Abstract
The aim of the present study was to conduct, in southern Australian waters, a preliminary epidemiological survey of five commercially significant species of fish (yellow-eye mullet, tiger flathead, sand flathead, pilchard and king fish) for infections with anisakid nematodes larvae using a combined morphological–molecular approach. With the exception of king fish, which was farmed and fed commercial pellets, all other species were infected with at least one species of anisakid nematode, with each individual tiger flathead examined being infected. Five morphotypes, including Anisakis, Contracaecum type I and II and Hysterothylacium type IV and VIII, were defined genetically using mutation scanning and targeted sequencing of the second internal transcribed spacer of nuclear ribosomal DNA. The findings of the present study provide a basis for future investigations of the genetic composition of anisakid populations in a wide range of fish hosts in Australia and for assessing their public health significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The move to healthier eating habits and increasing consumption of seafood has resulted in more frequent reports of health problems among consumers. Adverse reactions to seafood are often caused by allergic reactions to the seafood itself or various contaminants (Lopata and Lehrer 2009). Possible adverse reactions to ingested seafood can be caused by unexpected exposure to the emerging food-borne parasite of the genus Anisakis. These parasites occur throughout the world, but there is a marked increase in reported diseases through exposure to contaminated seafood products. While the parasites and their impact on human health have been investigated in many fish species worldwide, there have been very few studies of parasites in commercial fish in Australian waters. Fish parasites are very diverse, and among them are several species which can cause diseases in humans (Table 1). Anisakidosis is a well-known disease involving an infection with live larvae of anisakid nematodes following the consumption of infected seafood (Takahashi et al. 1998; Chai et al. 2005). Since the first reports demonstrating the pathogenic effects of Anisakis larva in humans (Van Thiel et al. 1960), there has been an increasing awareness of fish-borne parasitic diseases. In Australia, various species of anisakids have been reported based on the morphology of the adult stages (Beumer et al. 1982; Mawson et al. 1986; Bruce and Cannon 1989; Bruce 1990a, b; Bruce and Cannon 1990; Bruce et al. 1994; Speare 1999; Shamsi et al. 2008, 2009a, b); however, there is a paucity of knowledge about the epidemiology and ecology of anisakid infections, due mainly to the inability to identify larval stages to species using morphological characters. Molecular–genetic tools can overcome this limitation and allow the genus- or species-specific identification of anisakids (e.g. Li et al. 2005; Shamsi et al. 2008; 2009a, b).
The aim of the present study was to conduct an initial epidemiological study of occurrence and abundance of anisakid nematode larvae in selected fish species in southern Australian waters. Morphological studies were combined with the specific identification of specimens using a mutation scanning-coupled sequencing approach in order to investigate the prevalence and intensity of anisakid larval infections.
Materials and methods
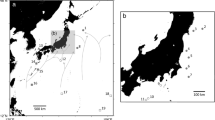
Third-stage anisakid larvae were sought from five species of fish, including Aldrichetta forsteri (yellow-eye mullet), Neoplatycephalus richardsoni (tiger flathead), Platycephalus bassensis (sand flathead), Sardinops sagax (pilchard) and Seriola lalandi (king fish) from Victoria (Melbourne and Lakes Entrance), and South Australia (Adelaide) (see Fig. 1 for locations). Individual parasites were thoroughly washed in physiological saline (pH 7.3) and head and tail cleared with lactophenol for morphological analysis. Larvae were identified to the genus level based on the morphological characters of the excretory and digestive systems (Cannon 1977; Deardorff and Overstreet 1981).
Genomic DNA was isolated from individual larvae by small-scale sodium dodecyl-sulphate/proteinase K treatment, column-purified (Wizard DNA Clean-Up, Promega) and eluted into 40 μl of water. Genomic DNA was also isolated from the musculature of fishes using the same method. PCR was used to amplify the internal transcribed spacer (ITS-2) region using primer pairs and conditions described previously (Shamsi et al. 2008, 2009b). Fish DNA and no-template (negative) controls were subjected to PCR; no amplicons were produced from these samples. An aliquot (4 μl) of each amplicon was examined on a 1.5% w/v agarose gel, stained with ethidium bromide and photographed using a gel documentation system.
