Abstract
Cysteine proteases play essential roles in parasite physiology as well as in host–parasite interactions through their modulation of various biological and pathobiological events. In the present study, a full-length sequence encoding cysteine protease of Clonorchis sinensis (CsCP) was isolated from our adult cDNA library. The open reading frame contains 984 bp encoding 327 amino acids. The present amino acid sequence shared 68% identity with two known CsCP genes and 29–49% identity with that of other species. Bioinformatics analysis showed that conserved domains and characteristic amino acid residues of cysteine proteases were observed in this sequence. Real-time PCR experiments revealed that CsCP was consecutively transcribed in various developmental stages of the parasite, including adult worm, excysted juvenile, metacercaria and egg. Recombinant CsCP (rCsCP) could be probed by rat anti-CsCP serum, rabbit anti-excretory–secretory products (ESP) serum and serum from human infected with Clonorchis sinensis in Western blot. The result of immunolocalization showed that CsCP was mainly located in the oral sucker, excretory bladder and tegument of cercariae and metacercariae, as well as the intestine of adult worm. The rCsCP-based IgG and its isotypes were all detected in sera from human infected with C. sinensis by enzyme-linked immunosorbent assay, and the level of IgG1 is the highest. The receiver-operating characteristic (ROC) analysis was used to determine the most appropriate cut-off value that yielded the high sensitivity (86.96%) and specificity (70.42%). These results revealed that CsCP may play an important role in the biology of C. sinensis and could be a diagnostic candidate for clonorchiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clonorchis sinensis is one of the causative agents of food-borne trematodiasis (Lun et al. 2005). It is estimated that 35 million people have been infected with C. sinensis globally, of which 15 million dwell in China (Fan et al. 2011). People acquire the infection by taking raw or undercooked fish (cyprinidae) and shrimps containing C. sinensis metacercariae. After entering the duodenum, the metacercariae excyst and migrate into the bile ducts and develop to adult worms (Keiser and Utzinger 2009). The adult worms residing in bile ducts cause clonorchiasis with the outcomes of cholecystitis, cholangectasis, cholelithiasis, hepatic fibrosis, and even liver cancer and cholangiocarcinoma (Choi et al. 2004; Lim et al. 2006).
Conventionally, microscopic examination of fecal samples for the presence of parasite eggs is considered the gold standard of screening clonorchiasis. However, omission and false detection is unavoidable due to the difficulty in observing and identifying the tiny eggs under microscope. Additionally, it is troublesome to collect stool samples in epidemiological surveys. Recently, PCR assays such as the nested, multiplex, and real-time PCR have been described in detection of both human and animal in C. sinensis infections (Le et al. 2006; Parvathi et al. 2007; Kim et al. 2009; Rahman et al. 2011). Yet, these techniques are inconvenient, especially in field conditions, for the paucity of precision thermocyclers and professional technologists. To overcome these deficiencies, immunological diagnostic methods relying on detecting specific antibodies in serum are becoming more and more prevalent due to their simplicity, practicality, high sensitivity and efficiency. Cysteine proteases (CPs) play important roles both in parasite physiology and pathogenicity events such as catabolic functions, protein decompozation, excystment/encystment, digesting host tissues for nutrient and immune evading (Sajid and McKerrow 2002; Delcroix et al. 2006; DuBois et al. 2008; Robinson et al. 2008; Kaewpitoon et al. 2008; Han et al. 2011). The importance of cysteine proteases in parasite survival or development makes them efficacious targets for vaccines and immunodiagnostic antigens. Reportedly, several recombinant cysteine protease antigens have been used for diagnosis of human fascioliasis (Carnevale et al. 2001), paragonimiasis (Ikeda et al. 1996) and schistosomiasis (Klinkert et al. 1991).
In the present study, we cloned and characterized a gene encoding a C. sinensis cysteine protease and investigated its diagnostic value for clonorchiasis using enzyme-linked immunosorbent assay (ELISA). The rCsCP-based IgG1 isotype was detected and yielded high sensitivity and specificity in sera from human infected with C. sinensis. These results revealed that CsCP may play an important role in the biology of C. sinensis and could be a diagnostic candidate for clonorchiasis.
