Abstract
The present work involves an ultrastructural study of the mature spermatozoon of the anoplocephalid cestode Gallegoides arfaai (Mobedi and Ghadirian, 1977) Tenora and Mas-Coma, 1978, obtained from the small intestine of naturally infected wood mice, Apodemus sylvaticus Linnaeus, 1758 (Rodentia, Muridae). The mature spermatozoon of G. arfaai is a filiform cell, tapered at both ends and lacking mitochondria. It is characterized by the presence of a 1,000-nm-long apical cone and two 140-nm-thick crest-like bodies in its anterior extremity. The axoneme, of the 9+’1’ trepaxonematan pattern, lacks a periaxonemal sheath, and disorganization occurs at the level of the nuclear region of the sperm cell. The cortical microtubules form two to four fields according to the different regions of the male gamete. They are twisted at an angle of about 35°, becoming parallel towards the posterior extremity of spermatozoon. The nucleus, spiralled around the axoneme, shows an irregular shape in both longitudinal and cross-sections. Numerous electron-dense granules were observed, which transform into an electron-dense material in the posterior extremity of the cell. Moreover, we describe for the first time the total length of the anterior region of sperm containing the helical crest-like bodies. This anterior extremity measures around 15 μm and presents two helical crest-like bodies of different lengths that describe 13–14 turns around the sperm body. Our ultrastructural results on the G. arfaai spermatozoon are compared with the ultrastructural organization of the spermatozoa of other previously studied species, with particular emphasis on the anoplocephalids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ultrastructural characters of the spermatozoa of Platyhelminthes are useful in the interpretation of the relationships within this group of parasites (Euzet et al. 1981; Swiderski 1986; Justine 1991, 1995, 1998, 2001; Bâ and Marchand 1995; Hoberg et al. 1997; Xylander 2001). Several ultrastructural characters present during spermiogenesis and in the spermatozoon have been established as synapomorphies for the major groups of Platyhelminthes. In fact, the absence of mitochondria as a synapomorphy for the Eucestoda has been used by Ehlers (1984, 1985a, 1985b, 1986) and Brooks (1989). Furthermore, the twisting pattern of cortical microtubules is a synapomorphy, in this case, for the Tetrabothriidea and Cyclophyllidea (Justine 1991, 2001). The presence of one or more crest-like bodies in the anterior extremity of the spermatozoon has also been considered as a synapomorphy for the Eucestoda (Bâ and Marchand 1995). However, there are presently several characters detected in the mature sperm, the usefulness of which as phylogenetic tools is yet to be described, for example the angle of rotation of the cortical microtubules, the morphology of the nucleus, the thickness of the crest-like bodies and the morphometry of the apical cone, among others. Nevertheless, several characters, such as the presence or absence of a periaxonemal sheath, electron-dense granules or intracytoplasmic walls will probably play an interesting role in the future interpretation of the relationships between the different families of cyclophyllideans (Justine 1998).
Within the order Cyclophyllidea, the family Anoplocephalidae Cholodkowsky, 1902 has already been extensively studied from the ultrastructural point of view. The family Anoplocephalidae comprises four subfamilies: Anoplocephalinae Blanchard, 1891, Inermicapsiferinae López-Neyra, 1943, Linstowinae Fuhrmann, 1907 and Thysanosomatinae Skrjabin, 1933. Ultrastructural studies on spermatology have been carried out on several species of these subfamilies, particularly in the Anoplocephalinae. To date, ultrastructural studies on the sperm of anoplocephalid cestodes refer to 13 species belonging to 11 genera (Swiderski 1968, 1984; MacKinnon and Burt 1984; Swiderski and Subilia 1985; Bâ et al. 1991, 2000; Bâ and Marchand 1992a, 1992b, 1994a, 1994b, 1994c, 1994d; Miquel and Marchand 1998a, 1998b; Li et al. 2003). The present paper describes the first ultrastructural study of the spermatozoon of a species belonging to the genus Gallegoides Tenora and Mas-Coma, 1978 and increases the available data on spermatology of the anoplocephalids.