Single-strand conformation polymorphism (SSCP) analysis was conducted to screen for nucleotide variation among samples representing all species for which multiple specimens were available (Shamsi et al. 2008, 2009b). Selected amplicons (n = 5–7 per species of fish) were purified over minicolumns (Wizard PCR Prep, Promega, WI, USA), eluted in 30 μl H2O and then subjected to automated sequencing (BigDye® chemistry, Applied Biosystems), in both directions, using the same primers as for PCR. Sequences were aligned using the computer programme ClustalX (Thompson et al. 1997) and then adjusted manually. Polymorphic sites were identified and designated using the International Union of Pure and Applied Chemistry.
Results
This initial survey was conducted to collect larval anisakid nematodes and to estimate prevalence and intensity of infection. Forty fish from five different species were examined at necropsy. Anisakids were collected from four of the five species of fish, including yellow-eye mullet, tiger flathead, sand flathead and pilchard (Table 1); 75% of all examined fish (n = 40) were infected with anisakids. None of the king fish were infected with any parasite. Between 20% and 100% of each of the remaining four fish species was infected. A summary of the results is given in Table 1. The intensity of infection varied from one to 112 larvae per fish. The intensity of infection in each species of fish is demonstrated in Fig. 2. Larvae were found mainly within the abdominal cavity.
Morphological examination showed that the anisakid larvae found in the present study included Anisakis larval type I of Cannon (1977), Contracaecum larval type I of Cannon (1977), Contracaecum larval type II of Cannon (1977), Hysterothylacium larval type IV of Cannon (1977) and Hysterothylacium larval type VIII of Shamsi (2009a, b) (Fig. 3).
Various anisakid morphotypes found in the present study. a–c Anisakis larval type I of Cannon (1977) from N. richardsoni, a anterior end showing boring tooth, excretion porus and nerve ring; b ventriculus; c posterior end showing anal glands and mucron; d–f Contracaecum larval type I from A. forsteri, d anterior end showing boring tooth, nerve ring and intestinal caecum; e ventriculus with ventricular appendix; f posterior end; g–i Contracaecum larval type II of Cannon (1977) from N. richardsoni, g anterior part showing nerve ring, intestinal caecum and ventricular organ; h posterior part, surface structure; i posterior end showing anal glands; j–l Hysterothylacium larval type IV of Cannon (1977) from A. forsteri (j) and N. richardsoni (k, l), j anterior end showing lips, nerve ring, intestinal caecum and ventricular organ; k anterior end showing lips, interlabia, nerve ring and excretion porus; l posterior end; m–n Hysterothylacium larval type VIII of Shamsi et al. (2009a, b) from S. sagax, m and n anterior part showing nerve ring, excretion porus, intestinal caecum and ventricular organ; o posterior end showing anal glands (scale bar = 100 μm). Scale bars 500 μm in (a–f) and 250 μm in (g–j), and (m–n), and 100 μm in (k) and (l)
Anisakis larval type I of Cannon (1977)
Third-stage larvae (n = 53) are usually found encysted in a coiled, spring-like state on the walls of intestines, stomach, gonads and rarely in the liver. All specimens obtained were from the tiger flathead, N. richardsoni (n = 4). Total body length and width of the larvae were 21.74 mm (14.99–27.12) and 0.48 mm (0.39–0.59), respectively. The boring tooth was present (Fig. 3a), and the excretory pore was located below the tooth. A nerve ring was located 0.31 mm (0.08–0.37) from the anterior end. The muscular oesophagus was 2.16 (1.67–2.66) long, and the glandular ventriculus was 0.84 (0.36–1.30) long. The ventriculus joined obliquely with the intestine (Fig. 3b). The anus was located 0.12 mm (0.08–0.15) from the posterior end. Three anal glands encircled the rectum. The tail was short and rounded, ending with a distinct mucron (Fig. 3c).
The ITS-2 region was PCR-amplified from genomic DNAs from all Anisakis larval type I morphotypes, and amplicons were subjected to SSCP analysis. Multiple amplicons were selected for sequencing based on SSCP profile and host species. The length of the ITS-2 sequence was 308 bp (GenBank accession no. FN556176 to FN556178). The alignment of the ITS-2 sequences revealed that they were identical to the sequence available for Anisakis pegreffii (GenBank accession no. AM503954; Zhang et al. 2007).