Materials and methods
Identification and molecular characterization of cDNA sequence encoding CsCP
From 3475 Unigenes in our C. sinensis cDNA library, a clone (No. CsC61E05) encoding a cysteine protease (accession number JN655695) was identified by BLASTx at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST). Characteristics of deduced protein sequence were predicted by online tools on Expasy website (http://web.expasy.org/), including molecular mass, isoelectric point, functional domain, and signal peptide. Based on similarities, the sequences from different species including Paragonimus westermani (P. w, DQ016547), Schistosoma japonicum (S. j, FN316422), Fasciola hepatica (F. h, EF407948), Echinococcus multilocularis (E. g, AB586073), Taenia solium (T. s, AB441815), Homo sapiens (H. s, BC010240) and C. sinensis (C. s, AY273802; C. s, AB020036), were aligned by the bioinformatics analysis software Vector NTI suite 8.0. The phylogenetic tree was constructed using the neighbor-joining method with MEGA4. Bootstrap proportions were used to assess the robustness of the tree with 500 bootstrap replications.
Preparation of parasites
Living metacercariae of C. sinensis were prepared and collected as previously described (Chen et al. 2011). Sprague–Dawley rats were infected orally with 50 metacercariae each and adult worms were harvested from the biliary tracts of rats 9 weeks after experimental infection. Excysted juveniles were obtained from metacercariae treated with trypsin (0.1% trypsin, pH 7.4). Living cercariae were directly collected from Parafossarulus striatulus in our ecologic pool.
Real-time PCR analysis of CsCP at different life stages of C. sinensis
Total RNA was extracted respectively from eggs, metacercariae, excysted juveniles and adult worms with TRIZOL reagent (Invitrogen, USA) according to the manufacturer's instructions. First-strand cDNA was performed with the reverse transcriptase superscript (TaKaRa, Japan) with oligo dT primers using total RNA as template. β-Actin (accession number EU109284) was used as transcriptional control (Yoo et al. 2009) and amplified with forward primer 5′-ACCGTGAGAAGATGACGCAGA-3′ and reverse primer 5′-GCCAAGTCCAAACGAAGAATT-3′. Real-time PCR amplification was carried out on LightCycler480 instrument (Roche, Switzerland) using SYBR Premix ExTaq Kit (TaKaRa, Japan). The program for amplification was 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 20 s. After amplification, a melting curve was performed using the following profile: 95°C for 30 s, 65°C for 15 s, followed by increase to 95°C while continuously collecting the fluorescence signal. The LightCycler480 software (version 1.5) was used to analyze the data according to 2−ΔΔCt method (Pfaffl 2001). The β-actin amplification signal was employed as transcriptional control, and signal of egg was used as the calibrator.
Cloning, expression, and purification of CsCP
The gene encoding cysteine protease was amplified by polymerase chain reaction (PCR) using No.CsC61E05 plasmid clone as the template. The primer pair is as follows: forward, 5′-GACGGATCCAGCAACATTCCTGAATC-3′, and reverse, 5′-GCCAAGCTTTCACAAGATGATCGAGG-3′. The forward and reverse primers incorporated the restriction enzyme sites BamHI and HindIII (underlined), respectively. PCR assay was carried out for 35 cycles at 94°C for 50 s, 57°C for 60 s, and 72°C for 60 s, and the reaction continued for 5 min at 72°C after the last cycle using ExTaq polymerase (TakaRa, Japan). The purified PCR products were cloned into the His6-tag expression vector pET28a(+) (Novagen, Germany) and subsequently transformed into Escherichia coli BL21 (DE3) (Promega, USA). Selected clones were grown and induced by isopropyl β-d-thiogalactopyranoside (IPTG) at a final concentration of 1 mM at 37°C for 5 h. Induced cells were collected by centrifugation at 8,000×g for 15 min at 4°C, and the inclusion bodies containing the recombinant fusion protein were solubilized thoroughly in phosphate-buffered saline (PBS) containing 6 M urea, and then the rCsCP was purified with His Bind Purification kit (Novagen) according to the manufacturer's instructions. Renaturation was followed by dialysis through gradient dilution of urea. Purified protein was analyzed by 12% sodium dodecyl sulphide-polyacrylamide gel electrophoresis (SDS-PAGE, 12% gel) and stained by Coomassie blue. Protein concentration was estimated using Bradford assay on nucleic acid/protein analyzer (Beckman, USA).
Acquirement of rat anti-rCsCP serum and Western blot analysis
Polyclonal antiserum to rCsCP was produced by immunizing Sprague–Dawley rats purchased from Sun Yat-sen University animal center under the Guide for the Care and Use of Laboratory Animals. Briefly, 200 μg purified rCsCP mixed with equal volume of complete Freund’s adjuvant (Sigma, USA) was injected subcutaneously into Sprague–Dawley rats, two boosters of 100 μg rCsCP with equal volume of incomplete Freund′s adjuvant were given at 2-week intervals. Immune sera were collected 2 weeks after the final injection. The antibody titers were confirmed by ELISA.
