fig. 4b)................................. Eristalinus
-
Anterior spiracles: facets arranged in one row at edge of the clear area (Fig. 4a)........................................... 3
-
-
3.
Anterior spiracles: clear area not well defined with less than 15 facets (see also Hartley 1961: figs 83, 86–87)........................................................ Helophilus
-
Anterior spiracles: clear area well defined with more than 15 facets............................................................. 4
-
-
4.
Pupal spiracles almost straight, 1–1.5 mm in length, projecting forward and then bending sharply downward (Fig. 4c)........................................................... .................Eristalis (including subgenus Eoseristalis)
-
Pupal spiracles curved, 2–2.5 mm in length, projecting upward and forward (Fig. 4d)....... Palpada scutellaris
-
Identification key to myiasis-causing flower fly genera based on adult morphology
-
1.
Metallic green, bluish, or purple flies (Fig. 5a, b). Vein R4+5 straight, not sinuate (Fig. 5a). Hind femur without basoventral patch of black setulae (Fig. 5b).......... Ornidia
-
2.
Cell r1 open to wing margin (Fig. 6a)............. Helophilus
-
Cell r1 petiolate, closed before reaching wing margin (Fig. 5b)..................................................................... 3
-
-
3.
Eyes with spots or with bands (Fig. 6b). Anepimeron with dorsomedial portion (triangular portion) pilose (see Thompson 2003: figs 1–5). Postalar pile tuft present........................................................ Eristalinus
-
4.
Katepimeron bare (Fig. 7a). Eye uniformly pilose (Fig. 6c).................................. Eristalis (Eoseristalis)
-
Katepimeron pilose (Fig. 7b). Eye pilosity uniform or with two bands of darker, contrasting hairs (Figs. 5c and 6d).....5
-
5.
Eye with contrasting bands of light and dark colored pile (Fig. 5c). Meron and metaepisternum without any pile near spiracle (Fig. 7c). Wing bare...... Eristalis (Eristalis)
-
Eye uniformly pilose (Fig. 6d). Meron and metaepisternum with pile anterior to and/or ventral to metathoracic spiracle (Fig. 7d). Wing with or without microtrichia.............................................. Palpada
Fig. 5 Habitus of flower flies. aOrnidia obesa (female, ZFMK-DIP-00061223), dorsal view. bO. obesa, hind leg, lateral view. cEristalis (Eristalis) tenax (male, ZFMK-DIP-00053688), dorsal view. dE. tenax, hind leg, lateral view. Arrow indicates the basoventral patch of black setulae. eE. tenax, hind leg, detail of the basoventral patch of black setulae. Scale for all is 1 mm, except in e that is 0.5 mm
Fig. 6 Habitus of flower flies. aHelophilus pendulus (male, ZFMK-DIP-00061222), dorsal view. bEristalinus taeniops (male, ZFMK-DIP-00015921), dorsal view. cEristalis (Eoseristalis) arbustorum (male, ZFMK-DIP-00053708), dorsal view. dPalpada scutellaris (male, from http://syrphidae.myspecies.info/taxonomy/term/979 ). Scale for all is 1 mm
Fig. 7 Detail of the pleuron, lateral view. aE. arbustorum. Arrow indicates the bare katepimeron. bE. tenax. Arrow indicates the pilose katepimeron. cE. tenax. Arrow indicates the bare metaepisternum. dPalpada mexicana (Macquart, 1847) (male, ZFMK-DIP-00061224). Arrow indicates the pile near the posterior respiratory spiracle
-
Discussion
Historical background of rat-tailed maggots as myiasis agents
The presence of dipteran larvae in the digestive tract of human and other animals dates back to the seventeenth century [see Del Río 1902 citing Redi’s work about human intestines in 1684, and Hall 1918 citing Blundeville’s work with horses in 1609], but the term myasis [sic] or “the fly-disease” was introduced by Hope (1840). The first documented case report of rat-tailed maggots as myiasis agents was published by the physician Johan Odhelius (1789) from Sweden. He received several larvae that had been passed by a young girl suffering from strong stomach pains. Larvae were expelled over several months and stopped after several weeks of treatment with large amount of mineral water to ingest medications. Odhelius hypothesized that the patient could have ingested the insect’s eggs in her food, which would have subsequently developed into the larval stage. Odhelius (1789) used the term “råtterumpor,” a translation of the French term “vers à queue de rat” proposed by Réaumur (1738) literally meaning rat-tailed maggot, and indicated the similarity of the expulsed larvae with the drawings of Réaumur (1738, p. 443, plate 30).
Years later, Canali (1808) published the presence of a mobile larva, with a very long and thin tail, excreted from the urethra of a woman. The specimen was initially identified as a “coda di sorcio” (rat-tailed) syrphid larva by Valeriano Luigi Brera, a reputed Italian physician expert on parasitic worms (helminths), who compared it with the drawings of Réaumur (1738), but after some microscopic examination, Brera (1811) decided to erect a new genus of helminth based on this specimen, Cercosoma Breral, 1811. Bremser (1819) pointed out the misidentification by Brera and stated that the “langgeschwänzte Figur” (long-tailed figure) was a syrphid larva of the genus Eristalis after asking his entomologist colleague Ziegler. Several authors published Cercosoma (misquoted by some authors as Conosoma) as a junior synonym of Eristalis (Dujardin 1845; Cobbold 1864; Davaine 1877; Hanby 1905; Stiles and Hassall 1905), but this synonymy is not listed in the latest database (Thompson 2013). Although species identification at this time was not possible, in 1908, Gilbert (1908) listed larvae of four “Eristalis species” reported to have been passed from the human bowels, namely E. dimidiatus (now E. dimidiata Wiedemann, 1830 because the nomenclatural gender of this name is currently considered as feminine; see Chandler et al. 2004; ICZN 2006), E. tenax, E. arbustorum (Linnaeus 1758), and E. pendulus (now Helophilus pendulus (Linnaeus 1758)).
There is a remarkable ambiguity when characterizing the identity of the species involved in the first works mentioning the myiasis caused by rat-tailed larvae (Odhelius 1789; Canali 1808; Hope 1840). This confusion has not been critically reviewed in the medical literature and errors or inaccuracies in the species identification have been repeated many times in subsequent papers. In fact, the taxonomical nomenclature of the species of Syrphidae with rat-tailed larvae was not fixed until many years after the first cases of myiasis were published and, even nowadays, the unequivocal morphological identification of Syrphidae larvae is difficult without the help of a specialist and the associated imago.
In this section, we review all syrphid species cited in the literature in connection with cases of myiasis and discuss the likelihood of the identifications based on taxonomic characters and the known biology of the immatures. The results of this review are divided into four parts: (1) myiasis caused by Eristalis tenax, the common drone fly; (2) myiasis caused by other species with rat-tailed larvae; (3) myiasis caused by non-rat-tailed larvae; and (4) myiasis wrongly attributed to rat-tailed larvae.
Myiasis caused by Eristalis tenax, the common drone fly
Eristalis tenax is a highly anthropophilic and almost ubiquitous species in a wide spectrum of habitats. This synanthropic species is highly migratory and almost cosmopolitan after being widely introduced by humans. It is now the most widely distributed flower fly species in the world, known from all regions except the Antarctic (Greenberg 1971; Speight 2018). The common drone fly is cited as a cause of myiasis in almost all medical and veterinary cases where flower fly species are involved reported around the world; however, unambiguous species identification based exclusively on larval morphology has been not possible until recently because the preimaginal stages of most eristaline species remain undescribed (Hartley 1961; Pérez-Bañón et al. 2013; Campoy et al. 2017). In the same line of argumentation, we agree with other authors (e.g., Metcalf 1916; Zumpt 1965; Goldsmid and Phelps 1977) that the name Eristalis tenax is used for all rat-tailed maggots found in connection with true or supposed cases of myiasis, and it would not be a surprise that under this name other species or even other genera of Eristalinae are involved.
There is an old taxonomical confusion between two valid species of flower flies, Helophilus pendulus and Eristalis tenax (type species of the genera Helophilus and Eristalis, respectively), originating with the first case reported of syrphids as myiasis agents (Odhelius 1789). Both species belong to the group of flower flies with rat-tailed larvae, but unfortunately, they had been considered as synonyms in many papers for a long time. Part of the confusion comes from the original descriptions made by Linnaeus in 1758, when he proposed the original names of Musca tenax and Musca pendula for each of them (Linnaeus 1758). In both cases, Linnaeus used information about the biological cycle of the “mouches abeilliformes” (bee-like flies) published 20 years earlier by Réaumur (1738), but referred only to the drawings of the larvae of Réaumur in his description of Musca pendula Linnaeus 1758 to indicate the larval biology and morphology of this species. As mentioned above, Odhelius (1789) compared the rat-tailed maggot of his study with the same drawings of Réaumur (1738) and identified the material as the same species that Linnaeus called Musca pendula and De Geer (1776) called “Mouche pendante”. Later, Musca pendula was transferred to the genus Helophilus and nowadays is known as Helophilus pendulus, but the work of Odhelius (1789) was cited by other authors and the species was referred under different names, with Eristalis pendulus the most common, but also as Helophilus pendulus, Helophilus pendulinus, Tubifera pendula, or Elophilus pendulus (Hall and Muir 1913). As an example, in his review of larvae occasionally found in the human body, Hope (1840) used Elophilus pendulus for the case reports of Odhelius (1789) and Canali (1808), and wrongly cited as case reports three other works that merely cited the case of Odhelius (1789). Bremser (1819) identified Cercosoma as a larva of Eristalis and added that most likely it was “Eristalis pendulus Fabric. (Syst. Entliat. n.7 p. 233)”. Fabricius (1805) listed Helophilus under Eristalis as a synonym and the taxonomic confusion continued with the validity of the names published by Meigen in two of his works (Meigen 1800; Meigen 1803). To summarize more than 40 years of disputes among taxonomists in one sentence (see Smart 1944; Sabrosky 1952, 1999; Melville 1961), Tubifera Meigen 1800 was suppressed by the International Code of the Zoological Nomenclature (ICZN 1963) and Elophilus Meigen 1803 was considered a junior synonym of Eristalis (Thompson 2013).