Contracaecum larval type I
Third-stage larvae (n = 26) were found exclusively inside the liver of the yellow-eye mullet, A. forsteri (n = 9). Body length and width were 22.71 mm (14.99–28.11) and 1.08 mm (0.92–1.18), respectively. Labia were weakly developed, and a boring tooth as present (Fig. 3d) with an excretory pore below it. A nerve ring was located 0.36 mm (0.29–0.45) from the anterior end. The oesophagus was long and slim, 3.97 mm (2.61–5.04). The ventriculus was 0.22 mm (0.14–0.31) long. The ventricular appendix and intestinal caecum were 1.16 mm (0.39–1.51) and 3.35 mm (1.95–4.42) long, respectively (Figs. 3d, e). The anus was located 0.18 mm (0.09–0.26) from the posterior end. The tail was conical, with a sharply pointed end and a spine (Fig. 3f).
The ITS-2 region was PCR-amplified from genomic DNAs from all Contracaecum larvae. Amplicons were subjected to SSCP analyses. Two samples were chosen for sequencing based on the SSCP profile. The ITS-2 region was 231-bp long for both samples (accession nos. FN556179 and FN556180). One polymorphism was detected at alignment position 180. An alignment of these sequences with those available in the GenBank database showed that they were identical to the ITS-2 sequence of Contracaecum multipapillatum D (accession no. AM940060).
Contracaecum larval type II of Cannon (1977)
Third-stage larvae (n = 26) were found in the liver and pancreas, and, in one case, in the intestines of the tiger flathead, N. richardsoni (n = 2); a single specimen was extracted from the liver of the yellow-eye mullet, A. forsteri (n = 1). Body length and width were 3.28 mm (1.49–4.77) and 0.23 mm (0.13–0.33), respectively. A boring tooth was present (Fig. 3g) with an excretory pore below it. A nerve ring was located 0.11 mm (0.05–0.25) from the anterior end. The muscular oesophagus was 0.55 mm (0.35–0.73) long. The ventriculus was 0.07 mm (0.03–0.20) long, respectively. The ventricular appendix and intestinal caecum were 0.37 mm (0.11–0.61) and 0.33 mm (0.13–0.50) long. The annulated cuticle had a ripple-like pattern (Fig. 3h). The anus was located 0.10 (0.08–0.13) from the posterior end. Three anal glands were present around the rectum. The tail was conical with a rounded tip, and although phasmids could not be identified, a pair of pyriform structures was visible in the tail (Fig. 3i). No molecular analyses were carried out on these specimens.
Hysterothylacium larval type IV of Cannon (1977)
All fourth-stage larvae (n = 63) were collected from the intestines, pyloric caeca, liver and pancreas from the tiger flathead, N. richardsoni (n = 4). One specimen was obtained from the intestines of the yellow-eye mullet, A. forsteri (n = 1). Its body length was 7.26 mm (2.49–19.25), and maximum body width was 0.23 mm (0.11–0.44). Lips were well developed, with small interlabia between them. A nerve ring was located 0.18 mm (0.06–0.33) from the anterior end. The excretory pore was near the nerve ring. The muscular oesophagus was 0.72 mm (0.45–1.16) long. The ventricular appendix and intestinal caecum were 0.39 mm (0.06–1.74) and 0.31 mm (0.10–1.05) long, respectively. The anus was located 0.14 mm (0.06–0.35) from the posterior end. The tail was short, with a cluster of spines resembling a crown at the posterior end (Fig. 3j–l).
The ITS-2 region was PCR-amplified from all genomic DNA samples using PCR. Amplicons were subjected to SSCP analysis. All amplicons have the same SSCP profile. Therefore, one specimen was selected for sequencing. The ITS-2 sequence was 345-bp long (GenBank accession no. FN556181). The ITS-2 sequence did not match any sequence in the GenBank database.
