Abstract
The mature Taeniarhynchus saginatus spermatozoon exhibits an apical cone of electron-dense material and one helicoidal crest-like body roughly 50 nm thick. The axoneme is of the 9 + “1” Trepaxonemata pattern. It is surrounded by a periaxonemal sheath of electron-dense material. The cytoplasm is electron lucent and divided into compartments by intracytoplasmic walls of electron-dense material in regions III and IV. The nucleus is an electron-dense cord 60–90 nm thick coiled in a spiral around the axoneme. It reaches the posterior extremity of the gamete where the axoneme is disorganized and is accompanied on all its posterior length by the nucleus. To our knowledge, such a posterior extremity has never been described before in a cyclophyllidean cestode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Taeniidae parasites of human belong to two genera and three species. These are Taenia solium Linnaeus,1758 which the scolex bears a rostellum armed of two rows of hooklets, Taenia asiatica (Eom and Rim 1993), and Taeniarhynchus saginatus (Goeze, 1782) Weinland, 1858 which the scoleces are unarmed. To our knowledge, in the genus Taenia, only six species have been up until now the subject of ultrastructural study of spermiogenesis and/or the spermatozoon. These are Taenia hydatigena (Featherston 1971), Taenia mustelae (Miquel et al. 2000), Taenia parva (Ndiaye et al. 2002), Taenia solium (Willms et al. 2003), Taenia crassiceps (Willms et al. 2004), and Taenia taeniaformis (Miquel et al. 2009a, b). In the present work, we describe for the first time the ultrastructure of the spermatozoon of Taeniarhynchus saginatus.
Materials and methods
Gravid proglottids of Taeniarhynchus saginatus were removed from human feces and kept in 0.9% NaCl solution. Portions of about 3 mm long including the genitalia were fixed in cold 2.5% glutaraldehyde with a 0.1 M sodium cacodylate buffer at pH 7.2 for 1 h, rinsed in a 0.1 M sodium cacodylate buffer at pH 7.2, postfixed in cold (4°C) osmium tetroxide in the same buffer for 1 h, rinsed in a 0.1 M sodium cacodylate buffer at pH 7.2, dehydrated in ethanol and propylene oxide, embedded in Epon, and polymerized at 60°C for 48 h. Ultrathin sections were cut on a Reichert-Jung Ultracut E ultramicrotome, stained with uranyl acetate and lead citrate, and examined with a Hitachi H-600 electron microscope at 75 kV.
Results
The mature spermatozoon of Taeniarhynchus saginatus (Figs. 1 (1, 2, 3, 4, 5 and 6), 2 (7, 8, 9, 10, 11, 12, 13, 14, 15 and 16), 3 (17)) is filiform, tapered at both ends, and lacks mitochondria. From front to back, we were able to distinguish five regions (I–V) exhibiting distinctive ultrastructural characters.