Due to this taxonomic confusion, many of the very old records of rat-tailed maggots causing myiasis were identified as Helophilus pendulus, and some workers cited the original reference of Odhelius (1789) as synonym of Tubifera tenax, now Eristalis tenax. We cannot discard the genus Helophilus as a potential myiasis agent because it is also present in polluted water close to human activities (Sasaki and Mikami 2007), but we agree with James (1947) and consider that identifications of H. pendulus based on larvae without emerged adults were most likely erroneous as immature stages of many species are unknown and very similar to the larvae of Eristalis tenax. The natural history of E. tenax, its presence in polluted water, and its close association with human activities [see Gil Collado 1961 as an example of a larval massive invasion of human dwellings or Guizzardi et al. 1989 for a larval outbreak in a dairy cattle farm] makes this species the most likely candidate to be involved in Odhelius’s case. This also fits with a critical examination of the drawings by Réaumur (1738), which makes us think that they were based on Eristalis larvae and not on immatures of Helophilus. Both taxa are separated by the presence (in Helophilus) of the three pairs of fleshy projections between the anal opening and the base of the breathing tube (Rotheray 1993, see also Fig. 1a). In the very detailed drawings of Réaumur (1738), there is no presence of these fleshy projections, but curiously, this feature is present in the figures of the larvae of Eristalis in classical papers (e.g., Miall 1895, p. 199, fig. 70; Natvig 1924) or specifically as Eristalis tenax in the medical and veterinary manual of Patton and Evans (1929, p. 330, fig. 193) and repeated by Gil Collado (1956) under the name of Tubifera tenax. Furthermore, there is other evidence that confirms that Eristalis species were called Helophilus at the nineteenth- to twentieth-century boundary; for example, Brumpt (1910, p. 586, fig. 432) illustrated the wing of a syrphid that he named Helophilus, but the figure shows clearly that the cell r1 is closed, not matching the Helophilus wing venation that has the cell r1 open. This wing venation with a closed cell r1, though, fits the genera Eristalinus and Eristalis among others. Therefore, until the appearance of new refutable cases, we consider all the quotations of H. pendulus in myiasis as doubtful and consider they be referred as E. tenax. At the end, it seems that Osten Sacken (1894) was right when, explaining the resemblance of E. tenax with the honeybee, he defined E. tenax as an insect “for man’s confusion born.”
Myiasis caused by other species with rat-tailed larvae
Wagner (1870) reported Eristalis arbustorum from larvae said to be passed with feces by a woman in Germany, after identifying the reared adults from larvae. In this publication and subsequent reviews (e.g., Austen 1912), the possibility is indicated of the flower fly eggs being swallowed by the patient with polluted drinking water. This Palaearctic species was introduced in the USA around 1885 and it is ubiquitous in that continent (Skevington et al. 2019). Due to human activities such as international trading and its larval development in anthropized environments, it is likely that E. arbustorum may be involved in other cases of human myiasis as this species is already involved in forensic cases (Lindgren et al. 2015).
Carpaneto and Vigna Taglianti (1995) reported the first case of Eristalinus taeniops (Wiedemann, 1818) from a larva that was passed by a 24-year-old woman in Italy, after identifying the reared adult. The most likely reason of this accidental myiasis was the ingestion of eggs or small larvae present in liquid media related with agricultural work and the use of manure by the woman. This taxon is widely distributed throughout Africa and the Oriental region (Knutson et al. 1975; Smith and Vockeroth 1980) and is also present in the Mediterranean basin (Speight 2018). It has been subsequently established in the New World (Thompson et al. 1990; Thompson 1999). To date, no new cases of myiasis with this species have been published, although Dutto and Maistrello (2017) reported the presence of a mature larva in the feces of a 4-year-old child from Piedmont (Italy), without indicating a relationship with a possible case of myiasis. This Eristalinus species shares the ecological plasticity of E. tenax and E. arbustorum, as well as its dispersal ability derived from the adaptation to human environs.
Riley (1890) reported larvae of Eristalis (Eoseristalis) dimidiata that were passed from the bowels of a young woman from Evansville (Indiana, USA). In the same paper, this author also reported a similar case with larvae of E. tenax. The life cycle of E. dimidiata is similar to the one of E. tenax and its distribution coincides with the location of the case report (Skevington et al. 2019). Until now, this is the only record of this species as a myiasis agent in the scientific literature. This record is probably a misidentification as the larval stages of E. dimidiata are still unknown (Campoy et al. 2017). It is interesting to note that a few years before the publication of Riley (1890), several papers were published indicating a rapid expansion of E. tenax throughout North America, mainly because of involuntary transport due to human activities and its high degree of adaptation to polluted water derived from the development of rural and urban areas. In the USA, E. tenax had a rapid expansion since 1870, possibly after its arrival from Eastern Siberia to the Pacific coast (Williston 1886). In all likelihood, the rapid east–west expansion could not be completed until rural and urban development (with drains, sewers, and cesspools for the development of the larvae) allowed the union of both areas (Osten Sacken 1894). Since 1875, E. tenax was a common species in the USA associated primarily with water media directly or indirectly related to human activity (Osten Sacken 1886). It is notable that all cases of myiasis caused by rat-tailed maggots in the USA were reported after 1870, which coincides with the beginning of the distribution of E. tenax throughout the country. Riley’s case report is dated in 1890, so the involved fly species in the myiasis is plausibly E. tenax and not E. dimidiata.
Soler Cruz (2000) listed Eristalis diminuta [sic] in her review of the myiasis in Spain as an occasional myiasis agent found in the entire Iberian Peninsula. There is no species with this name, and it might be a misspelling of E. dimidiata. But E. dimidiata does not occur in Europe (Speight 2017, 2018) and there is no record or case report of E. dimidiata as myiasis agent except the one mentioned in Riley (1890).
A very interesting report from the Caribbean Antigua (current Antigua and Barbuda) cited Eristalis vinetorum (Fabricius, 1799) [now Palpada vinetorum (Fabricius, 1799)] in a list of myiasis-producing flies in humans and other animals (Goodwin 1925). This report is probably the reason why Eristalis is listed by Berger (2019). Goodwin (1925) is a very uncommon publication, and after several attempts via different libraries, we conclude that this work is not held in North America or Europe. Without the original text, it is not possible to know if the species listed in the abstract by Goodwin (1925) were compiled from myiasis case reports, if the reports were from humans or other animals, or if the larvae were reared into adults for identification. There are no records of E. tenax from the Lesser Antilles and E. tenax is rarely collected in the Neotropics, but P. vinetorum is found in Antigua (Thompson 1981). Without adults to confirm the identification, we believe that a species name for a rat-tailed larva is not possible to assure, and was much more difficult back in 1925 with many species still to be described. Consequently, the identity of the material studied by Goodwin (1925) remains unknown, as well as the host and the type of myiasis.
Myiasis caused by non-rat-tailed larvae
Larvae of other species groups of Syrphidae (without a rat-tailed morphology) have been reported as causative of myiasis in humans and animals. There are two different flower fly groups reported in the literature: a species with saprophagous larvae, Ornidia obesa (Fabricius, 1775), and a group with nonsaprophagous larvae that are very likely unable to survive in the human digestive tube.
Several authors have reported Ornidia obesa as a myiasis agent in humans in the New World. Machado (1937) and Monteiro et al. (2008) reported O. obesa from larvae passed by patients in Brazil, while Yang (2014) reported this species in connection with an intestinal myiasis from Hawaii. More recently, López et al. (2017) cited two cases of myiasis caused by O. obesa. After the study of the original work, we confirm that only the case of a 70-year-old patient was due to O. obesa. The second reported case was clearly caused by some other dipteran as figures 2–7 in López et al. (2017) do not represent a syrphid larva. Martins et al. (2010) studied O. obesa breeding in pig carcasses in Brazil in order to evaluate the importance of this species in forensic entomology. López Millán et al. (2015) documented several cases of myiasis in pigs and cited Ornidia robusta [sic] as one of the three dipteran species collected. This species name does not exist. Adults of the so-called O. robusta are easy to identify from the pictures in the original publication and they belong to O. obesa.
Ornidia is a small group of brilliant metallic green or purple flies, originating from the New World, which has been spread extensively in the Pacific and across the Orient to the east of Africa due to human activities (Thompson 1991). Like rat-tailed larvae involved in myiasis, the larva of this species is known from numerous synanthropic habitats including human latrines, animal dung, decaying fruits, and other semiliquid media such as agri-food wastes (Lardé 1989; Whittington and Rotheray 1997). Due to its highly synanthropic habits, O. obesa might be a common species involved in human/animal myiasis, but the use of specific identification keys based on preimaginal morphology is recommended for unambiguous identification (see Rotheray et al. 2005; Da Silva Carvalho Filho and Esposito 2009).
On the other group with non-rat-tailed larvae, Austen (1912) reported three cases where the dipteran larvae causing the myiasis were identified as belonging to Syrphus Fabricius, 1775 or Scaeva Fabricius, 1805. In two cases, larvae were discharged through the rectum, while in the third one, a larva was removed from the ear of a boy. As already explained by Austen (1912) and later on by James (1947), the occurrence of a larva within the ear (auditory meatus) was probably accidental and should not be regarded as myiasis. The other two records are difficult to accept as the larvae of these genera are predaceous on soft-body insects, most commonly aphids (Metcalf 1916; Rojo et al. 2003). James (1947) guessed that larvae could be ingested with Brussels sprouts or other vegetables harboring aphid colonies, although it is highly doubtful whether they could cause enteric disturbances or pass through the digestive tract alive.
Myiasis wrongly attributed to rat-tailed larvae
There are several collations of myiasis agents based on previous reports [e.g., see Bernhardt et al. 2019 as the most recently published], and there are some works compiling the reports of Eristalis-caused myiasis from published literature (e.g., Desoubeaux et al. 2014; Singh and Singh 2015). No access to the original publications or simply citing other collations, especially if their main focus is the medical aspects of the reports, can perpetuate in the literature mistakes and inaccurate identifications of the species involved in myiasis. In the present review, the risk of the collated list (Table 1) is to convey the impression that the majority of myiasis-causing flower flies are Eristalis species (especially E. tenax) due to misidentifications of larvae in the literature and the fact that we cannot study that material for reassessment. For the elaboration of Table 1, some of these incorrect or inaccurate records, as well as country of origin of these myiasis, have been corrected based on the original texts of the publications, and in this section, we mention some myiasis reports that were wrongly attributed to syrphid flies. For example, Utsalo and Ahmed Khalifa (1985) presented an interesting case of urinary myiasis caused by rat-tailed larvae but also mentioned that Gil Collado (1961) encountered larvae of E. tenax as causing agents of intestinal myiasis. Gil Collado (1961) did not discuss myiasis and only reported a case of massive invasion of E. tenax larvae in several houses in Madrid, Spain, due to the prepupal migratory behavior of this species.
The work of Nagakura et al. (1991) is cited by other authors (e.g., Mumcuoglu et al. 2005; Desoubeaux et al. 2014; Lewis et al. 2015 as E. tenax) as an example of gastrointestinal myiasis caused by Eristalis-like or rat-tailed larvae. However, Nagakura et al. (1991) reported three cases of intestinal myiasis in Japan caused by larvae of two species of Sarcophagidae and by larvae of the black soldier fly, Hermetia illucens (Linnaeus, 1758) (Diptera: Stratiomyidae). In the original article, there is a photo of a black soldier fly mature larva that typically presents the long and narrow head capsule on the dorsal side.