The rCsCP was subjected to 12% SDS-PAGE gel and then electrotransferred to the polyvinylidene difluoride (PVDF) membrane (Millipore, USA) at 100 V for 1 h in a Trans-Blottransfer cell (Bio-Rad, USA). The PVDF membranes were blocked with PBS (pH 7.4) containing 5% skimmed milk overnight at 4°C and then probed with naive rat serum, rat anti-rCsCP serum (1:200 dilution), naive human serum, serum from human infected with C. sinensis (1:100 dilution) and rabbit anti-ESP serum (1:100 dilution) for 2 h at room temperature, respectively. After being washed three times with PBS, the membranes were incubated in horseradish peroxidase-conjugated goat anti-rat IgG, anti-rabbit IgG or anti-human IgG (1:2,000 dilution, Invitrogen, USA) for 1 h at room temperature. Both primary and secondary antibodies were diluted with 0.1% BSA in PBS. Bound antibodies were visualized using the 3,3′-diaminobenzidine (DAB) solution (Invitrogen, USA).
Immunolocalization of CsCP at adult worm, metacercaria and cercaria of C. sinensis
After washing several times with PBS (pH 7.4), the worms and metacercariae were fixed in 4% paraformaldehyde and then embedded with paraffin wax and sliced into 5-μm sections, respectively. The collected cercariae were immersed in PBS–0.3% TritonX-100 for 1 h at room temperature before being fixed with cold acetone on glass slide for immunohistochemical analysis. Deparaffinized sections of metacercariae and adult, and fixed cercarie were blocked with normal goat serum for 1 h at room temperature. After that, slides were incubated with rat anti-rCsCP serum (1:200 dilution) at 4°C overnight, and naive rat serum was employed as negative control. After being washed three times with PBS, slides were incubated with goat anti-rat IgG (1:400 dilution) labeled with red-fluorescent Cy3 (Jackson, USA) for 1 h at room temperature in the dark and then imaged under fluorescent microscope (Zeiss, Germany).
Source of human sera samples
Sera from patients with clonorchiasis used for ELISA were collected in Guangxi province and defined based on the presence of eggs in feces. Other sera samples from patients infected with C. sinensis, P. westermani, F. hepatica, S. japonicum, E. granulosus and T. solium were provided by the center of disease prevention and control of Nanning, Jiangsu, Gansu and Fujian province, respectively. Sera from healthy donors were judged by the absence of eggs in feces and no history of eating raw fresh water fish. All patients were singly infected with corresponding parasite.
Detection of antibody isotypes
In order to confirm the optimal detection index, 24 positive and 24 negative sera samples were randomly selected to measure the levels of IgG and its subclasses (IgG1, IgG2, IgG3, and IgG4) by ELISA. The optimal concentration of coating antigen and dilution of serum samples were determined by checker board titration. Briefly, microtiter plates (Corning, USA) were coated with 1, 3, 5, 7 μg/ml purified rCsCP antigen in 0.1 M carbonate–bicarbonate buffer (pH 9.6) and incubated at 4°C over night, respectively. Subsequently, the plates were blocked with 5% skimmed milk in PBS containing 0.05% Tween 20 (PBS-T, pH 7.4) for 2 h at 37°C, then serum samples were diluted at 1:50, 1:100, 1:200, 1:400 under each antigen concentration with PBS-T containing 0.1% BSA. Plates were incubated at 37°C for 2 h before adding HRP-conjugated mouse anti-human IgG and its subclasses (Southern Biotech, Germany). Plates were incubated for 1 h at 37°C and then developed color with substrate 3,3′,5,5′-tetramethylbenzidine (TMB, BD, USA). The absorbance was measured at 450 nm after adding 2 M H2SO4 (50 μl/well) to stop the reaction.
Serodiagnosis of clonorchiasis with rCsCP
A total of 188 human sera samples were used for the serodiagnosis of clonorchiasis. The levels of IgG1 in sera samples from patients infected with C. sinensis, P. westermani, F. hepatica, S. japonicum, E. granulosus and T. solium were detected by ELISA according to the method described above. Briefly, each well was sensitized with the optimal concentration of coating antigen, then the sera diluted in optimal concentration were reacted with rCsCP and incubated with mouse anti-human IgG1 successively. All assays were tested in triplicate and repeated twice.
Statistical analysis
All data in ELISA represented mean ± SD. Statistical analysis was determined by Student’s t-test, and p < 0.05 was considered significant difference. Scatter plots were performed using statistical software SPSS16.0.
