Abstract
This study was performed to determine the prevalence and genotypes of Cryptosporidium species among HIV patients and cattle in Thailand. Stool specimens were collected from 46 HIV patients from Prabat Nampu Temple, Lop Buri Province in central Thailand. Two hundred fecal samples from dairy cattle were collected from seven farms in Chon Buri Province, the eastern part of Thailand. Each sample was concentrated by Sheather’s sucrose flotation technique and stained by acid fast stain (AFS) for the identification of oocysts by microscopy. All HIV stool samples and 83 fecal specimens from cattle were further tested using nested polymerase chain reaction (PCR) targeting the 18S SSUrRNA gene to characterize the detected species. In HIV patient samples, the detection rate was 28.7% by AFS and 4.35% by nested PCR. In cattle samples, the detection rate was 13% by AFS and 9.63% by nested PCR. After DNA sequencing results, we identified the genotypes of the Cryptosporidium from seven of the PCR positive samples. All were found to be C. parvum. The findings presented here represent the first genetic identification of Cryptosporidium species in cattle in Thailand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryptosporidium spp. are among the most important coccidian parasites of humans, as well as of other animals, birds, reptiles, and fish. The ingestion of low numbers of oocysts can cause severe diarrheal disease (cryptosporidiosis). In humans, healthy individuals may clear the infection in less than a month, but those whose immune systems are compromised, particularly AIDS patients, transplant patients, and cancer patients, suffer prolonged and potentially fatal episodes of diarrhea. Cryptosporidiosis may occur sporadically or as outbreaks following zoonotic transmission from farm animals, person-to-person spread, or the contamination of water supplies (Casemore 1991).
New species and genotypes of Cryptosporidium have been identified in recent years and there is evidence that more than one Cryptosporidium species is involved in human disease (Hunter and Thompson 2005; Xiao et al. 2004). Several species of Cryptosporidium have been described including C. parvum and C. hominis which are the most common species causing human disease (Hunter and Thompson 2005). Other species have occasionally been reported to cause human illness including C. felis, C. meleagridis, C. canis, C. muris, C. andersoni, and C. suis (Read et al. 2004; Leoni et al. 2006). Currently, a total of 16 Cryptosporidium species are considered to be valid, but C. hominis and C. parvum appear to be most widely distributed (Slapeta 2006). An important difference between these two genotypes is that C. hominis appears largely responsible for human to human transmission, while C. parvum is typically responsible for animal to human transmission. Cattle have been described as major hosts for C. parvum, C. bovis, Cryptosporidium deer-like genotype, and C. andersoni, and several studies have shown host age-related susceptibility: C. parvum predominates in pre-weaned calves, C. bovis and the deer-like genotype in post-weaned calves, and C. andersoni in older calves and adult cattle (Santin et al. 2004; Slapeta 2006).
The presence of Cryptosporidium spp. oocysts in water is increasingly recognized as a problem throughout the world (Karanis et al. 2007a). Drinking water sources can become contaminated through introduction of infected feces from wild and/or domestic animals or from sewage discharged to surface waters. Outbreaks have been reported worldwide attributed to a combination of disinfection resistance of oocysts and water treatment deficiencies allowing passage of sufficient numbers of protozoa to cause illness (Karanis et al. 2007a).
In Thailand, the most prevalence information on Cryptosporidium in human and cattle has been based on microscopy (Uga et al. 1998; Saksirisampant et al. 2002; Tiangtip and Jongwutiwes 2002; Gatei et al. 2002). Accordingly, information on Cryptosporidium species or genotypes is rare. The aim of this work was to determine the genotypes of Cryptosporidium species among AIDS patients and dairy cattle in Thailand.
Materials and methods
Specimen collection, Cryptosporidium oocyst purification, and microscopic examination
Fecal samples were randomly collected from 46 HIV patients (ranged in age from 24 to 62 years) from Prabat Nampu Temple, Lop Buri Province in central Thailand. Further 200 samples from young dairy cattle (ranged in age from 10 days to 4 months) were collected from five farms in Chon Buri Province, in eastern Thailand. Samples were collected between January and August, 2007. Oocysts from each sample were concentrated by Sheather’s sucrose flotation technique and discontinuous sucrose gradient concentration. Harvested concentrates were washed in distilled water and sedimented by centrifuging. The pellets were stained by the acid fast stain (AFS). Microscopic examination was performed in all samples (Ongerth and Stibbs 1987). The fecal samples were preserved in 2.5% potassium dichromate and stored at 4°C. An aliquot of 200 μl of the sample suspension in 2.5% potassium dichromate was used for genotypic analysis.