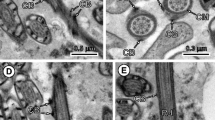
1 Longitudinal section of the anterior extremity of the mature spermatozoon of Taeniarhynchus saginatus showing the apical cone. Ac apical cone, Ax axoneme, C centriole, Cb crest-like body, Cm cortical microtubules. Bar = 0.2 μm. 2 Cross section of region I of the mature spermatozoon of Taeniarhynchus saginatus at the anterior extremity of the centriole. Cb crest-like body, Cm cortical microtubules. Bar = 0.1 μm. 3 Cross section of region I of the mature spermatozoon of Taeniarhynchus saginatus at the level of the centriole. Cb crest-like body, Cm cortical microtubules. Bar = 0.1 μm. 4 Cross section of region I of the mature spermatozoon of Taeniarhynchus saginatus at the level of the axoneme. Ax axoneme, Cb crest-like body, Cm cortical microtubules. Bar = 0.1 μm. 5 Cross section of region II of the mature spermatozoon of Taeniarhynchus saginatus. Cm cortical microtubules, Ps periaxonemal sheath. Bar = 0.1 μm. 6 Longitudinal section of regions I and II of the mature spermatozoon of Taeniarhynchus saginatus. Ax axoneme, Cb crest-like body, Ps periaxonemal sheath. Bar = 0.2 μm
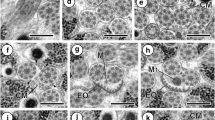
7 Longitudinal section of regions III and IV of the mature spermatozoon of Taeniarhynchus saginatus. Ax axoneme, Cm cortical microtubules, Iw intracytoplasmic wall, N nucleus, Ps periaxonemal sheath. Bar = 0.4 μm. 8 Cross section of region III of the mature spermatozoon of Taeniarhynchus saginatus. Cm cortical microtubules, Iw intracytoplasmic wall, Ps periaxonemal sheath. Bar = 0.1 μm. 9 Cross section of region IV of the mature spermatozoon of Taeniarhynchus saginatus showing the nucleus (N) in a horseshoe shape. Cm cortical microtubules, Iw intracytoplasmic wall, N nucleus, Ps periaxonemal sheath. Bar = 0.1 μm. 10 Cross section of region IV of the mature spermatozoon of Taeniarhynchus saginatus showing the nucleus (N), in an annular shape, interposed between the cortical microtubules (Cm) and the axoneme (Ax). Bar = 0.1 μm. 11 Cross section of region V of the mature spermatozoon of Taeniarhynchus saginatus showing the nucleus (N) partially interposed between the cortical microtubules (Cm). Bar = 0.1 μm. 12 Longitudinal section of region V of the mature spermatozoon of Taeniarhynchus saginatus. Ax axoneme, Cm cortical microtubules, N nucleus. Bar = 0.2 μm. 13 Cross section of region V of the mature spermatozoon of Taeniarhynchus saginatus in which the cortical microtubules have disappeared. Ax axoneme, N nucleus. Bar = 0.1 μm. 14–16 Cross sections of region V of the mature spermatozoon of Taeniarhynchus saginatus. The axoneme disorganizes progressively. Its central core first disappears, then, its doublets become singlets (S). Bar = 0.1 μm
17 Attempted reconstruction of the mature spermatozoon of Taeniarhynchus saginatus. Ac apical cone, Ape axonemal posterior extremity, Ase anterior spermatozoon extremity, Ax axoneme, C centriole, Cb crest-like body, Cm cortical microtubules, Iw intracytoplasmic wall, N nucleus, Ps periaxonemal sheath
Region I (Figs. 1 (1, 2, 3 and 4) and 3 (17)), roughly 0.2–0.3 μm wide, corresponds to the anterior extremity of the spermatozoon. It exhibits an apical cone of electron-dense material (Fig. 1 (1, 2, 3 and 4)) about 1.2 μm long and 230 nm wide at its base and one helicoidal crest-like body (Fig. 1 (2, 3 and 4)) about 50 nm thick, spiraled at an angle of about 40° to the spermatozoon axis (Fig. 1 (1 and 6)). The axoneme of the 9 + “1” pattern is situated in a central position and surrounded by a thin layer of electron-lucent cytoplasm and an electron-dense submembranous layer of cortical microtubules. The latter are spiraled at an angle of about 40° to the spermatozoon axis.
Region II (Figs. 1 (5 and 6) and 3 (17)) has a maximum width of 0.4 μm and lacks crest-like body. The axoneme is central (Fig. 1 (5 and 6)) and surrounded by a sheath of electron-dense material, an electron-lucent cytoplasm and submembranous cortical microtubules (Fig. 1 (5)).
Region III (Figs. 2 (7 and 8) and 3 (17)) is about 0.4–0.5 μm wide. In this region, the axoneme is central and surrounded by a sheath of electron-dense material and an electron-lucent cytoplasm (Fig. 2 (7)). The latter is divided into compartments by intracytoplasmic walls of electron-dense material which join the periaxonemal sheath to the cortical microtubules (Fig. 2 (8)).