Results
Sequence analysis of CsCP
The gene harboured an N-terminal hydrophobic signal peptide at the position of aa 1–18 according to the results of signalP analysis (http://www.cbs.dtu.dk/services/SignalP/). The complete coding sequence of CsCP was comprised of 984 bp encoding a putative protein of 327 amino acids with a predicted molecular mass of 37.1 kDa. The estimated half-life of rCsCP was more than 10 h in E. coli. The indexes of instability in solution, aliphatic, and grand average of hydropathicity were 30.59, 75.69, and −0.407, respectively, indicating that rCsCP was a stable protein with a hydrophobic core in solution. Blastx analysis revealed that the deduced amino acid sequence shared 68% identity with two known CsCP genes and 29−49% identity with other species. Rich B cell epitopes were found in the CsCP, suggesting that the rCsCP maintains good immunogenicity. Moreover, highly conserved amino acids were observed, including the Cys138, His274, Asn295 residues which form the catalytic triad, the Gln132, Cys138, His274, Asn295 and Trp297 residues which maintain the stabilization of the transition state, as well as Gly134 which was involved in substrate binding. Moreover, six critical cysteine residues that form disulphide bridges were also conserved among compared species (Fig. 1). The relationships displayed in the phylogenic tree were in good agreement with traditional taxonomy. The three cysteine proteae genes of C. sinensis were distributed in different branches, suggesting they have different functions (Fig. 2).
Alignment of CsCP deduced amino acid sequence (C. s, JN655695) from Clonorchis sinensis (C. s, AY273802; C. s, AB020036) and other species including P. westermani (P. w, DQ016547), S. japonicum (S. j, FN316422), F. hepatica (F. h, EF407948), E. multilocularis (E. g, AB586073), T. solium (T. s, AB441815), and H. sapiens (H. s, BC010240). The predicted signal peptide region was shown with a signal line. The gaps (dashes) were introduced to maximize alignment. Shaded sequences were highly conserved in sequence alignments. Asterisks indicate the conserved active site residues. Filled diamond indicates the substrate binding site. The six conserved cysteines (pound symbol) which formed disulphide bridges were linked. The B cell epitopes are indicated by dots
Phylogenetic analysis of CsCPs and other related enzymes. The tree was constructed with the neighbor-joining method using MEGA4 program. Numbers on the branches indicated bootstrap proportions (500 replicates). The relationships displayed in the phylogenic tree were in good agreement with traditional taxonomy. The three C. sinensis cysteine protease genes were distributed in different branches. Filled star indicates that sequences were previously reported (AB020036, AY273802). Empty star indicates that the sequence was submitted by our laboratory (JN655695)
Transcriptional level of CsCP at different developmental stages of C. sinensis
Real-time PCR was performed to detect the CsCP transcripts at life stages of egg, metacercaria, excysted juvenile and adult worm. The -fold changes of CsCP gene transcriptional level in adult worm, excysted juvenile and metacercaria were calculated compared with egg. The results showed that the expression levels of CsCP gene were 19.03-, 88.04-, and 177.71-fold in metacercaria, excysted juvenile and adult worm, respectively (Fig. 3).
Stage-specific expression levels of CsCP gene revealed by real-time PCR. DNA markers (M), CsCP PCR products using egg cDNA (E), metacercaria cDNA (M), excysted juvenile cDNA (EJ), and adult worm cDNA (A) as template. β-Actin was employed as the transcriptional control, and the sample of egg was used as the calibrator. Each bar represents the mean value from three experiments with standard deviation (*p < 0.05, **p < 0.01)
Expression and purification of rCsCP
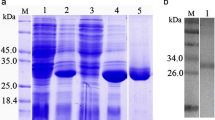
The recombinant pET-28a(+) plasmid containing the CP coding region (signal peptide removed) was confirmed by digestion with restriction enzyme. DNA sequencing revealed that the construct was correct with His6-tag at the N terminus of the recombinant protein. The rCsCP was expressed in E. coli (BL-21) after induction by IPTG, and a purified fusion protein with an approximate molecular weight around 38 kDa was detected, corresponding to the predicted size of 38.5 kDa (including 34 amino acids of vectors) originated from the primary gene sequence. After purification and renaturation, the concentration of the purified CsCP protein was about 0.4 mg/ml (Fig. 4, lane 7).