Hysterothylacium larval type VIII of Shamsi (2009a, b)
Third-stage larvae (n = 90) were collected from the intestines, pyloric caeca, liver, pancreas and the body cavity of the pilchard, S. sagax (n = 9) and tiger flathead, N. richardsoni (n = 3). Total body length and maximum body width were 5.97 mm (2.37–12.83) and 0.24 mm (0.11–0.51), respectively. A nerve ring was located 0.22 mm (0.06–0.46) from the anterior end, with the excretory pore being near the nerve ring. The oesophagus was slender and 0.91 mm (0.40–1.45) mm long, followed by a short ventriculus, 0.08 mm (0.02–0.17) long. The ventricular appendix and intestinal caecum were 0.30 mm (0.07–0.78) and 0.37 mm (0.12–0.98) long, respectively. The anus was located 0.14 mm (0.07–0.20) from the posterior end. The tail was conical, with a single terminal spine on the round tip (Fig. 3m, n).
Three and seven samples from N. richardsoni and S. sagax were subjected to molecular analysis. ITS-2 was PCR-amplified from these samples, and all amplicons were subjected to SSCP analysis. No variation in the SSCP pattern was detected. Therefore, one specimen was selected for sequencing. The ITS-2 was 348-bp long (accession no. FN556182). The ITS-2 sequence did not match any sequence in the GenBank database.
Discussion
Using both traditional morphological and molecular approaches, it has been possible to investigate the occurrence and abundance of various species of anisakid larvae in selected fish of southern Australian waters. Previous studies reported the occurrence of various morphotypes of anisakid nematodes in Australian fishes (Lebedev 1968; Hurst 1984; Bruce et al. 1994; Doupe et al. 2003); however, it is not yet clear which species occur in these fishes. The present epidemiological survey revealed five larval morphotypes representing three different genera of anisakid nematodes, including A. pegreffii, Contracaecum and Hysterothylacium, which have been reported to cause human anisakidosis (Im et al. 1995; Gorokhov et al. 1999; D’Amelio et al. 1999; D’Amelio 2003; Marques et al. 2006; Lopata and Lehrer 2009). Infection with larvae of Anisakis occurs relatively frequently (Kagei et al. 1995), whereas infection with Contracaecum or Hysterothylacium is less common (Dei-Cas et al. 1986; Im et al. 1995; Yagi et al. 1996).
In recent years, it has also been recognized that allergic responses can occur in humans against live anisakids or food in which worms have been killed by cooking or freezing (Moreno-Ancillo et al. 1997; Audicana et al. 2002). The allergenic proteins are exceptionally stable to denaturation, causing IgE antibody-mediated allergic reactions (Lopata and Lehrer 2009). Interestingly, several fish parasites are known to cause infections in consumers of infected fish; however, only several species among the anisakids have been associated with allergic sensitisation and reactions (Fig. 4). Therefore, it is of great importance to identify even low numbers of parasites present in infected fish (De Corres et al. 1996; Audicana and Kennedy 2008).
Since human infection occurs after eating raw, undercooked or improperly processed fish or seafoods, infection of fish with anisakid nematodes is important. Infection of fish with anisakid nematodes is of significance not only because of adverse health effects in humans, but also because of the affect that they have on the infected fish. Anisakid larvae, particularly when located in the musculature, can also affect the commercial value of fish and thus result in significant economic losses to the fishing industry (Smith and Wootten 1978; Angot and Brasseur 1995). Moreover, anisakids can cause disease in fish. The symptoms and severity of disease can vary considerably depending on factors, such as the species of fish, species and intensity of infecting parasite in the fish and the particular organs invaded (Woo 1995). The disease is most severe when the anisakid larvae infect the liver causing fibrosis of the liver which can lead to atrophy of this organ and significant loss in body weight. Other manifestations can also be granulomatous inflammation and necrosis of the muscularis externa of the pyloric, gall bladder, intestine and body cavity, which can cause substantial mortality in fish (Woo 1995).
It is also worth indicating that ansakids may not be host-specific at the larval stages, which means that a wide range of fish species can act as their intermediate or paratenic hosts. It has been shown that larval anisakids can pass through several fish species via predation and can be accumulated in larger fish (Burt et al. 1990; Jensen 1997). Hence, fish of different species can play an important role in the distribution of anisakids in the environment. Different species of fish are not only the source of infection to humans, but also infect a broad range of marine mammals and piscivorous birds.