Oluwatosin and Fadahunsi (2003) mentioned a cutaneous myiasis due to Eristalis luteola [sic] involving breast tissue invasion in a female. No species exist with this name and the name luteola might refer to Auchmeromyia luteola (Fabricius, 1805), currently known as Auchmeromyia senegalensis Macquart, 1851 (Diptera: Calliphoridae) or the “Congo floor maggot”, which causes sanguinivorous myiasis and can feed for up to 20 min on the human body (Zumpt 1965). Oluwatosin and Fadahunsi (2003) also reported a case of a 9-year-old girl who vomited two living larvae, identified as Eristalis sp. The morphological description of these immature stages, i.e., “larvae had globular anterior regions and tail-like retractile posterior ends fringed with setae”, fits with the general description of an unidentified rat-tailed maggot. Finally, this work also cited an enteric myiasis by Eristalis from Egypt originally reported by Mandour and Omran (1978). We read the original report by Mandour and Omran (1978) and the enteric myiasis was due to second instar larvae of Sarcophagidae from Sudan.
In the studied literature, we have found that sometimes rat-tailed maggots have been cited as nematode parasites and vice versa. Axe (1874) reported rat-tailed larvae being expulsed by horses, but Cobbold (1879) pointed out that this case report was spurious and the so-called rat-tailed maggot by Axe was “merely a very stout and pregnant Oxyuris curvula Rudolphi, 1803” [currently known as Oxyuris equi (Schrank, 1788)], the horse pinworm. We also found a case where the causative agent of the myiasis was a rat-tailed larva, but the authors of the case report attributed the myiasis to another species. Förstl et al. (2002) reported a 27-year-old woman with severe spasmodic abdominal pain after a 2-week trip to Tunis. Once back in Czech Republic, the patient passed a rat-tailed larva (photographed and illustrated in the article), which was erroneously identified as the human whipworm nematode parasite Trichuris trichiura (Linnaeus, 1771). Förstl (2003) used the same photographs in his book to illustrate T. trichiura, but the larva belongs to Eristalis sp.
Types of myiasis
In Table 1, we summarize all known literature of accidental myiasis caused by flower fly species including the original reference, the host, the syrphid species, and the myiasis type. To date, the present review is the most exhaustive regarding Syrphidae as myiasis agents, and in total, it comprises almost three and a half times the number of references with respect to the previous large review on E. tenax as a myiasis agent (Desoubeaux et al. 2014). In order to present the reviewed data in a more efficient way, we subdivided the myiasis into various types according to the tissue and region of the body infested (James 1947; Singh and Singh 2015): (i) traumatic myiasis or myiasis of wounds; (ii) oral myiasis or infestation of the oral cavity; (iii) nasal myiasis or infestation of the nose; (iv) aural myiasis or ear infestation; (v) ophthalmomyiasis or larvae in the eye; (vi) enteric or gastrointestinal myiasis, or infestation of the stomach and intestine; (vii) urogenital myiasis or the presence of maggots in or near the genitals or urethra; and (viii) rectal myiasis or anus infestation. Contrary to other fly larvae, syrphid species have not been reported for oral myiasis, aural myiasis, or ophthalmomyiasis.
Since Zumpt (1962), several authors cited the rat-tailed larvae of syrphids as causative of true rectal myiasis. Zumpt (1963) was skeptical about the so-called generically “intestinal myiasis” recorded from humans, as he considered that they were frequently wrongly interpreted if only the presence of larvae in the stool or the vomit was considered. He recognized that except the obligatory myiasis species such as botflies (Oestridae), the rest could live with difficulty in the human alimentary tract and only under certain circumstances were they able to survive. Thus, Zumpt (1963) affirmed that some cases of myiasis caused by Eristalis are due to contamination after defecation, as Hall (1918) did previously, and suggested that most dipteran larvae could not survive in the conditions of the digestive tube. The rectal myiasis as defined by Zumpt (1962, 1963) happens when adult flies lay the eggs into or near the anus and larvae penetrate into the posterior part of anus. Zumpt and other authors (e.g., Leclercq 1969) accepted that the typical morphology of the Eristalis larvae, with the anal segment extended, would help them to breathe once in the anus area as they do in nature. In the present review work we consider as enteric myiasis all case reports where syrphid larvae were passed in the feces or vomit of the patient (or animal). We doubt that rectal myiasis is possible for rat-tailed maggots and this explanation fails to explain how the larvae of Ornidia obesa (without “tail”) can survive in the digestive tract. If we assume that myiases involving syrphid flies are due to the accidental ingestion of eggs or first instar larvae, but expulsed maggots are 2.5–3 cm long and close to pupation, this can only be explained if those species complete the larval development inside the digestive system of vertebrates. Thus, syrphid larvae would need to be considered as true facultative myiasis agents instead of accidental agents or pseudomyiasis.
Myiasis-causing flower flies are reported in the literature from livestock (cattle, pig, and horse), pets (dog), and humans, but no records from wild animals exist. We found some works (Lever 1957; Richards 1977; Robertson and Whelan 1987; Urios 1990) where numerous rat-tailed maggots were mentioned from stomachs and scats of the wild red fox, Vulpes vulpes Linnaeus, 1758. Larvae of Eristalis tenax (Robertson and Whelan 1987) and empty eristaline puparia (Richards 1977) have been reported from fox scats and larvae of Myathropa florea (Linnaeus, 1758), a flower fly with rat-tailed larvae associated with wet decaying vegetation in rot-holes and in decaying heartwood (Rotheray 1993), were identified from the fox intestine (Lever 1957). The presence of syrphid larvae in the vulpine diet can be explained either by accidental ingestion while drinking or deliberate ingestion, as invertebrates are commonly reported in the fox’s diet (Southern and Watson 1941; Scott 1943; Richards 1977 among others). Independently of the reason of the ingestion, the massive amount of rat-tailed maggots in the large intestine (Lever 1957), the large amounts of “ghost skins” (= puparia) found in scats (Richards 1977), and the fact that they were identified to species level (i.e., the larvae preserve their morphology to be identifiable) indicate a possible myiasis in the red fox. Lever (1957) did not mention if the larvae were alive or not, and this topic deserves further investigation.
During the literature review, we corrected some errors or mistakes present in other reviews, for example, indicating the country where the myiasis or infection took place instead of citing the country where the patient received treatment, not including some works where the original text do not mention any flower fly but have been referred by subsequent authors to E. tenax (e.g., de Groot 1956; Bryan 1937 cited in Supple 1958), and corroborating the identifications when images were available. In some cases, we could not verify the identifications, i.e., myiases in South America reporting E. tenax as the agent are very unlikely, but see an exception by Desoubeaux et al. (2014). Based on the present work, only five flower fly species can be unambiguously assigned as the cause of myiasis in humans and animals: Eristalis tenax, Eristalis arbustorum, Eristalinus taeniops, Palpada scutellaris, and Ornidia obesa.
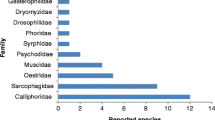
The majority of the reported myiases caused by flower flies are gastrointestinal or enteric (104 out of 126), with the other types much less reported: urogenital (13 and another 3 together with other myiasis types), nasal (4 and 1 together with a urogenital myiasis), and traumatic (3). Interestingly, urogenital myiasis in humans has been reported for male and female individuals, and 5 mammal species have been recorded as accidental hosts: cattle (cow) (8), pig (4), dog (1), horse (3), and human (114). The common drone fly is the causative species in ca. 68% of the reported cases, Eristalis sp. and other species with rat-tailed larvae represent ca. 27% of the cases, and Ornidia is involved in 4% of the cases. The identity of the causative species, though, cannot be corroborated without voucher specimens and detailed morphological studies of adults and/or larvae. We suggest that future studies of myiasis always deposit vouchers into national museums and that DNA barcodes of the agents are obtained and published as surrogate vouchers.
Species identification using DNA barcodes
DNA barcoding is a molecular tool that aims to help in the identification of biological species (Hebert et al. 2003a, 2003b). Although often used to support the description of new species (e.g., Ståhls et al. 2009; Grković et al. 2015 for flower flies), DNA barcodes also allow the association of all life stages and genders of a single species or to identify organism from parts/pieces (Casiraghi et al. 2010). For instance, DNA barcoding was successfully applied on Syrphidae to associate phytophagous larvae with adults and identify them as Merodon avidus Rossi, 1790 (Andrić et al. 2014), or to associate unknown sex to species for which only one of the sexes is known (Jordaens et al. 2015a). More frequently, DNA barcoding is used to identify species when the query DNA barcode is compared against a library of sequences. In this respect, Jordaens et al. (2015b) corroborated the identification of a Neotropical syrphid species in Benin and Cameroon (West and Central Africa), and GilArriortua et al. (2014) confirmed the presence of the obligate amphibian parasite Lucilia bufonivora Moniez, 1876 (Diptera: Calliphoridae) in the Basque Country (Spain). Moreover, DNA barcodes are more often used to identify myiasis species (Otranto and Stevens 2002; Severini et al. 2015; Zhang et al. 2017), and DNA barcode libraries have been established for other myiasis dipterans such as the species of Oestridae (Otranto et al. 2003) or dipteran species of forensic importance (Sonet et al. 2013; Fuentes-López et al. 2019).
We applied a similar approach and identified the pre-adult inside the puparium from the stool as Palpada scutellaris, reporting the first case of the genus Palpada causing an intestinal myiasis. We think that as long as the DNA barcode databases grow in number of sequences and species represented in them, the utility of DNA barcoding to identify biological species will improve and their use and accuracy will increase. Assembling a public dataset of barcodes for all myiasis agents will be very valuable for medical professionals. That way, matches can be assessed, and new agents can be clearly identified. We strongly believe that medical colleagues will benefit enormously from this molecular tool in order to obtain an accurate species identification and recommend their use and promotion.
Conclusions
The present case report is the first time where the genus Palpada, and the species P. scutellaris, has been shown to cause intestinal myiasis. Palpada larvae are filter-feeding “rat-tailed maggots” living in semi-aquatic environments rich in decaying organic matter, including dung or decaying plant material (Thompson et al. 2010), and they can be found in open dumpsite (Solange Sánchez et al. 2010) and in refuse dumps (detritus) of nests of the leaf-cutter ant Atta cephalotes (Linnaeus, 1758) (van Doesburg 1962). Moreover, saprophagous larvae of the genera Palpada and Ornidia have been used to recycle wastes from coffee and orange juice production (Thompson et al. 2010; Pérez-Bañón et al. 2013).