Expression and purification of CsCP by 12% SDS-PAGE. Protein molecular weight markers (M), lysate of E. coli with pET-28a(+) before induction with IPTG (lane 1), and after induction (lane 2), lysate of E. coli with pET28a(+)-CsCP before induction (lane 3) and after induction (lane 4), supernatant of induced pET28a(+)-CsCP (lane 5), and sediment (lane 6), purified rCsCP (lane 7)
Western blot analysis of rCsCP
The titers of rat anti-rCsCP antibody were 1:102,400 as determined by ELISA. Western blot analysis showed that rat anti-rCsCP serum (Fig. 5, lane 1), rabbit anti-ESP serum (Fig. 4, lane 2) and sera from patients infected with C. sinensis (Fig. 5, lane 5) could strongly react with purified rCsCP while the naive rat serum (Fig. 5, lane 3) and human healthy serum (Fig. 5, lane 4) could not.
Western blot analysis of rCsCP. Protein molecular weight markers (M); rCsCP probed with anti-CsCP rat serum (lane 1); rCsCP probed with serum from human infected with C. sinensis (lane 2); rCsCP probed with preimmune rat serum (lane 3); rCsCP probed with human normal serum (lane 4) and with ES-immuned rabbit serum (lane 5)
Immunolocalization of CsCP at adult worm, metacercaria and cercaria of C. sinensis
Using the rat anti-rCsCP serum as the primary antibody and red-fluorescent Cy3 labeling IgG as the secondary antibody, immunolocalization showed that CsCP distributed surrounded the intestine of C. sinensis adult worm, excretory bladder and tegument of metacercaria, as well as oral sucker, excretory bladder and tegument of cercaria (Fig. 6). No fluorescence was visualized in other organs or in controls treated with naïve serum.
Immunolocalization of CsCP in cercaria, metacercaria and adult worm of C. sinensis. Rat anti-CsCP serum was used as primary antibody and goat anti-rat IgG as secondary antibody. b, d, f, h, j, l Parts under fluorescence microscope and the same parts (a, c, e, g, i, k) under white light. Preimmune rat serum was applied as a negative control. a, b, e, f, i, g Negative control; c, d, g, h, k, l localization of CsCP. T tegument and tegumentary cell, os oral sucker, i intestine, eb excretory bladder, eg intrauterine egg, v vitellarium, E eye point. The images were magnified at × 100 for adult worm and × 200 for cercaria and metacercaria
Human IgG isotype response to CsCP
Optimal concentration of coating antigen and dilution of serum samples were 5 ug/ml and 1:200. IgG, IgG1, IgG2, IgG3 and IgG4 isotypes were all detected. The level of IgG (t = 2.563, p = 0.016), IgG1 (t = 3.667, p = 0.002), and IgG3 (t = 2.338, p = 0.025) in sera of patients with clonorchiasis showed significant difference comparing with those of negative sera (Fig. 7). The most significant index was IgG1, which seemed to be the most appropriate candidate for clonorchiasis serodiagnosis.
Sensitivity, specificity and cross-reactivity of rCsCP in IgG1 ELISA
A total of 188 human sera were used in indirect ELISA to test the reactivity of the rCsCP. The cut-off value determined by ROC curve was 0.255. The sensitivity and specificity of rCsCP were 86.96% and 70.42% (Table 1). The result of ELISA showed that rCsCP could react with human sera from clonorchiasis, fascioliasis, schistosomiasis, paragonimiasis, echinococciosis and cysticercosis (Table 2; Fig. 8).
Cross-reactivity of IgG1 ELISA based on rCsCP. 1 C. sinensis infection group (n = 46), 2 P. westermani infection group (n = 14), 3 S. japonicum infection group (n = 16), 4 F. hepatica infection group (n = 37), 5 E. granulosus infection group (n = 16), 6 T. solium infection group (n = 11), 7 egg-negative group (n = 46)
Discussion
Cysteine proteases are of great interest to parasitologists, as there is considerable evidence that they play a key role in the biology of parasites (McKerrow et al. 2006). In C. sinensis, cysteine proteases are considered to participate in essential biological processes such as immune evasion, stage transition and nutrient uptake from the host (Li et al. 2004; Na et al. 2006, 2008). In the present study, a full-length gene encoding a cysteine protease from C. sinensis was identified, cloned and overexpressed in E. coli. Sequence analysis showed that CsCP has a typical signal peptide at the position of aa1–18, as well the catalytic residue of cysteine, histidine and asparagine that is the signature of cysteine protease (Smooker et al. 2010). The Gln132, Cys138, His274, Asn295 and Trp297 residues, which play critical roles for stabilization of either the thiolate-imidazoliumi on pair or the transition states, were well conserved. Six cysteine residues which can form three conserved disulphide bonds were found among compared species, suggesting a similar tertiary structure. The putative amino acid sequence shared 68% identities with the two reported CsCPs (Nagano et al. 2004; Na et al. 2002), and B cell epitopes showed low similarity. Moreover, the three CsCP genes were distributed on different branches in phylogenic tree. Both results suggested that the sequence in our study was different from the two reported ones which have been applied for serodiagnostic biomarkers in clonorchiasis, encouraging us to assess the value of our rCsCP in serodiagnosis of clonorchiasis.