Materials and methods
Adult specimens of Gallegoides arfaai (Mobedi and Ghadirian, 1977) Tenora and Mas-Coma, 1978 were obtained live from the small intestine of naturally infected wood mice, Apodemus sylvaticus Linnaeus, 1758 (Rodentia, Muridae) captured in Mosset and in the Natural Reserve of Py (Pyrenean Mountains, France). The living cestodes were placed in a 0.9% NaCl solution. Mature proglottids of these cestodes were routinely processed for transmission electron microscopy examination; they were fixed in cold (4°C) 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer at pH 7.2 for 1 h, rinsed in a 0.1 M sodium cacodylate buffer at pH 7.2, postfixed in cold (4°C) 1% osmium tetroxide in the same buffer for 1 h, rinsed in a 0.1 M sodium cacodylate buffer at pH 7.2, dehydrated in an ethanol series and propylene oxide, and finally embedded in Spurr’s resin. Ultrathin sections were obtained using a Reichert-Jung Ultracut E ultramicrotome, placed on copper grids and double-stained with uranyl acetate and lead citrate according to Reynolds (1963). Ultrathin sections were examined using a Jeol 1010 transmission electron microscope.
Results
The observation of numerous sections of mature spermatozoa of G. arfaai contained in the seminal vesicle in the mature proglottids allows the establishment of five different regions in the mature sperm of this anoplocephalid according to its distinct ultrastructural features.
Region I
This region constitutes the anterior extremity of the spermatozoon. It measures about 15 μm in length (Fig. 1) and its maximum width is around 475 nm. It is characterized by the presence of a slightly electron-dense apical cone measuring 1,000 nm in length and 275 nm in width at the base (Figs. 1, 2, 3), and two crest-like bodies which have different lengths and one of which initiates its helical course around the sperm cell at the level of the apical cone (Figs. 2, 3, 4). These crest-like bodies are spiralled around the spermatozoon (Figs. 1, 3, 4, 5, 6, 7). They have a maximum thickness of 140 nm (Figs. 3, 6) and describe 13–14 turns around the sperm body (Fig. 1). In the apical cone, the cortical microtubules are parallel and grouped (Figs. 2, 4). Later, they become twisted at an angle of 35° to the hypothetical spermatozoon axis. The cortical microtubules constitute an electron-dense submembranous layer formed by two fields partially covering each other (Figs. 5, 6). These two fields of cortical microtubules are separated from each other by submembranous electron-dense material which constitutes the above mentioned crest-like bodies. The centrally located axoneme of the 9+’1’ pattern of the trepaxonematan Platyhelminthes lacks a periaxonemal sheath (Figs. 5, 6). At the base of the apical cone, the central core of the 9+’1’ axoneme appears apically from the peripheral doublets (Figs. 2, 3, 4). The crest-like bodies progressively reduce their thickness and disappear (Figs. 1, 5, 7).
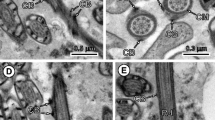
Longitudinal section of region I. Ase Anterior spermatozoon extremity, Cb crest-like bodies. Bar 2 μm
Cross-section of region I at the base of the apical cone. Notice the appearance of the central core (Cc) of axoneme before the doublets. A parallel group of cortical microtubules (Cm) is also present. Bar 0.2 μm
Longitudinal section of region I showing the apical cone (Ac). The arrowhead indicates the beginning of the longest crest-like body. Ase Anterior spermatozoon extremity, Ax axoneme, Cb crest-like bodies. Bar 0.5 μm
Another cross-section of region I at the level of the base of the apical cone showing the first crest-like body (Cb). Cm Cortical microtubules. Bar 0.2 μm
Cross-section of the mature spermatozoon at the level of the end of region I. Cb Crest-like bodies. Bar 0.2 μm
Cross-section of region I showing the maximum thickness of crest-like bodies (Cb). Ax Axoneme. Bar 0.2 μm
Longitudinal section of the transition between regions I and II. Cb Crest-like bodies. Bar 0.5 μm
Cross-section of region II showing the spiralled layer of cortical microtubules formed by three fields (arrowheads). Bar 0.2 μm
Region II
Figures 1, 7, 8, 9, 10 show region II, which is characterized by the absence of crest-like bodies. Its maximum width is around 425 nm. In this region, the twisted submembranous and electron-dense layer of cortical microtubules is divided into two to four discontinuous bundles (Figs. 8, 10).