Region IV (Figs. 2 (7, 9 and 10) and 3 (17)) is roughly 0.4–0.5 μm wide. It is characterized by the presence of a nucleus. This is a fine compact cord of electron-dense material approximately 60–90 nm thick coiled in a helix around the axoneme (Fig. 2 (7 and 9)). It interposes itself between the periaxonemal sheath and the cortical microtubules (Fig. 2 (7 and 9)). In cross sections depending on the level of the cut, it is in a horseshoe shape (Fig. 2 (9)) or in an annular shape (Fig. 2 (10)).
Region V (Figs. 2 (11, 12, 13, 14, 15 and 16) and 3 (17)) is roughly 0.3–0.2 μm. It corresponds to the posterior end of the gamete. It is characterized by the progressive disorganization of the axoneme: its central core disappears first, and later on, its peripheral doublets become singlets (Fig. 2 (15 and 16)). The nucleus, in cross sections, is annular shaped (Fig. 2 (11, 13, 14 and 15)) and is interposed between the cortical microtubules which are present only in the anterior area of this region (Fig. 2 (11)). It accompanies the axoneme on all its posterior length (Fig. 2 (11, 13, 14, 15 and 16)).
Discussion
The crest-like body or bodies always mark the anterior extremity of the cestode spermatozoon (Bâ et al. 1991). Consequently, the extremity with crest-like body of the Taeniarhynchus saginatus spermatozoon corresponds to its anterior extremity and the other one without crest-like body to its posterior extremity.
In the Cyclophyllidea, the number of crested bodies varies according to the species from one to 12 (Bâ et al. 2005a). Nevertheless, in the Taeniidae, only a single crested body was described in the studied species to date.
Intracytoplasmic walls of electron-dense material were described in the spermatozoon of several species of Cyclophyllidea: these are the anoplocephalidaeans Avitellina centripunctata (Bâ and Marchand 1994c), Inermicapsifer guineensis (Bâ and Marchand 1994b), and Inermicapsifer madagascariensis (Bâ and Marchand 1994b); the davaineidaeans Raillietina (R) tunetensis (Bâ and Marchand 1994a), Cotugnia polyacantha (Bâ and Marchand 1994d), Raillietina (R) baeri (Bâ et al. 2005a), and Paroniella reynoldsae (Bâ et al. 2005b); and the taeniidaeans Taenia hydatigena (Featherston 1971), Taenia mustelae (Miquel et al. 2000), Taenia solium (Willms et al. 2003), Taenia parva (Ndiaye et al. 2002) Taenia crassiceps (Willms et al. 2004), and Taenia taeniaformis (Miquel et al. 2009a,b).
In the Cyclophyllidea, the shape of the nucleus varies according to the species. In Retinometra serrata (Bâ and Marchand 1993), Echinocotyle dolosa (Bâ et al. 2002), and Sudarikovina taterae (Bâ et al. 2000), in particular, it is straight and situated between the axoneme and the cortical microtubules. In most cyclophyllideans, it is coiled around the axoneme. Thus, in cross section, depending on the level of the cut, it appears in a horseshoe shape or in an annular shape. In Taeniarhynchus saginatus, it is both in a horseshoe shape and in an annular shape.
The degree of spiralization of the front and of the back of the nuclear area around the axoneme varies according to the cyclophyllidean species. In Taenia parva (Ndiaye et al. 2002), Raillietina (R) baeri (Bâ et al. 2005a), and Paroniella reynoldsae (Bâ et al. 2005b), the nucleus is more coiled in its anterior area. On the other hand in Taenia taeniaformis (Miquel et al. 2009a,b), Taenia mustelae (Miquel et al. 2000), and Taenia crassiceps (Willms et al. 2004), like as in Taeniarhynchus saginatus, it is more coiled in its posterior area.