In immunolocalization, CsCP mainly distributed in excretory–secretory organs such as intestine of adult worm, excretory bladder of cercaria and metacercaria. Western blot analysis showed that rCsCP could be probed by rabbit ESP-immunized serum. Additionally, a typical signal peptide was predicted by signalP analysis. All these results suggested that CsCP mainly be synthesized in the intestinal epithelia, guided by the signal peptide and secreted into the intestinal lumen or released outside the parasite as one component of the ESPs of C. sinensis. The ESPs of parasitic organisms are generally considered to play important roles in host–parasite interactions (Lightowlers and Rickard 1988). In C. sinensis, the ESPs have been investigated for several decades with regard to their antigenic and pathogenic functions and have been shown to be highly sensitive and specific antigens for the diagnosis of clonorchiasis (Choi et al. 2003; Zhao et al. 2004; Ju et al. 2009; Wang et al. 2011). CP has already been found in ESPs of C. sinensis and some other helminths (Smooker et al. 2010). In addition, the results above revealed that CsCP was a component of ESPs, and CsCP could react with C. sinensis-infected rat serum. In ELISA results, rats injected with recombinant CsCP developed high antibody titers. These results collectively showed that CsCP might be a potential candidate for clonorchiasis serodiagnosis as a component of ESPs with antigenicity and immunogenicity. In multicellular parasites such as trematodes, the intestine is a major source of secreted proteases and also a place for nutrition digestion and absorption. According to results of immunolocalization in adult worms, it is reasonable to conclude that CsCP might be involved in digestion of host protein and nutrient uptake for this parasite itself. In real-time PCR experiments, CsCP transcripts were detected at the life stages of egg, metacercaria, excysted juvenile and adult worm. As a secreted protease, these observations suggested that CsCP may play an important role in the biology of this parasite.
The immune interactions between parasites and hosts during parasites invasion are extremely complex and likely triggered both humoral and cell-mediated immune response (Wynn and Hoffmann 2000). As immunoglobulin molecules, IgG2 responded to antigens of parasite surface glycoproteins (Schur 1987). Low IgG2 titer in our study might due to lack of glycosylation sites on the rCsCP. In S. japonicum, there was evidence that IgG4 was related to the susceptive status of the host (Hagan et al. 1991). In C. sinensis, however, antibody titer of rCsCP-specific IgG4 was low to detect, the weak response of rCsCP-specific IgG4 implied that rCsCP might involved in resistance of C. sinensis infection. Generally, IgG1 and IgG3 have functional activity of opsonization, cell-dependent cytotoxicity, and the ability to activate the classical complement pathway thus be involved in host resistance to parasites infection. Our results revealed that the levels of IgG1 and IgG3 were higher than those in the other subgroups. It is reasonable to speculate that CsCP might take part in resistant mechanism during C. sinensis infection. Furthermore, the level of IgG1 was the most significant in sera of patients comparing with that of healthy people (t = 3.667, p = 0.002). Therefore, IgG1 may be more promising than other IgG subgroups in clonorchiasis serodiagnosis. Before the evaluation of application, the optimal concentration of coating antigen and dilution of serum samples were determined by checker board titration, and the cut-off value was evaluated by receiver operating characteristic (ROC) curve. Then IgG1 was chosen as the optimal detection index by IgG isotypes detecting. At last, 188 human sera were used to test the serodiagnosis value with rCsCP. The sensitivities of the test using CsCP antigen was 86.96%. The specificity of this test was analyzed by measuring the reactivity of sera from 48 healthy people and 94 patients infected with other parasites. Cross-reactivity with parasites sharing similar antigens reduce the specificity of the serological test (Bruneau and Bonin 1983). Regarding less similarity about the amino acid sequences between CsCP and other parasites, cross reactions may result from the common conformational epitopes and conserved domain that displayed in sequence analysis.