This study also corroborates the arguments of James (1947) and Zumpt (1965) when saying that the name Eristalis tenax was indiscriminately used for any rat-tailed larva involved in a myiasis, and that other eristaline genera may be involved. Due to the lack of immature descriptions from Central and South America, almost nothing is known about the larval morphology of some eristaline genera (but see Thompson et al. 2010), and the use of out-of-date literature (see Rojas Soto et al. 2017) perpetuates the name of E. tenax in the medical literature as myiasis agent. We strongly suggest that samples from myiasis are examined by a taxonomic specialist and, if possible, to try to rear the immatures into adults in order to corroborate the taxonomic identification. In case of unsuccessful rearing, we advise to preserve the immatures or their remnants into 95% or pure ethylic alcohol for molecular analysis. It is also recommended to publish a photograph of the larvae for each medical case report.
References
Achenjang F et al (2003) Rat-tailed maggot, RTM Pearls. Dr. Fidelis Achenjang website at Union College. https://www.unionky.edu/directory/dr-fidelis-achenjang/rat-tailed-maggot-rtm-pearls. Accessed 26 July 2019
Adam AA (2012) Nasal expulsion of rat-tailed maggot: the first reported case in Sudan. Al Neelain Med J 2:43–47
Aguilera A, Cid A, Regueiro BJ, Prieto JM, Noya M (1999) Intestinal myiasis caused by Eristalis tenax. J Clin Microbiol 37:3082
Altincicek B, Vilcinskas A (2007) Analysis of the immune-inducible transcriptome from microbial stress resistant, rat-tailed maggots of the drone fly Eristalis tenax. BMC Genomics 8:326. https://doi.org/10.1186/1471-2164-8-326
Ambrosi M (1995) Un epidodio di gastromiasi accidentale collettiva nei suini. Praxis Vet Milano 16:13–15
Andrić A, Šikoparija B, Obreht D, Dan M, Preradović J, Radenković S, Pérez-Bañón C, Vujić A (2014) DNA barcoding applied: identifying the larva of Merodon avidus (Diptera: Syrphidae). Acta Entomol Mus Natl Pragae 54:741–757
Archer MS, Ranson DL (2005) Potential contamination of forensic entomology samples collected in the mortuary. Med Sci Law 45:89–91. https://doi.org/10.1258/rsmmsl.45.1.89
Atkins EL (1948) Mimicry between the drone-fly, Eristalis tenax (L.), and the honeybee, Apis mellifera L. its significance in ancient mythology and present-day thought. Ann Entomol Soc Am 41:387–392. https://doi.org/10.1093/aesa/41.3.387
Austen EE (1912) British flies which cause myiasis in man. Reports to the local goverment board on public health and medical subjects 66:5-15
Axe JW (1874) Larva of the Helophilus, an equine parasite. Vet Lond 47:10–11
Bacigalupo J, Pérez Vuidepot G, Didiego EP (1941) Primera observación argentina de miasis intestinal por Eristalis tenax L. Sem Med 48:555–556
Berger S (2019) Infectious diseases of Antigua and Barbuda, 2019th edn. GIDEON Informatics, Los Angeles
Bernhardt V, Finkelmeier F, Verhoff MA, Amendt J (2019) Myiasis in humans—a global case report evaluation and literature analysis. Parasitol Res 118:389–397. https://doi.org/10.1007/s00436-018-6145-7
Bhaibulaya M (1982) Intestinal myiasis caused by Eristalis tenax larva: a case report. J Parasitol Trop Med Assoc Thai 5:97–99
Boughalmi M, Pagnier I, Aherfi S, Colson P, Raoult D, La Scola B (2013) First isolation of a Marseillevirus in the Diptera Syrphidae Eristalis tenax. Intervirol 56:386–394. https://doi.org/10.1159/000354560
Bremser JG (1819) Über lebende Würmer im lebenden Menschen. Bei Carl Schaumburg et Comp, Wien
Brera VL (1811) Memorie fisico-mediche sopra i principali vermi del corpo umano vivente; e le cosi dette malattie verminose. Presso Antonio Ronna, Crema
Brossard M, Moddle H (1985) Le diagnostic parasitaire dans le canton de Neuchâtel – Rapport d’activité 1984. Bull Soc Neuchl Sci Nat 108:191–193
Bruce EA (1917) A new parasite for cattle. The larvae of Eristalis tenax L (drone-fly). J Am Vet Med Assoc 52:66–68
Brumpt E (1910) Précis de parasitologie. Masson et Cie, Paris
Bryan WJ (1937) Myiasis. JAMA 109:573–574. https://doi.org/10.1001/jama.1937.92780340001010
Cameron TWM (1924) The pig and human disease. Proc R Soc Med 17:31–37. https://doi.org/10.1177/003591572401701509
Campoy A, Pérez-Bañón C, Nielsen TR, Rojo S (2017) Micromorphology of egg and larva of Eristalis fratercula, with an updated key of Eristalis species with known third instar larvae (Diptera: Syrphidae). Acta Entomol Mus Natl Pragae 57:215–427. https://doi.org/10.1515/aemnp-2017-0070
Canali L (1808) Lettera sopra un verme uscito vivo dall’ uretra d’ una Donna. Gior Pisano Lett Sci ed Arti 8:35–47
Canese A (1970) Miasis intestinal por Tubífera tenax. Rev parag Microbiol 5:26
Carpaneto GM, Vigna Taglianti A (1995) Un caso di miasi intestinale prodotta da Eristalinus taeniops in Italia Diptera, Syrphidae. Boll Assoc Rom Entomol 49:119–126
Casiraghi M, Labra M, Ferri E, Galimberti A, De Mattia F (2010) DNA barcoding: a six-question tour to improve users’ awareness about the method. Brief Bioinform 11:440–453. https://doi.org/10.1093/bib/bbq003
Cassamagnaghi A (1945) Miasis intestinal en un bovino por larvas de Eristalis. Anal Fac Vet - Montevideo 4:517–519
Cazorla Perfetti DJ, Morales Moreno P, Acosta M, Bermúdez S (2011) Primer reporte de pseudomiasis intestinal humana por Eristalis tenax (Diptera, Syrphidae) en zona semiárida urbana del estado Falcón, Venezuela. Bol Malariol Salud Ambient 51:225–228
Chabasse D, Maurice JJ, Beaucournu JC, Hocquet P (1981) Myiase digestive. Ouest Med 34:961–962
Chagnon G (1949) Le diptère Eristalis tenax parasite accidentel de l’home. Ann ACFAS (Ass Canad-Fran Avanc Sci) 15:93
Chagnon G, Leclercq M (1949) Myiase intestinale à Eristalis tenax L. (Diptère Syrphidae). Rev Med Liege 4:634–635
Chandler AC (1943) Additional records of human intestinal myiasis caused by Eristalis. J Parasitol 29:425. https://doi.org/10.2307/3272858
Chandler PJ, Wakeham-Dawson A, Mccullough A (2004) Case 3259. Eristalis Latreille, 1804, (Insecta, Diptera): proposed confirmation that the gender is feminine; Musca nemorum Linnaeus, 1758, M. arbustorum Linnaeus, 1758 and M. horticola De Geer, 1776 (currently Eristalis nemorum, E. arbustorum and E. horticola): proposed conservation of usage of the specific names by designation of neotypes. Bull Zool Nomencl 61:241–245
Clavel A, Toledo M, Goñi P, Aspiroz C (2011) Intestinal myiasis due to Eristalis tenax: report of a new case in Spain. New Microbiol 34:335–336
Cobbold TS (1864) Entozoa: an introduction to the study of helminthology, with refrence more particulary, to the internal parasites of man. Groombridge and Sons, London
Cobbold TS (1879) Parasites; a treatise on the Entozoa of man and animals, including some account of the Ectozoa. J & A Churchill, London. https://doi.org/10.5962/bhl.title.22473
Cookson HA, Oldroyd H (1937) Intestinal infestation by larvae of drone fly. Lancet 230:804. https://doi.org/10.1016/S0140-6736(00)71305-5
Croll NA, Gyorkos T, Faubert GM (1976) A case of Eristalis tenax myiasis in Quebec. Can Dis Wkly Rep 2:175
Da Silva Carvalho Filho F, Esposito MC (2009) A review of the flower fly genus Ornidia Lepeletier & Serville (Diptera: Syrphidae) with the description of a new species from Brazil. Zootaxa 2014:59–64. https://doi.org/10.11646/zootaxa.2014.1.6
Davaine C (1877) Traite des Entozoaires et des maladies vermineuses de l’homme et des animaux domestiques. Librairie J.-B. Bailliere et fils, Paris
de Geer C (1776) Memoires pour servir a l'histoire des insectes vol 6. P Hesselberg, Stockholm
de Groot HB (1956) Myiasis in Canada. Can Med Assoc J 75:673–674
Del Río L (1902) Miasa. Notable caso de parasitismo accidental de una larva viva de múscido en el intestino de un niño. Rev Ibero-Amer Cienc méd 7:183–192
Delmastro B, Casabianca A, Bordino C, Chichino G, Carnevale G (1989) Miasi intestinale da Eristalis tenax in ambiente rurale. G Mal Infett Parassit 41:194–196
Derraik JGB, Heath ACG, Rademaker M (2010) Human myiasis in New Zealand: imported and indigenously-acquired cases; the species of concern and clinical aspects. N Z Med J 123:21–38
Desoubeaux G, Gaillard J, Borée-Moreau D, Bailly E, Andres CR, Chandenier J (2014) Gastrointestinal symptoms resembling ulcerative proctitis caused by larvae of the drone fly Eristalis tenax. Pathog Glob Health 108:158–163. https://doi.org/10.1179/2047773214Y.0000000135
D'Ignacio C, Giaquinto Mira M (1941) Miasi intestinale da larve di Eristalis in donna di razza bianca in Addis Abeba. Arch ital Sci Med Colon 22:325–331
Dik B, Uslu U, Işik N (2012) Myiasis in animals and humanbeings in Turkey. Kafkas Univ Vet Fak Derg 18:37–42. https://doi.org/10.9775/kvfd.2011.4654
Drisdelle R, Forward KR (2006) Doctor, there’s a tadpole in my feces! Can J Infect Dis Med Microbiol 17:189–191
Dubois E, Durieux M, Franchimont MM, Hermant P (2004) Un cas exceptionnel en Belgique de myiase intestinale due a Eristalis tenax. Acta Clin Belg 59:168–170. https://doi.org/10.1179/acb.2004.025