In most of the Cyclophyllidea, the axoneme becomes disorganized after the nuclear region. On the other hand, in Paranoplocephala omphalodes (Miquel and Marchand 1998), E. dolosa (Bâ et al. 2002), Gallegoides arfaai (Miquel et al. 2004), and Taeniarhynchus saginatus (present work), it becomes disorganized at the level of the posterior region of the nucleus which never reaches the posterior extremity of the gamete. Taeniarhynchus saginatus spermatozoon is distinguished by the fact that its axoneme is accompanied on all its posterior length by a nucleus that stops its helicoidal course at the posterior extremity of the spermatozoon.
The rostellum is a structure of taxonomic importance in Taeniidae. Taeniarhynchus has been established for the Taeniidae having (or not) a rudimentary rostellum but lacking rostellar hooklets that are typical to the genus Taenia. Thus, Taenia saginata becomes Taeniarhynchus saginatus by the absence of rostellar hooklets. The validity of the genus Taeniarhynchus has been largely debated by several authors. Verster (1969) did not accept the validity of the genus Taeniarhynchus. He considered that a single character could not justify the erection of a new genus. According to Khalil et al. (1994), the genera Taenia and Taeniarhynchus are synonyms. On the other hand, Wardle and McLeod (1952) and Schmidt (1986), considering the fact that Taenia saginata does not have any rostellum nor hooklets on its scolex, accepted the validity of the genus Taeniarhynchus.
Our work seems to support the validity of the genus Taeniarhynchus, since it is the only Taeniidae, known until now, to have the nucleus at the posterior extremity of the spermatozoon.
References
Bâ CT, Marchand B (1993) Ultrastructure of the Retinometra serrata spermatozoon (Cestoda), intestinal parasite of turtle doves in Senegal. J Submicrosc Cytol Pathol 25:233–238
Bâ CT, Marchand B (1994a) Ultrastructure of spermiogenesis and the spermatozoon of Raillietina (R.) tunetensis (Cyclophyllidea, Davaineidae) intestinal parasite of turtle doves in Senegal. Int J Parasitol 24:237–248
Bâ CT, Marchand B (1994b) Comparative ultrastructure of the spermatozoa of Inermicapsifer guineensis and I. madagascariensis (Cestoda, Anoplocephalidea, Inermicapsiferinae), intestinal parasites of rodents in Senegal. Can J Zool 72:1633–1638
Bâ CT, Marchand B (1994c) Ultrastructure of the spermatozoon of Avitellina centripunctata (Cestoda, Cyclophyllidea) a parasite of the small intestine of cattle in Senegal. Acta Zool-Stockholm 75:167–175
Bâ CT, Marchand B (1994d) Simulitude ultrastructurale des spermatozoïdes de quelques Cyclophyllidea. Parasite 1:51–55
Bâ CT, Marchand B, Mattei X (1991) Demontration of the orientation of the Cestoda spermatozoon illustrated by the ultrastructural study of spermiogenesis and the spermatozoon of a Cyclophyllidea: Thysaniezia ovilla Rivolta, 1874. J Submicrosc Cytol Pathol 23:605–612
Bâ A, Bâ CT, Marchand B (2000) Ultrastructure of spermiogenesis and the spermatozoon of Sudarikovina taterae (Cestoda, Cyclophyllidea, Anoplocephalidae) intestinal parasite of Tatera gambiana (Rodentia, Gerbillidae). J Submicrosc Cytol Pathol 24:29–34
Bâ A, Bâ CT, Marchand B (2002) Ultrastructural study of the spermatozoon of Echinocotyle dolosa (Cestoda, Cyclophyllidea, Hymenolepididae). Acta Parasitol 47:131–136