In conclusion, a gene encoding CsCP was cloned, expressed and characterized. We first discovered that CsCP was localized in oral sucker and tegument of cercaria, indicating that CsCP may take part in the process of cercaria penetrating into the second intermediate host. Furthermore, the value of its serodiagnosis in clonorchiasis was assessed. Our research implied that rCsCP could be a candidate for serodiagnosis of clonorchiasis. Further investigations are required to improve the sensitivity and specificity rates.
References
Bruneau C, Bonin H (1983) Evidence for a disease specific antigen in circulating immune complexes in ankylosing spondylitis. Clin Exp Immunol 53(3):529–535
Carnevale S, Rodriguez MI, Guarnera EA, Carmona C, Tanos T, Angel SO (2001) Immunodiagnosis of fasciolosis using recombinant procathepsin L cystein proteinase. Diagn Microbiol Infect Dis 41(1–2):43–49
Chen W, Wang X, Deng C, Lv X, Fan Y, Men J, Liang C, Yu X (2011) Molecular cloning and characterization of a novel ras-related protein (rap2) from Clonorchis sinensis. Parasitol Res 108(4):1021–1026
Choi MH, Park IC, Li S, Hong ST (2003) Excretory–secretory antigen is better than crude antigen for the serodiagnosis of clonorchiasis by ELISA. Korean J Parasitol 41(1):35–39
Choi BI, Han JK, Hong ST, Lee KH (2004) Clonorchiasis and cholangiocarcinoma: etiologic relationship and imaging diagnosis. Clin Microbiol Rev 17(3):540–552, table of contents
Delcroix M, Sajid M, Caffrey CR, Lim KC, Dvorak J, Hsieh I, Bahgat M, Dissous C, McKerrow JH (2006) A multienzyme network functions in intestinal protein digestion by a platyhelminth parasite. J Biol Chem 281(51):39316–39329
DuBois KN, Abodeely M, Sakanari J, Craik CS, Lee M, McKerrow JH, Sajid M (2008) Identification of the major cysteine protease of Giardia and its role in encystation. J Biol Chem 283(26):18024–18031
Fan Y, Wang X, Deng C, Huang Y, Wang L, Chen W, Liang C, Li X, Wu Z, Yu X (2011) Molecular cloning, expression, and immunolocalization of the NAD(+)-dependent glycerol 3-phosphate dehydrogenase (GPD) from Clonorchis sinensis. Parasitol Res 109(3):621–626
Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA (1991) Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349(6306):243–245
Han YP, Li ZY, Li BC, Sun X, Zhu CC, Ling XT, Zheng HQ, Wu ZD, Lv ZY (2011) Molecular cloning and characterization of a cathepsin B from Angiostrongylus cantonensis. Parasitol Res 109(2):369–378
Ikeda T, Oikawa Y, Nishiyama T (1996) Enzyme-linked immunosorbent assay using cysteine proteinase antigens for immunodiagnosis of human paragonimiasis. Am J Trop Med Hyg 55(4):435–437
Ju JW, Joo HN, Lee MR, Cho SH, Cheun HI, Kim JY, Lee YH, Lee KJ, Sohn WM, Kim DM, Kim IC, Park BC, Kim TS (2009) Identification of a serodiagnostic antigen, legumain, by immunoproteomic analysis of excretory–secretory products of Clonorchis sinensis adult worms. Proteomics 9(11):3066–3078
Kaewpitoon N, Laha T, Kaewkes S, Yongvanit P, Brindley PJ, Loukas A, Sripa B (2008) Characterization of cysteine proteases from the carcinogenic liver fluke, Opisthorchis viverrini. Parasitol Res 102(4):757–764
Keiser J, Utzinger J (2009) Food-borne trematodiases. Clin Microbiol Rev 22(3):466–483
Kim EM, Verweij JJ, Jalili A, van Lieshout L, Choi MH, Bae YM, Lim MK, Hong ST (2009) Detection of Clonorchis sinensis in stool samples using real-time PCR. Ann Trop Med Parasitol 103(6):513–518
Klinkert MQ, Bommert K, Moser D, Felleisen R, Link G, Doumbo O, Beck E (1991) Immunological analysis of cloned Schistosoma mansoni antigens Sm31 and Sm32 with sera of schistosomiasis patients. Trop Med Parasitol 42(4):319–324
Le TH, Van De N, Blair D, Sithithaworn P, McManus DP (2006) Clonorchis sinensis and Opisthorchis viverrini: development of a mitochondrial-based multiplex PCR for their identification and discrimination. Exp Parasitol 112(2):109–114
Li S, Chung YB, Chung BS, Choi MH, Yu JR, Hong ST (2004) The involvement of the cysteine proteases of Clonorchis sinensis metacercariae in excystment. Parasitol Res 93(1):36–40