Extraction of Cryptosporidium genomic DNA
Altogether, 129 samples (46 human and 83 cattle) were subjected to DNA extraction and nested polymerase chain reaction (PCR) analysis of the SSUrRNA gene according to Nichols et al. (2003), Plutzer and Karanis (2007), and Karanis et al. (2007b). DNA was extracted from fecal samples using the QIAmp DNA Stool Mini Kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer’s descriptions, with the addition of three 10-min freeze–thaw cycles after resuspension in lysis solution in order to rupture the Cryptosporidium oocysts. Liquid nitrogen was used for freezing, and thawing was carried out at 70°C in a Dry Thermo unit (DTU-2B, Taitec). DNA was eluted in 100 μl buffer (Qiagen) and stored at −20°C until use.
Amplification of Cryptosporidium DNA by nested PCR
The 18S SSUrRNA nested PCR was performed as described by Nichols et al. (2003) and reported in previous investigations in our laboratory (Plutzer and Karanis 2007; Karanis et al. 2007b). Briefly, first the PCR product of a 655–667-bp segment, and second, the PCR product of a 435-bp segment were amplified in standard mixtures of 25 μl containing 400 nmol (1 μl) of each SSUrRNA specific primer, 200 μM dNTP (2.5 μl), 1.5 mM MgCl2 (3 μl) and 2.5 U (0.25 μl) HotStarTaq DNA polymerase (Qiagen), and 5 μl of DNA template. The templates were subjected to 35 amplification cycles (94°C for 30 s, 68°C at primary PCR and 60°C at secondary PCR for 60 s, 72°C for 45 s) followed by 1 cycle of 7 min at 72°C and held at 4°C. The PCR products were analyzed by 1.5% horizontal agarose gel electrophoresis with TAE buffer, then stained with 0.5 μg/ml ethidium bromide. Finally, the gels were visualized and photographed under ultraviolet light.
Sequencing and Cryptosporidium genotyping
Direct sequencing of PCR products was performed for genotyping of Cryptosporidium species. Out of ten positive samples by nested PCR, eight were successfully sequenced. The 18S PCR products were purified using the QIAquick PCR Purification Kit (Qiagen GmbH, Germany) and applied as templates for sequencing using the forward and reverse primers of the nested (secondary) PCR. Sequencing was conducted using the Big Dye Terminator V3.1 Cycle Sequencing Kit (Applied Biosystems, USA) according to the manufacturer’s protocol. An ABI Prism 3100 Genetic Analyzer automated sequencer (Applied Biosystems, USA) was used to analyze the sequencing reactions. The obtained sequences were compared with published sequences of Cryptosporidium species and genotypes on the NCBI server (http://www.ncbi.nlm.nih.gov/BLAST/). Data on the nucleotide sequences reported in this paper are available in the GenBank database under accession numbers EU606194–EU606201.
Results
Cryptosporidium identification in HIV patient specimens
Cryptosporidium oocysts in positive samples from HIV patients when acid-fast-stained appeared bright red, round to ovoid bodies against a pale green background, often showing elongated naked sporozoites. Cryptosporidium oocysts were found in 13 of 46 samples examined from HIV patients (eight females and five males; Table 1). The overall prevalence was 28.7%. Out of 46 HIV patients, 13 (28.26%) were exhibiting diarrhea and 33 (71.74%) were not. Five of the patients with diarrhea (38.46%) and eight without diarrhea (61.53%) were found positive for Cryptosporidium. The rate of Cryptosporidium finding in patients with diarrhea (38.46%) was not significantly different (p > 0.05) than in patients without diarrhea (61.53%). No significant association was found between Cryptosporidium positivity and age or sex in HIV patients (p > 0.05). No further information is available from the patients investigated. By nested PCR, Cryptosporidium species infection was diagnosed in two of 46 (4.35%) HIV patients (Table 1). Sequencing data indicated that the species found in each case was C. parvum.