Bâ CT, Bâ A, Marchand B (2005a) Utrastructure of the spermatozoon of Raillietina (R) baeri (Cyclophyllidea, Davaineidae) an intestinal parasite of the multimammate rat, Mastomys huberti (Rodentia, Muridae). Parasitol Res 97:173–178
Bâ CT, Bâ A, Marchand B (2005b) Ultrastructure of the spermatozoon of Paroniella reynoldsae (Cyclophyllidea, Davaineidae) an intestinal parasite of Corvus albus (Aves, Corvidae). Acta Parasitol 50:208–214
Eom KS, Rim HJ (1993) Morphologic description of Taenia asiatica n. sp. Korean J Parasitol 31:1–6
Featherston DW (1971) Taenia hydatigena. III. Light and electron microscope study of spermiogenesis. Parasitol Res 37:148–168
Khalil LF, Jones A, Bray RA (1994) Keys to the cestode parasites of vertebrates. Cambridge University press, Wallingford
Miquel J, Marchand B (1998) Ultrastructure of the the spermatozoon of the bank vole tapeworm, Paranoplocephala omphalodes (Cestoda, Cyclophyllidea, Anoplocephalidae). Parasitol Res 84:239–245
Miquel J, Hidalgo C, Feliu C, Marchand B (2000) Sperm ultrastructure of Taenia mustelae (Cestoda, Taeniidae), an intestinal parasite of the weasel, Mustela nivalis (Carnivora). Ivertebr Reprod Dev 38:43–51
Miquel J, Swiderski Z, Mlocicki D, Marchand B (2004) Ultrastructure of the spermatozoon of the anoplocephalid Cestode Gallegoides arfaai (Mobedi and Ghadirian 1977) Tenora and Mas-coma, 1978, an intestinal parasite of the wood mouse (Apodemus sylvaticus Linnaeus,1758). Parasitol Res 94:460–467
Miquel J, Hidalgo C, Foronda P, Torres J, Swiderski Z, Feliu C (2009a) Ultrastructural study of the spermatozoon of Taenia taeniaeformis (Batsch, 1786) (Cestoda, Cyclophyllidea, Taeniidae), an intestinal parasite of Felis catus from La Palma (Canary islands, Spain). Parasitol Res 104:1477–1483
Miquel J, Swiderski Z, Foronda P, Torres J, Feliu C (2009b) Ultrastructure of spermatogenesis of Taenia taeniaeformis (Batsch, 1786) (Cestoda, Cyclophyllidea, Taeniidae) and comparison of spermatological characters in the family Taeniidae Ludwig, 1886. Acta Parasitol 54:230–243
Ndiaye PI, Miquel J, Marchand B (2002) Ultrastructure of spermiogenesis and spermatozoa of Taenia parva Baer, 1926 (Cestoda, Cyclophyllidea, Taeniidea), a parasite of the common genet (Genetta genetta). Parasitol Res 89:34–43
Schmidt GD (1986) Handbook of tapeworm identification. CRC Press, Inc, Boca, Ranton Florida
Verster A (1969) A taxonomic revision of the genus Taenia Linnaeus, 1758 Sensu strictu. Onderstepoort J Vet Res 36:3–58
Wardle RA, McLeod JA (1952) The zoology of tapeworm. University of Minnesota Press, Minneapolis
Willms K, Caro JA, Robert L (2003) Ultrastructure of spermatogonia and spermatocyte lobules in Taenia solium strobilae (Cestoda, Cyclophyllidea, Taeniidae) from golden hamsters. Parasitol Res 90:479–488
Willms K, Robert L, Jiménez JA, Everhart M, Kuhn RE (2004) Ultrastructure of spermiogenesis and the spermatozoon in Taenia crassiceps Strobilae WFU Strain (Cestoda, Cyclophyllidea, Taeniidae) from golden hamsters. Parasitol Res 93:262–267
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bâ, A., Bâ, C.T., Quilichini, Y. et al. Ultrastructure of the spermatozoon of Taeniarhynchus saginatus (syn. Taenia saginata) (Goeze, 1782) Weinland, 1858 (Cestoda, Taeniidae) an intestinal parasite of human. Parasitol Res 108, 831–836 (2011). https://doi.org/10.1007/s00436-010-2125-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2125-2