This study investigated flatheads which is a particularly popular fish in the Australian cuisine and demonstrated considerable infections with potentially zoonotic parasites, including A. pegreffii, and C. multipapillatum D and Hysterothylacium types IV and VIII. Another type of Hysterothylacium was also discovered in the sand flathead, P. bassensis, based on ITS-2 sequence data.
A striking finding of this study was the high prevalence, intensity of infection and species diversity of anisakid nematodes in tiger flathead, N. richardsoni. Interestingly, the closely related sand flathead P. bassensis, which has a similar biology and prey preference, demonstrated a comparative low level of infection. Such infection differences between these two flathead species may be a reflection of the region where these fish were collected, the different diet and/or host preference of anisakids. Seasonal variation of the abundance of anisakid larvae in intermediate hosts is also possible, as observations have shown for A. simplex in fish in Norwegian waters (Strmnes and Andersen 2000). Another remarkable discovery was the absence of any anisakids from king fish, S. lalandi, originating from an aquaculture farm from South Australia. In contrast, larval stages of Anisakis, Contracaecum and Hysterothylacium spp. are known to infect wild king fish in southern Australian waters (Hutson et al. 2007; Shamsi et al. 2009a). It is not clear if the absence of Anisakis larvae is a reflection of the feed method used (pellets); however, there are reports of Anisakis infections in other cultured marine fish (Yoshinaga et al. 2006).
An additional objective of this survey was to evaluate the usefulness of both morphological examination and molecular analysis and whether the reliance on a single approach brings equally adequate results compared with a combined approach. Microscopic characterization minimizes costs and is simple to perform, but is time consuming and also prone to subjective interpretation (Thompson 1982). Furthermore, the latter approach does not allow the specific identification of larval stages of anisakids (Oshima 1972). In contrast, SSCP and targeted DNA sequencing of ITS-2 is accurate and efficient for large-scale studies (Gasser et al. 2006) newly discovered species, information on morphology has to be compared with available molecular data. Since larval forms of anisakid nematodes do not allow unambiguous identification to the species level (Fagerholm 1988), the retrieval of adult specimens, preferably males, which possess more characteristic traits (Hartwich 1974; Fagerholm 1991), would alleviate some problems with specific identification. Genetic levelling of marker regions between validated species of anisakids, given they are species-specific, enables immediate identification of the species. The use of additional genetic loci, such as the ITS-1 region and perhaps mitochondrial gene loci might support further the specific identification of anisakid nematodes (Shamsi et al. 2008, 2009a, b). This is particularly important, given that many species of anisakids remain to be studied taxonomically. In addition, the public health importance of anisakids in commonly consumed fish in Australia warrants detailed investigation.
References
Angot V, Brasseur P (1995) Les larves d’anisakides et leur incidence sur la qualite des poissons et produits de poisson. Rev Med Vet 146:791–804
Audicana MT, Kennedy MW (2008) Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev 21(2):360–379
Audicana MT, Ansotegui IJ, Fernandez de Corres L, Kennedy MW (2002) Anisakis simplex: dangerous—dead and alive? Trends in Parasitology. Trends Parasitol 18:20–25
Beumer JP, Ashburner LD, Burbury ME, Jette E, Latham DJ (1982) A checklist of the parasites of fishes from Australia and its adjacent Antarctic territories. Commonwealth Agricultural Bureaux, Technical Communication No. 48 of the Commonwealth Institute of Parasitology