Dujardin MF (1845) Historie naturelle des Helminthes ou vers intestinaux. Librairie encyclopédique de Roret, Paris
Dušek J (1971) Key to larvae. In: Greenberg B (ed) Flies and disease vol 1. Ecology, classification, and biotic associations. Princeton University Press, New Jersey, pp 163–199
Dutto M, Maistrello L (2017) Osservazioni sulla presenza di Eristalinus (Eristalodes) taeniops (Wiedemann, 1818) (Diptera, Syrphidae) in Piemonte (Italia) e nel Canton Ticino (Svizzera). Quader Mus Civ Stor Nat Ferrara 5:69–71
Fabricius JC (1805) Systema Antliatorum secundum ordines, genera, species adiectis synonymis, locis, observationibus, descriptionibus. C Reichard (publisher), Brunsvigae [=Brunswick]
Faggioli R (1927) Eristalis tenax, parásito accidental del cuerpo humano. Sem Med 34:887
Fernandes LF, Pimenta FC, Fernandes FF (2009) First report of human myiasis in Goiás State, Brazil: frequency of different types of myiasis, their various etiological agents, and associated factors. J Parasitol 95:32–38. https://doi.org/10.1645/GE-1103.1
Ferrer Bradley I, Navarro Pérez L, Maroto Arce N, López Serrano A, Montón Rodríguez C, Jiménez Mayordomo M, Hinojosa del Val J (2010) Miasis por Eristalis tenax en enfermedad de Crohn. Gastroenterol Hepatol 33:616–617. https://doi.org/10.1016/j.gastrohep.2010.02.010
Fischer OA, Mátlová L, Dvorská L, Švástová P, BartoŠ M, Weston RT, Kopecna M, Trcka I, Pavlík I (2005) Potential risk of Mycobacterium avium subspecies paratuberculosis spread by syrphid flies in infected cattle farms. Med Vet Entomol 19:360–366. https://doi.org/10.1111/j.1365-2915.2005.00585.x
Fischer OA, Mátlová L, Dvorská L, Švástová P, BartoŠ M, Weston RT, Pavlík I (2006) Various stages in the life cycle of syrphid flies (Eristalis tenax; Diptera: Syrphidae) as potential mechanical vectors of pathogens causing mycobacterial infestions in pig herds. Folia Microbiol (Praha) 51:147–153. https://doi.org/10.1007/BF02932171
Fleischmann A, Rivadavia F, Gonella PM, Pérez-Bañón C, Mengual X, Rojo S (2016) Where is my food? Brazilian flower fly steals prey from carnivorous sundews in a newly discovered plant-animal interaction. PLoS One 11:e0153900. https://doi.org/10.1371/journal.pone.0153900
Förstl M (2003) 4. Lékařská Parazitologie. In: Vermes Praktický atlas lékařské parazitologie. Nucleus HK, Hradec Králové, pp 45–127
Förstl M, Čermák P, Čermáková Z, Pellantová V, Toralová V (2002) Tenkohlavec lidský. Interní medicína pro praxi 4:199–201
Francesconi F, Lupi O (2012) Myiasis. Clin Microbiol Rev 25:79–105. https://doi.org/10.1128/CMR.00010-11
Francuski L, Matić I, Ludoški J, Milankov V (2011) Temporal patterns of genetic and phenotypic variation in the epidemiologically important drone fly, Eristalis tenax. Med Vet Entomol 25:135–147. https://doi.org/10.1111/j.1365-2915.2011.00956.x
Francuski L, Djurakic M, Ludoški J, Hurtado P, Pérez-Bañón C, Ståhls G, Rojo S, Milankov V (2014) Shift in phenotypic variation coupled with rapid loss of genetic diversity in captive populations of Eristalis tenax (Diptera: Syrphidae): consequences for rearing and potential commercial use. J Econ Entomol 107:821–832. https://doi.org/10.1603/EC13243
Fuentes-López A, Ruiz C, Galián J, Romera E (2019) Molecular identification of forensically important fly species in Spain using COI barcodes. Sci Justice. https://doi.org/10.1016/j.scijus.2019.12.003
Galli-Valerio B (1931) Notes de Parasitologie. Zentralbl Bakteriol Orig 120:98–106
Garcia-Zapata MTA, Júnior ESS, Fernandes FF, Santos SFO (2005) Human pseudomyiasis caused by Eristalis tenax (Linnaeus) (Diptera: Syrphidae) in Goiás. Rev Soc Bras Med Trop 38:185–187
Gil Collado J (1956) Dípteros productores de miasis humanas en España y sus colonias. Med Colon 27:323–343
Gil Collado J (1961) Una invasión de habitaciones humanas por larvas de Eristalis tenax. Med Trop (Madr) 37:73–77
GilArriortua M, de Pancorbo MM, Saloña Bordas M (2014) Confirmación de la presencia de Lucilia bufonivora (Diptera, Calliphoridae) en la Comunidad Autónoma del País Vasco (Norte de España). Bol Asoc Esp Entomol 38:25–31
Gilbert NC (1908) Infection of man by dipterous larvae, with report of four cases. Arch Intern Med 3:226–240
Golding YC, Ennos AR, Edmunds M (2001) Similarity in flight behaviour between the honeybee Apis mellifera (Hymenoptera: Apidae) and its presumed mimic, the dronefly Eristalis tenax (Diptera: Syrphidae). J Exp Biol 204:139–145
Goldsmid JM, Phelps RJ (1977) A review of myiasis of man in Rhodesia. Cent Afr J Med 23:174–179
Goldstein PZ, DeSalle R (2011) Integrating DNA barcode data and taxonomic practice: determination, discovery, and description. BioEssays 33:135–147. https://doi.org/10.1002/bies.201000036
González MM, Comte MG, Monárdez PJ, Díaz de Valdés LM, Matamala CI (2009) Miasis genital accidental por Eristalis tenax. Rev Chil Infectol 26:270–272. https://doi.org/10.4067/S0716-10182009000400012
Goodwin H (1925) Report on the general health of the stock of Antigua for the year ending 31st March 1925. Antigua, 6 pp. [Abstract published in Rev Appl Entomol, ser B Med Vet (1926) vol XIII: page 165]
Graham-Smith GS (1913) Flies in relation to disease: non-bloodscucking flies. Cambridge University Press, New York. https://doi.org/10.5962/bhl.title.16264
Greenberg B (1971) Flies and disease: ecology, classification and biotic associations, vol 1. Princenton University Press, New Jersey
Grković A, Vujić A, Radenković S, Chroni A, Petanidou T (2015) Diversity of the genus Eumerus Meigen (Diptera, Syrphidae) on the eastern Mediterranean islands with description of three new species. Ann Soc Entomol Fr 51:361–373. https://doi.org/10.1080/00379271.2016.1144483
Guizzardi F, Prestini A, Rossi L (1989) Infestione da larve di Eristalis tenax nell’allevamento bovino. Obiett Doc Vet 10:27–30
Hall MC (1918) A note regarding myiasis, especially that due to syrphid larvae. Arch Intern Med (Chic) 21:309–312. https://doi.org/10.1001/archinte.1918.00020010001001
Hall MC, Muir JT (1913) A critical study of a case of myiasis due to Eristalis. Arch Intern Med (Chic) 11:193–203. https://doi.org/10.1001/archinte.1913.00060260074005
Hall MJR, Smith GV (1993) Diptera causing myiasis in man. In: Lane RP, Crosskey RW (eds) Medical insects and arachnids. Chapman & Hall, London, pp 429–469
Hall M, Wall R (1995) Myiasis of humans and domestic animals. Adv Parasitol 35:257–334. https://doi.org/10.1016/S0065-308X(08)60073-1
Hamed RAR, Hamid RAR, Hamid N (2017) Second report of accidental intestinal myiasis due to Eristalis tenax (Diptera: Syrphidae) in Iran, 2015. Case Rep Emerg Med. https://doi.org/10.1155/2017/3754180
Hanby ELK (1905) Case of infection with the rat-tailed larva of the drone fly. JAMA 45:1800. https://doi.org/10.1001/jama.1905.52510240032002a
Hartley JC (1961) A taxonomic account of the larvae of some British Syrphidae. Proc Zool Soc London 136:505–573. https://doi.org/10.1111/j.1469-7998.1961.tb05891.x
Hebert PDN, Cywinska A, Ball SL, DeWaard JR (2003a) Biological identifications through DNA barcodes. Proc R Soc Lond B 270:313–321. https://doi.org/10.1098/rspb.2002.2218
Hebert PDN, Ratnasingham S, DeWaard JR (2003b) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc Lond B (Suppl) 270:S596–S599. https://doi.org/10.1098/rsbl.2003.0025
Heo CC, Rahimi R, Mengual X et al (2019) Eristalinus arvorum (Fabricius, 1787) (Diptera: Syrphidae) in human skull: a new fly species of forensic importance. J Forensic Sci. https://doi.org/10.1111/1556-4029.14128
Hira PR (1977) Rectal myiasis: first report on a case due to the rat-tailed larva of Eristalis tenax in Africa. East Afr Med J 54:224–226
Hope FW (1840) On insects and their larvae occasionally found in the human body. Trans Ent Soc Lond 2:256–271
Howe K, Bateman A, Durbin R (2002) QuickTree: building huge neighbor-joining trees of protein sequences. Bioinformatics 18:1546–1547. https://doi.org/10.1093/bioinformatics/18.11.1546
Iches L (1914) Un cas original d’hospitalisation rectale. Arch parasitol 3:473–474
ICZN (1963) Opinion 678. The suppression under the plenary powers of the pamphlet published by Meigen, 1800. Bull Zool Nomencl 20:339–342
ICZN (2006) Opinion 2153 (case 3259) Eristalis Latreille, 1804 (Insecta, Diptera): confirmation that the gender is feminine; Musca nemorum Linnaeus, 1758, M. arbustorum Linnaeus, 1758 and M. horticola De Geer, 1776 (currently Eristalis nemorum, E. arbustorum and E. horticola): usage of the specific names conserved by designation of neotypes. Bull Zool Nomencl 63:146–147
Inouye DW, Larson BMH, Ssymank A, Kevan PG (2015) Flies and flowers III: ecology of foraging and pollination. J Pollinat Ecol 16:115–133. https://doi.org/10.26786/1920-7603%282015%2915
Jabbar Khan R (1987) Seasonal prevalence of myiasis producing larvae (Diptera) in human stools in Karachi, Pakistan. Proc Parasitol (currently Pakistan J Parasitol) 4:16–21
Jabbar Khan R, Jabbar Khan MA (1987) Gastrointestinal myiasis caused by the maggots of synanthropic flies in human. Proc Parasitol (currently Pakistan J Parasitol) 3:24–27