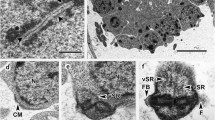
Longitudinal sections of regions II and III. G Dense granules. Bar 0.5 μm
Cross-section of the region II showing the spiralled layer of cortical microtubules formed by four fields (arrowheads). Bar 0.2 μm
Cross-section of region III. The spiralled layer of cortical microtubules is formed by three fields. G Dense granules. Bar 0.2 μm
Cross-section of region IV showing the simultaneous presence of dense granules (G) and nucleus (N). The cortical microtubules form three fields. Bar 0.2 μm
Longitudinal section of the transition between regions III and IV. G Dense granules, N nucleus. Bar 0.5 μm
Longitudinal section of the transition of regions IV and V. Notice the disruption of the axoneme at the level of the nuclear area. Ape Axonemal posterior extremity, G dense granules, N nucleus. Bar 0.5 μm
Cross-section of region IV showing the beginning of the disorganization of the axoneme. Notice the irregular morphology of the nucleus (N). Bar 0.2 μm
Cross-section of region V. Notice that several cortical microtubules (Cm) are parallel to the spermatozoon axis. N Nucleus. Bar 0.2 μm
Longitudinal section of region V. The nuclear posterior extremity (Npe) nearly reaches the posterior spermatozoon extremity (Pse). The cortical microtubules (Cm) become parallel to the spermatozoon axis. Bar 0.5 μm
Cross-section of region V near the posterior spermatozoon extremity. Cm Cortical microtubules, Pd posterior dense material. Bar 0.2 μm
Region III
Region III is characterized by the presence of electron-dense granular material (Figs. 9, 11). In this region, the maximum width of the spermatozoon increases to around 750 nm. The submembranous and spiralled layer of cortical microtubules forms three fields (Fig. 11).
Region IV
Region IV constitutes the principal nuclear region of the sperm, in which both the axoneme and nucleus coexist (Figs. 12, 13, 14, 15). It also contains electron-dense granules (Figs. 12, 13, 14, 15). Its maximum width is around 800 nm. In this region the twisted cortical microtubules form three fields (Figs. 12, 15). The nucleus is spiralled around the axoneme and shows an irregular shape in both cross and longitudinal sections (Figs. 12, 13, 14, 15). In certain areas of this region, the nucleus surrounds the axoneme more than once (Fig. 15). In the posterior areas the axoneme becomes disorganized and disappears (Fig. 14).
Region V
Region V (Figs. 14, 16, 17, 18) constitutes the posterior extremity of the spermatozoon presenting a maximum width of about 650 nm. It lacks an axoneme, and in this region it is possible to observe the posterior nuclear extremity (Fig. 17). The postnuclear area of the mature spermatozoon of G. arfaai measures around 1.4 μm in length (Fig. 17). The spiralled cortical microtubules progressively become parallel reaching the posterior tip of the spermatozoon (Figs. 16, 17, 18). In this extremity, the electron-dense granules progressively transform into a posterior electron-dense material (Figs. 17, 18).
Schematic diagram
Figure 19 is a schematic diagram of the entire mature spermatozoon showing regions I–V.