Bruce NL (1990a) Hysterothylacium Ward and Magath, 1917, and Ichthyascaris Wu, 1949, ascaridoid nematodes from Australian demersal fishes. Mem Queensl Mus 28:389–426
Bruce NL (1990b) Redescription of the Ascaridoid nematode Hysterothylacium scomberomori (Yamaguti) from Australian Spanish mackerel Scomberomorus commerson (Lacepede). Mem Queensl Mus 28:427–434
Bruce NL, Cannon LRG (1989) Hysterothylacium, Iheringascaris and Maricostula new genus, nematodes (Ascaridoidea) from Australian pelagic marine fishes. J Nat Hist 23:1397–1441
Bruce NL, Cannon LRG (1990) Ascaridoid nematodes from sharks from Australia and the Solomon Islands, southwestern Pacific Ocean. Invertebr Syst 4:763–783
Bruce NL, Adlard RD, Cannon LRG (1994) Synoptic checklist of ascaridoid parasites (Nematoda) from fish hosts. Invertebr Taxon 8:583–674
Burt MDB, Campbell JD, Likely CG, Smith JW (1990) Serial passage of larval Pseudoterranova decipiens (Nematoda: Ascaridoidea) in fish. Can J Fish Aquat Sci 47:693–695
Cannon LRG (1977) Some larval ascaridoids from south-eastern Queensland marine fishes. Int J Parasitol 7:233–243
Chai J, Murrell KD, Lymbery AJ (2005) Fish-borne parasitic zoonoses: status and issues. Int J Parasitol 35:1233–1254
D’Amelio S (2003) Phylogeny of anisakid nematodes: a review. Helminthologia 40:87–91
D’Amelio S, Mathiopoulos KD, Brandonisio O, Lucarelli G, Doronzo F, Paggi L (1999) Diagnosis of a case of gastric anisakidosis by PCR-based restriction fragment length polymorphism analysis. Parassitologia (Roma) 41:591–593
Deardorff TL, Overstreet RM (1981) Larval Hysterothylacium (=Thynnascaris) (Nematoda: Anisakidae) from fishes and invertebrates in the Gulf of Mexico. Proc Helminthol Soc Wash 48:113–126
De Corres LF et al (1996) Anisakis simplex induces not only anisakiasis: report on 28 cases of allergy caused by this nematode. J Investig Allergol Clin Immunol 6:315–319
Dei-Cas E et al (1986) Human anisakiasis. Five new cases in the northern France. Gastroenterol Clin Biol 10:83–87
Doupe RG, Lymbery AJ, Wong S, Hobbs RP (2003) Larval anisakid infections of some tropical fish species from north-west Australia. J Helminthol 77:363–365
Fagerholm HP (1988) Incubation in rats of a nematodal larva from cod to establish its specific identity: Contracaecum osculatum, (Rudolphi). Parasitol Res 75:57–63
Fagerholm HP (1991) Systematic implications of male caudal morphology in ascaridoid nematode parasites. Syst Parasitol 19:215–228
Gasser RB et al (2006) Single-strand conformation polymorphism (SSCP) for the analysis of genetic variation. Nat Protoc 1(6):3121–3128
Gorokhov VV, Sergiev VP, Romanenko NA (1999) Anisakiasis as a growing ecological and social problem. Med Parazitol (Mosk) (4):50–54
Hartwich G (1974) Keys to genera of the Ascaridoidea. In: Anderson RC, Chabaud AG, Willmott S (eds) CIH keys to the nematode parasites of vertebrates. Commonwealth Agricultural Bureaux, Bucks, pp 1–15
Hurst RJ (1984) Identification and description of larval Anisakis simplex and Pseudoterranova decipiens (Anisakidae: Nematoda) from New Zealand waters. NZ J Mar Freshw Res 18:177–186
Hutson KS, Ernst I, Whittington ID (2007) Risk assessment for metazoan parasites of yellowtail kingfish Seriola lalandi (Perciformes: Carangidae) in South Australian sea-cage aquaculture. Aquaculture 271:85–99
Im K, Shin H, Kim B, Moon S (1995) Gastric anisakiasis cases in Cheju-do, Korea Republic. Korean J Parasitol 33:179–186
Jensen T (1997) Experimental infection/transmission of sculpins (Myoxocephalus scorpius) and cod (Gadus morhua) by sealworm (Pseudoterranova decipiens) larvae. Parasitol Res 83:380–382
Kagei N et al (1995) A case of hepatic anisakiasis with a literal survey for extra-intestinal anisakiasis. Jpn J Parasitol 44:346–351
Lebedev BI (1968) Helminth fauna of carangid fish in the Pacific Ocean. Soobshch. Dal’nevost. Fil. V. L. Komarova sib. Otdel. Akad. Nauk SSSR 26:80–85
Li A et al (2005) Genetic evidence for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) and the validity of Contracaecum septentrionale (Kreis, 1955) (Nematoda: Anisakidae). Parasitol Res 96:361–366