Jabbar Khan R, Jabbar Khan MA (1992) Seasonal prevalence of myiasis producing larvae (Diptera) in human stools in Karachi, Pakistan. Proc Parasitol (currently Pakistan J Parasitol) 13:52–58
James MT (1947) The flies that cause myiasis in man. Misc Publ 631 / USDA, Government Printing Office, Washington. https://doi.org/10.5962/bhl.title.65688
Jensen T (1929) Eristalis-larver som Parasitter i Svinets Columna thoracica. Maanedsskr Dyrlaeger 41:50–58
Jordaens K, Goergen G, Virgilio M, Backeljau T, Vokaer A, Meyer D (2015a) DNA barcoding to improve the taxonomy of the Afrotropical hoverflies (Insecta: Diptera: Syrphidae). PLoS One 10:e0140264. https://doi.org/10.1371/journal.pone.0140264
Jordaens K, Goergen G, Kirk-Spriggs AH, Vokaer A, Backeljau T, De Meyer M (2015b) A second New World hoverfly, Toxomerus floralis (Fabricius) (Diptera: Syrphidae), recorded from the Old World, with description of larval pollen-feeding ecology. Zootaxa 4044:567–576. https://doi.org/10.11646/zootaxa.4044.4.6
Knutson LV, Thompson FC, Vockeroth JR (1975) Family Syrphidae. In: Delfinado MD, Hardy DE (eds) A catalog of the Diptera of the Oriental region, Suborder Brachycera through Division Aschiza, vol 2. Suborder Cyclorrhapha, The University Press of Hawaii, Honolulu, pp 306–373
Korzets Z, Bernheim J, Lengy J, Gold D (1993) Human urogenital myiasis due to Eristalis larva: an unusual cause of ureteric obstruction. Nephrol Dial Transplant 8:874–876. https://doi.org/10.1093/ndt/8.9.874
Kruatrachu M, Chinachoti N (1957) A case of myiasis due to Eristalis tenax larvae. J Med Assoc Thail 40:31–34
Kun M, Kreiter A, Semenas L (1998) Myiasis gastrointestinal humana por Eristalis tenax. Rev Saude Publica 32:367–369
Lakshminarayana CS, Kanchana MV, Janakavalli R, Mallika M (1975) Intestinal myiasis due to Eristalis tenax. J Indian Med Assoc 65:234–235
Lardé G (1989) Investigation on some factors affecting larval growth in a coffe-pulp bed. Biol Wastes 30:11–19
Larson BMH, Kevan PG, Inouye DW (2001) Flies and flowers: taxonomic diversity of anthophiles and pollinators. Can Entomol 133:439–465. https://doi.org/10.4039/Ent133439-4
Leclercq M (1969) Myiases. In: Alexander P, Bacq ZM (eds) Entomological parasitology. The relations between entomology and the medical sciences. Pergamon Press, Oxford, pp 74–82
Leclercq M (1981) Pseudomyiase intestinale à larves d’Eristalis tenax (L.) en Belgique. Bull ann Soc r belge entomolog 117:226–227
Lee DJ (1968) Human myiasis in Australia. Med J Aust 1:170–173. https://doi.org/10.5694/j.1326-5377.1968.tb28850.x
Lee HL (1989) A case of human urogenital myiasis caused by the drone fly Eristalis species (Diptera: Syrphidae) in Malaysia. Trop Biomed 6:49–51
Lee HL (1994) Larvae of Eristalis spp. (family: Syrphidae) found in human cadaver in Malaysia. J Biosci (Penang) 5:67–68
Leidy J (1874) Abstract of remarks. Proc Acad Natl Sci Phila 25:365
Lever RA (1957) Two records of foxes eating larval hover flies and door beetles. Proc Zool Soc London 128:596–597
Lewis JM, Winslow H, Jones J, Taegtmeyer M, O’Dempsey T, Nsutebu E (2015) Cramping and diarrhea in a 44-year-old man. Clin Infect Dis 61(432 supl):480–481. https://doi.org/10.1093/cid/civ181
Lia R, Otranto D, Paradies P, Puccini V (1999) Pseudomiasi intestinale da larve di Eristalis tenax (Diptera, Syrphidae) in un equino. Atti Soc Ital Sci Vet 53:195–196. [Annual meeting Societa’Italiana delle Scienze Veterinarie, Montecatini Terme, Pistoia 16-18 Sep 1999]
Lindgren NK, Sisson MS, Archambeault AD, Rahlwes BC, Willett JR, Bucheli SR (2015) Four forensic entomology case studies: records and behavioral observations on seldom reported cadaver fauna with notes on relevant previous occurrences and ecology. J Med Entomol 52:143–150. https://doi.org/10.1093/jme/tju023
Linnaeus C (1758) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis, Vol 1, 10th edn. Laurentii Salvii, Stockholm
López Millán CL, Olea MS, Dantur Juri MJ (2015) Unusual presence of Ornidia robusta (Diptera: Syrphidae) causing pig myiasis in Argentina. Parasitol Res 114:4731–4735. https://doi.org/10.1007/s00436-015-4755-x
López VG, Romero MI, Parra-Henao G (2017) Gastric and intestinal myiasis due to Ornidia obesa (Diptera: Syrphidae) in humans. First report in Colombia. Rev MVZ Cordoba 22:5755–5760. https://doi.org/10.21897/rmvz.935
Machado O (1937) Parasitismo accidental pela larva de Volucella obesa. Rev Flumin Med 2:239–242
Magalhães PS (1908) Notes d’helminthologie brésilienne. Arch Parasitol 12:218–223
Mandour AM, Omran LAAM (1978) A case report of intestinal myiasis accompanied with pruritus ani. J Egypt Soc Parasitol 8:105–108
Marjolet M (1983) Myiases et pseudo-myiases humaines: à propos de trois cas récents de myiases digestives. Bull Soc Sci Nat Ouest Fr 5:188–193
Martins E, Neves JA, Moretti TC, Godoy WAC, Thyssen PJ (2010) Breeding of Ornidia obesa (Diptera: Syrphidae: Eristalinae) on pig carcasses in Brazil. J Med Entomol 47:690–694. https://doi.org/10.1603/ME09254
Mathison BA, Pritt BS (2014) Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev 27:48–67. https://doi.org/10.1128/CMR.00008-13
McCampbell EF, Corper HJ (1909) Myiasis intestinalis due to infection with three species of dipterous larvae. JAMA 53:1160–1162. https://doi.org/10.1001/jama.1909.92550150001001c
McCulloch EC, McCoy JE (1941) Eristalis tenax genital myiasis in a cow. J Am Vet Med Assoc 99:293–294
McOrist S, Blunt R, Gebhart CJ (2011) Pig-associated Lawsonia intracellularis in various on-farm dipterous fly stages. J Swine Health Prod 19:277–283
Meigen JW (1800) Nouvelle classification des mouches a deux ailes (Diptera L.) d'apres un plan tout nouveau. Perronneau, Paris
Meigen JW (1803) Versuch einer neuen Gattungs-Eintheilung der europaischen zweiflugligen Insekten. Mag Insektenkunde 2:259–281
Meissner E (1950) Die “Rattenschwanzmade” der Schwirrfliege (Eristalis tenax) als Schmarotzer in menschlichen Darm. Med Klin 46:1474–1475
Melville RV (1961) Report on Mr. C. W. Sabrosky’s proposal for the suppression under the Plenary Powers of the pamphlet entitled “Nouvelle classification des mouches a deux ailes” by J. W. Meigen, 1800. Bull Zool Nomencl 18:9–64
Mengual X, Ståhls G, Rojo S (2008) First phylogeny of predatory flower flies (Diptera, Syrphidae, Syrphinae) using mitochondrial COI and nuclear 28S rRNA genes: conflict and congruence with the current tribal classification. Cladistics 24:543–562. https://doi.org/10.1111/j.1096-0031.2008.00200.x
Mengual X, Ståhls G, Rojo S (2012) Is the mega-diverse genus Ocyptamus (Diptera, Syrphidae) monophyletic? Evidence from molecular characters including the secondary structure of 28S rRNA. Mol Phylogenet Evol 62:191–205. https://doi.org/10.1016/j.ympev.2011.09.014
Mengual X, Ståhls G, Rojo S (2015) Phylogenetic relationships and taxonomic ranking of pipizine flower flies (Diptera: Syrphidae) with implications for the evolution of aphidophagy. Cladistics 31:491–508. https://doi.org/10.1111/cla.12105
Metcalf CL (1916) Syrphidae of Maine. Maine Agr Exp Stat 253:193–264. https://doi.org/10.5962/bhl.title.86683
Miall LC (1895) The natural history of aquatic insects. Macmillan, London
Monteiro SG, Faccio L, Anderson Otto M, Soares JF, Da Silva AS, Mazzanti A (2008) Miíase acidental por Ornidia obesa em humanos. Rev Bras Parasitol Vet 17:95–98
Moutaj R, Aoufi S, Agoumi A, Balouch L, Sbiti M, Benjelloun DB (2000) Un cas de myiase gastrique due à la larve d’Eristalis tenax (Linné, 1758) (Insecta: Diptera). Parasite 7:56–57
Muller J (1946) Notes entomologiques: Larve d’Eristale, parasite interne de l’homme. Eristalis sp. Diptère, Syrphide. Natural Amat 3:87–88
Mumcuoglu I, Aral Akarsu G, Balaban N, Keles I (2005) Eristalis tenax as a cause of urinary myiasis. Scand J Infect Dis 37:942–943. https://doi.org/10.1080/00365540510043275
Mumford EP (1926) Three new cases of myiasis in man in the North of England. Parasitol 18:375–383. https://doi.org/10.1017/S0031182000005369
Nagakura K, Kawauichi-Kato Y, Tachibana H, Kaneda Y, Shinonaga S, Kano R (1991) Three cases of intestinal myiasis in Japan. J Infect Dis 163:1170–1171. https://doi.org/10.1093/infdis/163.5.1170
Nash R (2005) Two unpublished cases of myiasis. Diptera.info website. Articles: interesting Diptera. https://diptera.info/articles.php?article_id=8
Natvig LR (1924) Fakultativ parasitisme av Eristalis-larver hos en ko. Norks Veterinaer Tidsskrift 36:348–356
Natvig LR (1939) Fliegenlarven als fakultative Parasiten bei Menschen und Tieren in Norwegen. VII Internationaler Kongresz fur Entomologie, Berlin 15-20 August 1938, v.3:1641-1655
Noutsis C, Millikan LE (1994) Myiasis. Dermatol Clin 12:729–736. https://doi.org/10.1016/S0733-8635(18)30136-0
Nunes Morales M, Marinoni L (2009) Cladistic analysis and taxonomic revision of the scutellaris group of Palpada Macquart (Diptera: Syrphidae). Invertebr Syst 23:301–347. https://doi.org/10.1071/IS09006