Diagram showing the ultrastructural organization of the mature spermatozoon of G. arfaai. Aae Axonemal anterior extremity, Ac apical cone, Ase anterior spermatozoon extremity, Ape axonemal posterior extremity, Ax axoneme, Cb crest-like bodies, Cm cortical microtubules, D doublets, G dense granules, N nucleus, Npe nuclear posterior extremity, Pd posterior dense material, Pm plasma membrane, Pse posterior spermatozoon extremity
Discussion
The mature spermatozoon of G. arfaai, as with other cyclophyllideans, is characterized by the presence of a single axoneme of the 9+’1’ pattern of the trepaxonematan Platyhelminthes (Ehlers 1984, 1985a, 1985b, 1986). It also presents crest-like bodies and lacks mitochondria as in all of the Eucestoda studied to date (Bâ and Marchand 1995).
The crest-like body or bodies always characterize the anterior extremity of the spermatozoon. They constitute a synapomorphy for the Eucestoda (Bâ and Marchand 1995). In the anoplocephalids, most of the species have one or two crest-like bodies in the anterior tip of the sperm. Only Aporina delafondi (Bâ and Marchand 1994a) with five crest-like bodies and Sudarikovina taterae (Bâ et al. 2000) with seven crest-like bodies differ from this pattern. Other than these, all of the species of the subfamily Anoplocephalinae present two crest-like bodies (Table 1). The anterior tip of the spermatozoon presently studied is very similar to two other Anoplocephalinae species: Anoplocephaloides dentata (Miquel and Marchand 1998a) and Paranoplocephala omphalodes (Miquel and Marchand 1998b). Both morphology and morphometry of the anterior spermatozoon extremity are quite similar in these three species (1,000 nm in G. arfaai versus 1,400 nm and 900 nm in A. dentata and P. omphalodes, respectively). They are also very similar in terms of their crest-like bodies, with a thickness of between 140 nm in A. dentata (Miquel and Marchand 1998a), and 180 nm in P. omphalodes (Miquel and Marchand 1998b), and G. arfaai (this study).
The anterior extremity of the spermatozoon of G. arfaai constitutes the most interesting aspect of this study. Its total length and the number of turns described by the crest-like bodies around the sperm body were observed for the first time. To date, even though the ultrastructure of the mature spermatozoon has been studied in more than 75 species of Eucestoda (see Ndiaye 2003) this character (length and number of turns described by the crest-like bodies) has never been observed. It has been demonstrated in several studies that the spermatozoon of Platyhelminthes measures around 300 μm in length. Considering that the anterior extremity (region I) of the spermatozoon of G. arfaai measures about 15 μm, it is relatively short compared to the total length of the mature spermatozoon. Another interesting aspect observed in the mature spermatozoon of G. arfaai is the occurrence of the central element of the 9+’1’ trepaxonematan axoneme before the formation of the nine peripheral doublets. This occurs at the base of the apical cone where a single crest-like body (the longest one) is present. The same was described in A. dentata and P. omphalodes (Miquel and Marchand 1998a, 1998b).
The spiralled pattern of cortical microtubules has been postulated as a synapomorphy for the Tetrabothriidea and Cyclophyllidea (Justine 1991, 2001). However, there are two species which seem to disrupt this concept, the mesocestoidid cyclophyllidean Mesocestoides litteratus (Miquel et al. 1999), which exhibits a parallel disposition of cortical microtubules, and the proteocephalidean Sandonella sandoni (Bâ and Marchand 1994e), which presents twisted cortical microtubules. In fact, except for M. litteratus (see Miquel et al. 1999), all of the cyclophyllideans studied to date present spiralled cortical microtubules. The angle of the cortical microtubules to the hypothetical spermatozoon axis ranges from 15° to 60° in species of the order Cyclophyllidea (see Ndiaye 2003). In the case of anoplocephalids, this angle of rotation varies from 15° in A. delafondi (Bâ and Marchand 1994a) to 50° in Stilesia globipunctata and Thysaniezia ovilla (Bâ et al. 1991, Bâ and Marchand 1992a). The angle of cortical microtubules in the mature spermatozoon of G. arfaai is within this range with a value of 35°. In G. arfaai, the submembranous layer of cortical microtubules is constituted by several fields (two to four, according to the different regions) which cover each other. A similar pattern is observed in three other anoplocephalids, Moniezia benedeni, M. expansa and T. ovilla (Bâ et al. 1991, Bâ and Marchand 1992b).