Lopata AL, Lehrer SB (2009) New insights into seafood allergy. Curr Opin Allergy Clin Immunol 9:270–277
Marques JF, Cabral HN, Busi M, D’Amelio S (2006) Molecular identification of Anisakis species from Pleuronectiformes off the Portuguese coast. J Helminthol 80:47–51
Mawson PM, Angel M, Edmonds SJ (1986) A checklist of helminths from Australian birds. Rec South Aust Mus 19:219–325
Moreno-Ancillo A, Caballero MT, Cabanas R (1997) Allergic reactions to Anisakis simplex parasitizing seafood. Ann Allergy Asthma Immunol 79:246–250
Oshima T (1972) Anisakis and Anisakiasis in Japan and adjacent area. In: Progress of medical parasitology in Japan, pp 301–393
Shamsi S, Gasser R, Beveridge I, Shabani AA (2008) A description of C. multipapillatum (von Drasche, 1882) from the Australian pelican, Pelecanus conspicillatus. Parasitol Res 103:1031–1039
Shamsi S, Norman R, Gasser R, Beveridge I (2009a) Genetic and morphological evidences for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) (Nematoda: Anisakidae) in Australia. Parasitol Res 105:529–538
Shamsi S, Norman R, Gasser R, Beveridge I (2009b) Redescription and genetic characterization of selected Contracaecum spp. (Nematoda: Anisakidae) from various hosts in Australia. Parasitol Res 104:1507–1525
Smith JW, Wootten R (1978) Anisakis and anisakiasis. Adv Parasitol 16:93–163
Speare P (1999) Parasites from east coast Australian billfish. Mem Queensl Mus 43:837–848
Strmnes E, Andersen K (2000) “Spring rise” of whaleworm (Anisakis simplex; Nematoda, Ascaridoidea) third-stage larvae in some fish species from Norwegian waters. Parasitol Res 86:619–624
Takahashi S, Ishikura H, Kikuchi K (1998) Anisakidosis: global point of view. In: Ishikura H, Aikawa M, Itakura H, Kikuchi K (eds) Host response to international parasitic zoonoses. Springer-verlag, Tokyo, pp 109–120
Thompson RCA (1982) Intraspecific variation and parasite epidemiology. In: Parasites—their world and ours. Proceedings of the 5th International Congress of Parasitology, Toronto, Canada, 7–14 August, 1982, under the auspices of the World Federation of Parasitologists. Elsevier Biomedical Press, Amsterdam Netherlands, pp 369–378
Thompson JD, Gibson TJ, Plewniac F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Van Thiel PH, Kuipers FC, Roskam RT (1960) A nematode parasitic to herring, causing acute abdominal syndromes in man. Trop Geogr Med 2:97–113
Woo PTK (1995) Fish diseases and disorders volume 1: protozoan and metazoan infections. In: Fish diseases and disorders volume 1: protozoan and metazoan infections. CAB International, Wallingford, UK
Yagi K, Nagasawa K, Ishikura H, Nakagawa A, Sato N, Kikuchi K, Ishikura H (1996) Female worm Hysterothylacium aduncum excreted from human: a case report. Jpn J Parasitol 45:12–23
Yoshinaga T, Kinami R, Hall KA, Ogawa K (2006) A preliminary study on the infection of anisakid larvae in juvenile greater amberjack Seriola dumerili imported from China to Japan as mariculture seedlings. Fish Pathology (Gyobyo Kenkyu) 41:123–126
Zhang L et al (2007) The specific identification of anisakid larvae from fishes from the Yellow Sea, China, using mutation scanning-coupled sequence analysis of nuclear ribosomal DNA. Mol Cell Probes 21:386–390
Acknowledgements
This study was financially supported by the Australian Biological Resources Study (ABRS), Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nucleotide sequence data reported in this paper are available in the GenBank database under the accession numbers FN556176 to FN556182.
Rights and permissions
About this article
Cite this article
Shamsi, S., Eisenbarth, A., Saptarshi, S. et al. Occurrence and abundance of anisakid nematode larvae in five species of fish from southern Australian waters. Parasitol Res 108, 927–934 (2011). https://doi.org/10.1007/s00436-010-2134-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2134-1