Odhelius JL (1789) Et sållsynt slags Larver, utdrifne ifrån et ungt Fruntimmer under en Brunscur. Kongliga Vetenskaps Academiens Nya Handlingar 10:221–224
Oliva A (2002) Miasis en la Argentina. In: Salomón OD (ed) Actualizaciones en artropodología sanitaria argentina. Fundación Mundo Sano, Buenos Aires, pp 45–50
Oluwatosin MA, Fadahunsi IF (2003) Cutaneous and intestinal myiases in Lagelu L.G.A of Oyo State. Afr J Clin Exp Microbiol 4:44–47
OPS, Organización Panamericana de la Salud (1962) Moscas de importancia en salud pública y su control. Publicaciones Científicas 61, Washington 44 pp
Osten Sacken CR (1886) Some new facts concerning Eristalis tenax. The Entomologist’s Monthly Magazine 23:97–99
Osten Sacken CR (1894) On the oxen-born bees of the ancients (bugonia) and their relation to Eristalis tenax, a two-winged insect. Enlarged edition of the essay published in the Bullet Soc Ent Ital, 1893). Hoerning J, Heidelberg, 1-80 pp
Otranto D, Stevens JR (2002) Molecular approaches to the study of myiasis-causing larvae. Int J Parasitol 32:1345–1360. https://doi.org/10.1016/S0020-7519(02)00095-4
Otranto D, Traversa D, Guida B, Tarsitano E, Fiorente P, Stevens J (2003) Molecular characterization of the mitochondrial cytochrome oxidase I gene of Oestridae species causing obligate myiasis. Med Vet Entomol 17:307–315. https://doi.org/10.1046/j.1365-2915.2003.00442.x
Patton WS (1921) Notes on the myiasis-producing Diptera of man and animals. Bull Entomol Res 12:239–261. https://doi.org/10.1017/S0007485300040190
Patton WS, Evans AM (1929) Insects, ticks, mites and venomous animals of medical and veterinary importance. Part I: Medical. HR Grubb Ltd, Croydon
Pérez-Bañón C, Rojo S, Ståhls G, Marcos-García MA (2003a) Taxonomy of European Eristalinus (Diptera: Syrphidae) based on larval morphology and molecular data. Eur J Entomol, 100:417–428. https://doi.org/10.14411/eje.2003.064
Pérez-Bañón C, Rotheray G, Hancock G, Marcos-García MA, Zumbado MA (2003b) Immature stages and breeding sites of some Neotropical saprophagous syrphids (Diptera: Syrphidae). Ann Entomol Soc Am 96:458–471. https://doi.org/10.1603/0013-8746(2003)096[0458:ISABSO]2.0.CO;2
Pérez-Bañón C, Hurtado P, García-Gras E, Rojo S (2013) SEM studies on immature stages of the drone flies (Diptera, Syrphidae): Eristalis similis (Fallen, 1817) and Eristalis tenax (Linnaeus, 1758). Microsc Res Tech 76:853–861. https://doi.org/10.1002/jemt.22239
Pérez-Lachaud G, Jervis MA, Reemer M, Lachaud JP (2014) An unusual, but not unexpected, evolutionary step taken by syrphid flies: the first record of true primary parasitoidism of ants by Microdontinae. Biol J Linn Soc Lond 111:462–472
Pérez-Zúñiga E (1908) Notas y comunicaciones – Sesion del 3 Junio de 1908. Bol R Soc Esp Hist Nat Sec Bio:8–259
Pick WM (1984) Rectal myiasis. S Afr Med J 65:832–834
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
Pumpelly WC (1925) Case of human infestation by larvae of the fly Eristalis tenex. JAMA 84:37. https://doi.org/10.1001/jama.1925.26620270002012a
Raffray L, Malvy D (2014) Accidental intestinal myiasis caused by Eristalis tenax in France. Travel Med Infect Dis 12:109–110. https://doi.org/10.1016/j.tmaid.2013.03.010
Rajamohanan K (1987) Intestinal myiasis caused by Eristalis tenax larvae in Kerala cattle. Kerala Vet Sci 18:109–111
Rajković Janje R, Vinković B, Matković K, Auslender V, Gojmerac T, Čović A (2010) O pojavi “rat-tail” ličinki (Diptera: Syrphidae) nađenih u organima uginulih farmskih životinja i u okolišu farme. Stočarstvo 63:41–47
Rathe M, Ozeraityte A (2009) Et dansk tilfælde af intestinal pseudomyiasis forårsaget af Eristalis tenax. Ugeskr Laeger 171:2922–2923
Ratnasingham S, Hebert PDN (2007) bold: the barcode of Life Data System (http://www.barcodinglife.org). Mol Ecol Notes 7:355-364. DOI:https://doi.org/10.1111/j.1471-8286.2007.01678.x
Ratnieks F, Carreck N (2010) Clarity on honey bee collapse? Science 327:152–153. https://doi.org/10.1126/science.1185563
Réaumur RAF (1738) Onzième Mémoire: Des Mouches à deux aîles qui ont l’air d’Abeilles, et de celles qui ont l’air d’Guêpes et de Frelons. In: Mémoires pour servir à l'histoire des insectes Volume 4. L’Imprimerie Royale, Paris, pp 439–496
Richards DF (1977) Observations on the diet of the Red fox (Vulpes vulpes) in South Devon. J Zool (Lond) 183:495–504. https://doi.org/10.1111/j.1469-7998.1977.tb04201.x
Riley CV (1890) Communications of the Entomological Society of Washington. Insect Life 2:261–262
Riley WA (1939) The possibility of intestinal myiasis in man. J Econ Entomol 32:875–876. https://doi.org/10.1093/jee/32.6.875
Rivarola JB (1944) Miasis intestinal humana por larva de Eristalis sp. Rev Bras Biol 4:239–241
Rivera Barquero A, Sánchez Rodríguez C (2015) Miasis gastrointestinal por Eristalis tenax en Costa Rica. Rev Méd Univ Costa Rica 9:100–104
Rivero de Rodríguez Z, Díaz Anciani I, Villalobos R (2007) Importancia del estudio epidemiológico en el diagnóstico de las miasis intestinales humanas: A propósito de un caso. Kasmera 35:65–69
Robertson PA, Whelan J (1987) The food of the red fox (Vulpes vulpes) in Co. Kildare, Ireland. J Zool (Lond) 213:740–743. https://doi.org/10.1111/j.1469-7998.1987.tb03739.x
Rojas Soto G, Zumbado Salazar A, Gómez Murillo C, Zamora Cruz M (2017) Miasis gastrointestinal accidental causada por Eristalis tenax. Rev méd Costa Rica Centroam 73:45–48
Rojo S, Gilbert F, Marcos-García MA, Nieto JM, Mier MP (2003) A world review of predatory hoverflies (Diptera, Syrphidae: Syrphinae) and their prey. Centro Iberoamericano de la Biodiversidad (CIBIO), Alicante
Rotheray GE (1993) Colour guide to hoverfly larvae (Diptera, Syrphidae) in Britain and Europe. Dipterists Digest 9:1–156
Rotheray GE, Gilbert F (2011) The natural history of hoverflies. Forrest Text, Tresaith, Wales
Rotheray GE, Hancock EG, Marcos-García MA, Zumbado M (2005) Early stages and breeding sites of three species of Neotropical Ornidia (Diptera, Syrphidae). Studia dipterol 12:419–427
Rozo-Lopez P, Mengual X (2015) Mosquito species (Diptera, Culicidae) in three ecosystems from the Colombian Andes: identification through DNA barcoding and adult morphology. ZooKeys 513:39–64. https://doi.org/10.3897/zookeys.513.9561
Sabrosky CW (1952) Meigen, 1800: a proposal for stability and uniformity. Bull Zool Nomencl 6:131–141
Sabrosky CW (1999) Family-group names in Diptera. An annotated catalog. Myia 10:1–360
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Salimi M, Edalat H, Jourabchi A, Oshaghi MA (2010) First report of human nasal myiasis caused by Eristalis tenax in Iran (Diptera: Syrphidae). Iran J Arthropod Borne Dis 4:77–80
Salleh AFM, Marwi MA, Jeffery J, Hamid NAA, Zuha RM, Omar B (2007) Review of forensic entomology cases from Kuala Lumpur Hospital and Hospital Universiti Kebangsaan Malaysia. J Trop Med Parasitol 30:51–54
Sarandeses AA (1951) Miasis intestinal por larvas de Eristalis tenax. Galicia Clin 9:629–633
Sasaki H, Mikami A (2007) Droneflies (Diptera: Syrphidae) ocurring from manure and effluent of manure in Hokkaido, Japan. Eisei Dobutsu (=Med Entomol Zool) 58:63–71. https://doi.org/10.7601/mez.58.63_1
Scott TG (1943) Some food coactions of the Northern Plains Red Fox. Ecol Monogr 13:427–479 https://www.jstor.org/stable/1948591
Scott HG (1964) Human myiasis in North America (1952-1962 inclusive). Fla Entomol 47:255–261. https://doi.org/10.2307/3493743
Scuderi G (1964) Un caso de miasi intestinale da Eristalis tenax nell’uomo. Arch Med Interna 16:135–139
Severini F, Nocita E, Tosini F (2015) Myiasis of the tracheostomy wound caused by Sarcophaga (Liopygia) argyrostoma (Diptera: Sarcophagidae): molecular identification based on the mitochondrial cytochrome c oxidase I gene. J Med Entomol 52:1357–1360. https://doi.org/10.1093/jme/tjv108
Shattock SG (1908) Three specimens of the larvae of Eristalis tenax, passed by the Bowel. Proc R Soc Med 1:147–152. https://doi.org/10.1177/003591570800101012
Silva-Campos R (1950) Hallazgos de adultos y larvas de artrópodos en deposiciones. Bol Inf Parasit Chil 5:7
Silva-Campos R (1955) Hallazgos de larvas de Tubifera tenax (Eristalis tenax). Bol Chil Parasitol 10:75–77
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P (1994) Evolution, weighting and phylogenetic utility of mitochondrial gene-sequences and a compilation of conserved polymerase chain-reaction primers. Ann Entomol Soc Am 87:651–701. https://doi.org/10.1093/aesa/87.6.651
Singh A, Singh Z (2015) Incidence of myiasis among humans—a review. Parasitol Res 114:3183–3199. https://doi.org/10.1007/s00436-015-4620-y
Siripoonya P, Tesjaroen S, Viravan C (1993) Intestinal myiasis: a case report. J Med Assoc Thail 76:229–231
Skevington JH, Locke MM, Young AD, Moran K, Crins WJ, Marshall SA (2019) Field guide to the flower flies of northeastern North America. Princeton University Press, New Jersey
Smart J (1944) An annotated bibliography-chronology of the literature and events relating to the generic names of Meigen, 1800. Ann Mag Nat Hist 11:261–272