According to Justine (1998), there is mutual exclusion between the character dense granules and the paired characters periaxonemal sheath and transverse intracytoplasmic walls. In our study, the presence of electron-dense granules and the simultaneous absence of a periaxonemal sheath and transverse intracytoplasmatic walls are in agreement with previous findings by other authors (see Justine 1998). For the Anoplocephalinae, all of the species studied to date present the same characters. These are A. dentata (Miquel and Marchand 1998a), A. delafondi (Bâ and Marchand 1994a), M. benedeni and M. expansa (Bâ and Marchand 1992b), Monoecocestus americanus (MacKinnon and Burt 1984), P. omphalodes (Miquel and Marchand 1998b) and S. taterae (Bâ et al. 2000). The same characters are also present in the mature spermatozoon of the Thysanosomatinae T. ovilla (Bâ et al. 1991). On the other hand, the opposed features are observed in the two representatives of Inermicapsiferinae studied to date, Inermicapsifer guineensis and I. madagascariensis (Bâ and Marchand 1994b). In the present work, we corroborate the homogeneity of these three features in species of the subfamily Anoplocephalinae.
Concerning the posterior areas of spermatozoon within the anoplocephalids, the disorganization of the axoneme at the level of the nuclear region has been previously described only for the mature spermatozoon of P. omphalodes (Miquel and Marchand 1998b). In general, most of Platyhelminthes show the disappearance of the central core of the axoneme before disorganization of the peripheral doublets. Nevertheless, in G. arfaai, in an initial stage of disorganization of the 9+’1’ axoneme, the displacement of doublets occurs before the disappearance of the central core. A posterior extremity constituted by electron-dense material, such as occurs in G. arfaai, has been observed in the following species: A. dentata (Miquel and Marchand 1998a), A. delafondi (Bâ and Marchand 1994a), P. omphalodes (Miquel and Marchand 1998b), I. guineensis and I. madagascariensis (Bâ and Marchand 1994b), Mathevotaenia herpestis (Bâ and Marchand 1994c), and T. ovilla (Bâ et al. 1991). In all of these species, with the exception of I. guineensis, I. madagascariensis and M. herpestis (Bâ and Marchand 1994b, 1994c), this posterior electron-dense material seems to result from the condensation of the granular material.
In conclusion, more ultrastructural studies on spermatozoa are needed to establish possible differences in the ultrastructural organization of sperm between the different families of Cyclophyllidea in general and, more particularly, to the subfamilies of the Anoplocephalidae. Nevertheless, according to the present ultrastructural studies, the presence of granular material and the absence of both a periaxonemal sheath and intracytoplasmic walls are three constant characters in all of the species of the subfamily Anoplocephalinae.