Lightowlers MW, Rickard MD (1988) Excretory–secretory products of helminth parasites: effects on host immune responses. Parasitology 96(Suppl):S123–S166
Lim MK, Ju YH, Franceschi S, Oh JK, Kong HJ, Hwang SS, Park SK, Cho SI, Sohn WM, Kim DI, Yoo KY, Hong ST, Shin HR (2006) Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. Am J Trop Med Hyg 75(1):93–96
Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY (2005) Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis 5(1):31–41
McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M (2006) Proteases in parasitic diseases. Annu Rev Pathol 1:497–536
Na BK, Lee HJ, Cho SH, Lee HW, Cho JH, Kho WG, Lee JS, Song KJ, Park PH, Song CY, Kim TS (2002) Expression of cysteine proteinase of Clonorchis sinensis and its use in serodiagnosis of clonorchiasis. J Parasitol 88(5):1000–1006
Na BK, Kim SH, Lee EG, Kim TS, Bae YA, Kang I, Yu JR, Sohn WM, Cho SY, Kong Y (2006) Critical roles for excretory–secretory cysteine proteases during tissue invasion of Paragonimus westermani newly excysted metacercariae. Cell Microbiol 8(6):1034–1046
Na BK, Kang JM, Sohn WM (2008) CsCF-6, a novel cathepsin F-like cysteine protease for nutrient uptake of Clonorchis sinensis. Int J Parasitol 38(5):493–502
Nagano I, Pei F, Wu Z, Wu J, Cui H, Boonmars T, Takahashi Y (2004) Molecular expression of a cysteine proteinase of Clonorchis sinensis and its application to an enzyme-linked immunosorbent assay for immunodiagnosis of clonorchiasis. Clin Diagn Lab Immunol 11(2):411–416
Parvathi A, Sanath Kumar H, Kenchanna Prakasha B, Lu J, Xu X, Hu W, Feng Z, Karunasagar I (2007) Clonorchis sinensis: development and evaluation of a nested polymerase chain reaction (PCR) assay. Exp Parasitol 115(3):291–295
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Rahman SM, Bae YM, Hong ST, Choi MH (2011) Early detection and estimation of infection burden by real-time PCR in rats experimentally infected with Clonorchis sinensis. Parasitol Res 109(2):297–303
Robinson MW, Dalton JP, Donnelly S (2008) Helminth pathogen cathepsin proteases: it's a family affair. Trends Biochem Sci 33(12):601–608
Sajid M, McKerrow JH (2002) Cysteine proteases of parasitic organisms. Mol Biochem Parasitol 120(1):1–21
Schur PH (1987) IgG subclasses—a review. Ann Allergy 58(2):89–96, 99
Smooker PM, Jayaraj R, Pike RN, Spithill TW (2010) Cathepsin B proteases of flukes: the key to facilitating parasite control? Trends Parasitol 26(10):506–514
Wang X, Chen W, Li X, Zhou C, Deng C, Lv X, Fan Y, Men J, Liang C, Yu X (2011) Identification and molecular characterization of a novel signaling molecule 14-3-3 epsilon in Clonorchis sinensis excretory/secretory products. Parasitol Res. doi:10.1007/s00436-011-2642-7
Wynn TA, Hoffmann KF (2000) Defining a schistosomiasis vaccination strategy — is it really Th1 versus Th2? Parasitol Today 16(11):497–501
Yoo WG, Kim TI, Li S, Kwon OS, Cho PY, Kim TS, Kim K, Hong SJ (2009) Reference genes for quantitative analysis on Clonorchis sinensis gene expression by real-time PCR. Parasitol Res 104(2):321–328
Zhao QP, Moon SU, Lee HW, Na BK, Cho SY, Kong Y, Jiang MS, Li AH, Kim TS (2004) Evaluation of Clonorchis sinensis recombinant 7-kilodalton antigen for serodiagnosis of clonorchiasis. Clin Diagn Lab Immunol 11(4):814–817
Acknowledgements
This work was supported by Development Program of China (973 program; No. 2010CB530000) and the Sun Yat-sen University innovative talents cultivation program for excellent tutors.
Competing interests
All authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
X. Lv and W. Chen contributed equally to this study.
Rights and permissions
About this article
Cite this article
Lv, X., Chen, W., Wang, X. et al. Molecular characterization and expression of a cysteine protease from Clonorchis sinensis and its application for serodiagnosis of clonorchiasis. Parasitol Res 110, 2211–2219 (2012). https://doi.org/10.1007/s00436-011-2751-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2751-3