Smith KGV, Vockeroth JR (1980) 38. Family Syrphidae. In: Crosskey RW (ed) Catalogue of the Diptera of the Afrotropical region. Publications of the British Museum (Natural History), London, pp 488–510
Solange Sánchez M, Bedoya A, Barahona R (2010) Estudio preliminar de la fauna en el morro de basuras de Moravia y presencia de metales pesados en artrópodos y roedores. Univ Sci (Bogota) 15:49–58. https://doi.org/10.11144/javeriana.SC15-1.psot
Soler Cruz MD (2000) El estudio de las miasis en España durante los últimos cien años. Ars Pharm 41:19–26
Sonet G, Jordaens K, Braet Y, Bourguignon L, Dupont E, Backeljau T, De Meyer M, Desmyter S (2013) Utility of GenBank and the Barcode of Life Data Systems (BOLD) for the identification of forensically important Diptera from Belgium and France. Zookeys 30:307–328. https://doi.org/10.3897/zookeys.365.6027
Southern HN, Watson JS (1941) Summer food of the Red Fox (Vulpes vulpes) in Great Britain: a preliminary report. J Anim Ecol 10:1–10 https://www.jstor.org/stable/1338
Spångberg J (1886) Résumés. Entomol Tidskr 7:121
Speight MCD (2017) Fauna Europaea: Syrphidae. In: Beuk P, Pape T (2017) Fauna Europaea: Diptera. Fauna Europaea version 2017.06, https://fauna-eu.org
Speight MCD (2018) Species accounts of European Syrphidae, 2018. In: Syrph the Net, the database of European Syrphidae (Diptera), vol 103. Syrph the Net publications, Dublin
Ståhls G, Vujić A, Pérez-Bañón C, Radenković S, Rojo S, Petanidou T (2009) COI barcodes for identification of Merodon hoverflies (Diptera, Syrphidae) of Lesvos Island, Greece. Mol Ecol Resour 9:1431–1438. https://doi.org/10.1111/j.1755-0998.2009.02592.x
Stevens JR, Wallman JF (2006) The evolution of myiasis in humans and other animals in the Old and New Worlds (part I): phylogenetic analyses. Trends Parasitol 22:129–136. https://doi.org/10.1016/j.pt.2006.01.008
Stevens JR, Wallman JF, Otranto D, Wall R, Pape T (2006) The evolution of myiasis in humans and other animals in the Old and New Worlds (part II): biological and life-history studies. Trends Parasitol 22:181–188. https://doi.org/10.1016/j.pt.2006.02.010
Stiles GW, Cleland WS (1953) Intestinal myiasis from rat-tailed maggots in a woman. Arch Intern Med 91:812–816. https://doi.org/10.1001/archinte.1953.00240180121015
Stiles CW, Hassall A (1905) The determination of generic types and a list of roundworm genera, with their original and type species. Government Printing Office, Washington
Supple CJ (1958) Genitourinary myiasis due to Eristalis tenax. JAMA 167:1838–1839. https://doi.org/10.1001/jama.1958.72990320009007c
Swartzwelder JC, Cali SJ (1942) Human intestinal myiasis due to syrphid larvae—report of an additional case (Eristalis tenax). Am J Trop Med Hyg 22:159–163. https://doi.org/10.4269/ajtmh.1942.s1-22.159
Thompson FC (1981) The flower flies of the West Indies (Diptera: Syrphidae). Mem Entomol Soc Wash 9:1–200 https://repository.si.edu/handle/10088/21160
Thompson FC (1991) The flower fly genus Ornidia (Diptera: Syrphidae). Proc Entomol Soc Wash 93:248–261
Thompson FC (1997) Revision of the Eristalis flower flis (Diptera: Syrphidae) of the Americas South of the United States. Proc Entomol Soc Wash 99:209–237
Thompson FC (1999) A key to the genera of the flower flies (Diptera: Syrphidae) of the Neotropical Region including descriptions of new genera and species and a glossary of taxonomic terms used. Contr Entomol, Int 3:321–378
Thompson FC (2003) Austalis, a new genus of flower flies (Diptera: Syrphidae) with revisionary notes on related genera. Zootaxa 246:1–19. https://doi.org/10.11646/zootaxa.246.1.1
Thompson FC (2013) Syrphidae. Systema Dipterorum. Version 1.5, 13,354 records. http://www.diptera.dk. Accessed 31 April 2019
Thompson FC, Rotheray GE (1998) Family Syrphidae. In: Papp L, Darvas B (eds) Contributions to a Manual of Palaeartic Diptera (with special reference to flies of economic importance) Vol 3. Budapest, Science Herald, pp 81–139
Thompson FC, Fee FD, Berzark LG (1990) Two immigrant synanthropic flower flies (Diptera: Syrphidae) new to North America. Entomol News 101:69–74
Thompson FC, Rotheray GE, Zumbado MA (2010) Syrphidae (flower flies). In: Brown et al (eds) Manual of Central American Diptera: volume 2. NRC Research Press, Ottawa pp 763-792
Thomson JA (1896) Note on the occurrence of the larva of the drone-fly (Eristalis tenax, Linn.) as a temporary endoparasite in man. Proc R Phys Soc Edinb 8:160–161
Töreci K, Kasımoğlu Ö, Büget E (1980) Tubifera (T. tenax?) larvaları ile oluşan iki barsak miyazı vakası. Turk Parasitol Derg 3:38–43
Urios V (1990) Consideraciones sobre la ecología del zorro (Vulpes vulpes). In: Gómez López JA et al (eds) Medi Natural, Conselleria d’Agricultura i Pesca, vol 2. Generalitat Valenciana, Valencia, pp 219–142
Utsalo SJ, Ahmed Khalifa RM (1985) Urinary myiasis presenting as urinary tract infection in a Nigerian adult male. Assiut Med J 9:43–54
van den Berghe L, Boné G (1944) Cas de myiase intestinale à Eristalis. Ann Soc Belg Med Trop 24:69–70
van Doesburg PH (1962) Preliminary list of Syrphidae known from Suriname and British and French Guiana. In: Studies on the fauna of Suriname and other Guyanas Geijskes DC and Wagenaar Hummerlinck P (eds) vol 5, Martinus Nijhoff, The Hague, pp 1-33
Verdura S, Romeo V (1968) On a case of intestinal myiasis from Eristalis. Rass Ital Gastroenterol 14:53–59
Verhelst X, Watelle M, Thomaes S, Moerman F, Colle I (2013) Anale last: vreemde duikers. Tijdschr Geneeskd 69:402–403. https://doi.org/10.2143/TVG.69.08.2001377
Vogelsang EG (1926) Caso de parasitismo humano por larvas de Syrphidae. Rev Med Vet - Montevideo 2:435–436
Wagner B (1870) Die Made von Eristalis arbustorum L. als Parasit im menschlichen Darmkanale. Entomol Zeit Stettin 31:71–80
Whish-Wilson PB (2000) A possible case of intestinal myiasis due to Eristalis tenax. Med J Aust 173:652
Whittington AE, Rotheray GE (1997) Afrotropical distribution and larval description of Ornidia obesa (Fabricius, 1775) (Diptera; Syrphidae). J Afr Zool 111:365–372
Williston SW (1886) Synopsis of the North American Syrphidae. Bull US Natl Mus 31:1–335. https://doi.org/10.5962/bhl.title.40963
Wilson DJ, Gerard PJ, De Villiers JE (2009) Preventing rat-tailed maggot incursion into dairy sheds. N Z Plant Prot 62:99–102
Yalçınkaya F (1976) Yurdumuzda Tubifera tenax larvalarının neden olduğu ilk naso-myiasis vakası. Türk Hijiyen ve Deneysel Biyoloji Dergisi 36:40–44
Yang P (2014) Two records of intestinal myiasis caused by Ornidia obesa and Hermetia illucens in Hawaii. Proc Hawaiian Entomol Soc 46:29
Youssefi MR, Sefidgar SAA, Abouhosseini Tabari M (2010) First report of intestinal myiasis due to Eristalis tenax in Iran. Iran J Parasitol 5:77–79
Zhang D, Yan L, Zhang M, Chu H, Cao J, Li K, Hu D, Pape T (2016) Phylogenetic inference of calyptrates, with the first mitogenomes for Gasterophilinae (Diptera: Oestridae) and Paramacronychiinae (Diptera: Sarcophagidae). Int J Biol Sci 12:489–504. https://doi.org/10.7150/ijbs.12148
Zhang B, Wang L, Liu J, Xu L, Song L, Wu X, Sun X, Wu Z (2017) Case report: A rare case of urinary myiasis induced by the fourth instar larvae of Telmatoscopus albipunctatus. PLoS Negl Trop Dis 11:e0006016. https://doi.org/10.1371/journal.pntd.0006016
Zumpt F (1962) Zum Problem der intestinalen Myiasis beim Menschen. Zeitschr angew Zool 49:7–14
Zumpt F (1963) The problem of intestinal myiasis in humans. S Afr Med J 37:305–307
Zumpt F (1965) Myiasis in man and animals in the Old World. A textbook for physicians, veterinarians and zoologists. Butterworths, London
Acknowledgments
We thank Javier Oviedo Cervantes and Labitec S.A. Laboratories for letting us study the larvae of Palpada scutellaris. We thank Jeff H. Skevington (Canadian National Collections) for the English language editing; any remaining error is ours. We also thank Claudia Etzbauer (ZFMK) for her help in the molecular lab to obtain DNA sequences and Andrés Campoy (University of Alicante) for the drawings. We are indebted to Marcela López (Asociación Médica Argentina), Chong Chin Heo (Universiti Teknologi MARA), Bilal Dik (Selçuk University), Refaat Khalifa (Assiut University), Naudy Trujillo (Universidad Centroccidental Lisandro Alvarado), Mercedes París (Museo Nacional de Ciencias Naturales, Madrid), Xavier Verhelst (Ghent University Hospital), Giovanni Poglayen (Alma Mater Studiorum-University of Bologna), Kurt Jordaens (Royal Museum for Central Africa), Libor Mazanek (Palacký University Olomouc), and Francis Gilbert (University of Nottingham) for their help with obtaining valuable bibliographic information.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pérez-Bañón, C., Rojas, C., Vargas, M. et al. A world review of reported myiases caused by flower flies (Diptera: Syrphidae), including the first case of human myiasis from Palpada scutellaris (Fabricius, 1805). Parasitol Res 119, 815–840 (2020). https://doi.org/10.1007/s00436-020-06616-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06616-4