References
Bâ CT, Marchand B (1992a) Ultrastructural particularities of the spermatozoon of Stilesia globipunctata (Cestoda) parasite of the small intestine of sheep and goats in Senegal. J Submicrosc Cytol Pathol 24:29–34
Bâ CT, Marchand B (1992b) Étude ultrastructurale sur le spermatozoïde de Moniezia expansa et M. benedeni (Cestoda, Cyclophyllidea, Anoplocephalidae). Ann Parasitol Hum Comp 67:111–115
Bâ CT, Marchand B (1994a) Ultrastructure of spermiogenesis and the spermatozoon of Aporina delafondi (Cyclophyllidea, Anoplocephalidae) intestinal parasite of turtle doves in Senegal. Int J Parasitol 24:225–235
Bâ CT, Marchand B (1994b) Comparative ultrastructure of the spermatozoa of Inermicapsifer guineensis and I. madagascariensis (Cestoda, Anoplocephalidae, Inermicapsiferinae) intestinal parasites of rodents in Senegal. Can J Zool 72:1633–1638
Bâ CT, Marchand B (1994c) Ultrastructure of spermiogenesis and the spermatozoon of Mathevotaenia herpestis (Cestoda), intestinal parasite of Atelerix albiventris in Senegal. Acta Zool (Stockh) 75:167–175
Bâ CT, Marchand B (1994d) Ultrastructure of the spermatozoon of Avitellina centripunctata (Cestoda, Cyclophyllidea), a parasite of the small intestine of cattle in Senegal. Acta Zool (Stockh) 75:161–166
Bâ CT, Marchand B (1994e) Ultrastructure of the spermatozoon of Sandonella sandoni (Cestoda, Proteocephalidea, Sandonellinae). Invert Reprod Dev 25:9–17
Bâ CT, Marchand B (1995) Spermiogenesis, spermatozoa and phyletic affinities in the Cestoda. In: Jamieson BGM, Ausió J, Justine JL (eds) Advances in spermatozoal phylogeny and taxonomy. Mem Mus Natl Hist Nat 166:87–95
Bâ CT, Marchand B, Mattei X (1991) Demonstration of the orientation of the Cestodes spermatozoon illustrated by the ultrastructural study of spermiogenesis of a Cyclophyllidea: Thysaniezia ovilla, Rivolta, 1874. J Submicrosc Cytol Pathol 23:606–612
Bâ A, Bâ CT, Marchand B (2000) Ultrastructure of spermiogenesis and the spermatozoon of Sudarikovina taterae (Cestoda, Cyclophyllidea, Anoplocephalidae) intestinal parasite of Tatera gambiana (Rodentia, Gerbillidae). J Submicrosc Cytol Pathol 32:137–144
Brooks DR (1989) A summary of the database pertaining to the phylogeny of the major groups of parasitic platyhelminths, with a revised classification. Can J Zool 67:714–720
Ehlers U (1984) Phylogenetisches System der Plathelminthes. Verh Naturwiss Ver Hamb (NF) 27:291–294
Ehlers U (1985a) Phylogenetic relationships within the Platyhelminthes. In: Conway Morris S, George JD, Gibson R, Platt HM (eds) The origins and relationships of lower invertebrates. Oxford University Press, Oxford, pp 143–158
Ehlers U (1985b) Das Phylogenetische System der Plathelminthes. G Fischer, Stuttgart
Ehlers U (1986) Comments on a phylogenetic system of the Platyhelminthes. Hydrobiologia 132:1–12
Euzet L, Swiderski Z, Mokhtar-Maamouri F (1981) Ultrastructure comparée du spermatozoïde des Cestodes. Relations avec la phylogénèse. Ann Parasitol (Paris) 56:247–259
Hoberg EP, Mariaux J, Justine J-L, Brooks DR, Weekes PJ (1997) Phylogeny of the orders of the Eucestoda (Cercomeromorphae) based on comparative morphology: historical perspectives and a new working hypothesis. J Parasitol 83:1128–1147
Justine J-L (1991) Phylogeny of parasitic Platyhelminthes: a critical study of synapomorphies proposed on the basis of the ultrastructure of spermiogenesis and spermatozoa. Can J Zool 69:1421–1440
Justine J-L (1995) Spermatozoal ultrastructure and phylogeny of the parasitic Platyhelminthes. In: Jamieson BGM, Ausió J, Justine JL (eds) Advances in spermatozoal phylogeny and taxonomy. Mem Mus Natl Hist Nat 166:55–86
Justine J-L (1998) Spermatozoa as phylogenetic characters for the Eucestoda. J Parasitol 84:385–408
Justine J-L (2001) Spermatozoa as phylogenetic characters for the Platyhelminthes. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor and Francis, London, pp 231–238
Li H-Y, Brennan JP, Halton DW (2003) Spermatogenesis, spermiogenesis and spermatozoon in the cestode (Moniezia expansa) (Cyclophyllidea, Anoplocephalidae). Acta Zool Sin 49:370–379
MacKinnon BM, Burt MDB (1984) The comparative ultrastructure of spermatozoa from Bothrimonus sturionis Duv. 1842 (Pseudophyllidea), Pseudanthobothrium hanseni Baer, 1956 (Tetraphyllidea), and Monoecocestus americanus Stiles, 1895 (Cyclophyllidea). Can J Zool 62:1059–1066
Miquel J, Marchand B (1998a) Ultrastructure of spermiogenesis and the spermatozoon of Anoplocephaloides dentata (Cestoda, Cyclophyllidea, Anoplocephalidae), an intestinal parasite of Arvicolidae rodents. J Parasitol 84:1128–1136
Miquel J, Marchand B (1998b) Ultrastructure of the spermatozoon of the bank vole tapeworm, Paranoplocephala omphalodes (Cestoda, Cyclophyllidea, Anoplocephalidae). Parasitol Res 84:239–245
Miquel J, Feliu C, Marchand B (1999) Ultrastructure of spermiogenesis and the spermatozoon of Mesocestoides litteratus (Cestoda, Mesocestoididae). Int J Parasitol 29:499–510
Ndiaye PI (2003) Systématique et phylogénie de Plathelminthes parasites (Trematoda et Cestoda): apports des études ultrastructurales de la reproduction. PhD Thesis, University of Barcelona, Barcelona, http://www.tdx.cesca.es/
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Swiderski Z (1968) The fine structure of the spermatozoon of sheep tapeworm, Monieza expansa (Rud., 1810) (Cyclophyllidea, Anoplocephalidae). Zool Pol 18:475–486
Swiderski Z (1984) Ultrastructure of the spermatozoon of the davaineid cestode Inermicapsifer madagascariensis. Proceedings of the Electron Microscopy Society of South Africa, 23th Annual Conference, Stellenbosch, South Africa, pp 131–132
Swiderski Z (1986) Three types of spermiogenesis in cestodes. Proceedings of the XIth International Congress on Electron Microscopy, Kyoto, Japan, pp 2959–2960
Swiderski Z, Subilia L (1985) Ultrastructure of the spermatozoon of the cestode Oochoristica agamae (Cyclophyllidea, Linstowiidae). Proceedings of the Electron Microscopy Society of South Africa, 24th Annual Conference, Pietermaritzburg, South Africa, pp 185–186
Xylander WER (2001) The Gyrocotylidea, Amphilinidea and the early evolution of Cestoda. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor and Francis, London, pp 103–111
Acknowledgements
The authors wish to thank IDES (Mosset) and the staff of the Nature Reserve of Py (Claude Guisset and David Morichon, in particular) (Pyrenean Mountains, France) for their hospitality and valuable help in the fieldwork. We also thank “Serveis Científico-Tècnics” of the University of Barcelona for their support in the preparation of samples. This study was partially supported by the Spanish projects 2001-SGR-00088 from the “Departament d’Universitats, Recerca i Societat de la Informació (Generalitat de Catalunya)” and HF2002-0063 from the “Ministerio de Ciencia y Tecnología”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miquel, J., Swiderski, Z., Młocicki, D. et al. Ultrastructure of the spermatozoon of the anoplocephalid cestode Gallegoides arfaai (Mobedi and Ghadirian, 1977) Tenora and Mas-Coma, 1978, an intestinal parasite of the wood mouse (Apodemus sylvaticus Linnaeus, 1758). Parasitol Res 94, 460–467 (2004). https://doi.org/10.1007/s00436-004-1238-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-004-1238-x