Cryptosporidium identification in cattle samples
Cryptosporidium oocysts were found in 26 out of 200 (13%) cattle samples examined by AFS (Table 2). Using the nested PCR method in 83 samples, Cryptosporidium species infections were identified in eight out of 83 (9.63%) cattle samples (Table 2).
Genotyping of Cryptosporidium species from HIV patient and cattle samples
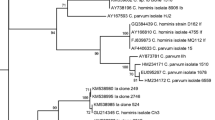
From two of the HIV patient samples, the DNA sequence of the 18S rRNA gene from the nested PCR positive samples matched the published sequence of C. parvum (Table 1). Table 2 shows five of eight successfully sequenced samples out of the eight nested PCR positive cattle samples and all matched the published sequence for C. parvum.
Discussion
In the present study, the prevalence of Cryptosporidium species in HIV positive patients from Prabat Nampu Temple by using acid fast stain was 28.7%. No significant differences were observed between the infection rates observed in both males and females. According to our AFS results, the prevalence of cryptosporidiosis was higher (3.7%) than reported as for example in 1,500 children with acute diarrhea in Thailand (Thamlikitkul et al. 1987). Cryptosporidium was reported in 17 children (8%) from 205 institutionalized orphans 1–61 months of age in Bangkok using the same method (Janoff et al. 1990). Cryptosporidium oocysts were detected in fecal specimens from orphanage children in Nonthaburi Province by a modified cold Kinyoun acid-fast technique (Jongwutiwes et al. 1990; Jirapinyo et al. 2002). Other researchers have found Cryptosporidium oocysts in 10% of Cryptosporidium spp. in HIV seropositive and 2% of those in HIV seronegative subjects in Thailand (Uga et al. 1998; Waywa et al. 2001; Moolasart et al. 1995; Saksirisampant et al. 2002).
In previous studies, in HIV seropositive patients from other countries, ZN staining indicated Cryptosporidium in 37.3% in France (Cotte et al. 1993), 14% in Zambia (Chintu et al. 1995), 21.5% in South Italy (Brandonisio et al. 1999), 50% in Malaysia (Oguntibeju 2006), and 2.2% in Australia (Stark et al. 2007). A recent study from Malaysia (Zaidah et al. 2008) which identified C. parvum infections in 16% stool specimens collected from HIV-infected hospitalized patients by nested PCR highlights the importance of C. parvum in HIV patients. Other reports from India using ZN staining indicated Cryptosporidium in 8.5% from Manipal (Ballal et al. 1999), 4.6% from Mumbai (Cotte et al. 1993), 10% from Chandigarh (Mohandas et al. 2002), and 17% in acute and 11.86% chronic diarrhea from Chennai (Kumar et al. 2002a, b). Ramakrishnan et al. (2007) reported prevalence of C. parvum infection 28.7% in HIV/AIDS patients with diarrhea in Madurai, South India and Guk et al. (2005) reported C. parvum infections of 10.5% in HIV-infected patients who visited Seoul National University Hospital, Korea.
In our study, the number of Cryptosporidium positive patients by AFS technique was 28.7% and 4.35% by PCR in HIV patients with or without diarrhea, respectively. The number of Cryptosporidium positive patients with diarrhea was not significantly different from the number of Cryptosporidium positive patients without diarrhea (p > 0.05). This result corresponds to reports of other authors according to which asymptomatic cryptosporidiosis is increasing (Houpt et al. 2005; Kaushik et al. 2008). This is illustrated by the report from Tanzania indicating cryptosporidiosis in 16.7% in asymptomatic as compared to 18% in symptomatic HIV-infected patients (Houpt et al. 2005). There, 14.9% asymptomatic HIV seropositive patients without diarrhea were positive for Cryptosporidium infection (Kaushik et al. 2008), and from Bolivia (Esteban et al. 1998) and Korea (Yu et al. 2004), cryptosporidiosis was found in 8–32% of healthy individuals. Cryptosporidium was reported in significantly higher number (22.6%) of HIV seropositive patients with diarrhea as compared to 0.5% in patients without diarrhea by ZN staining from Vellore, India (Mukhopadhya et al. 1999). Cryptosporidium positivity was 9.8% and 6% in HIV seropositive patients with and without diarrhea, respectively, by using safranine methylene blue staining, whereas in a recent study from Vellore (Muthusamy et al. 2006), there were 4.6% in diarrheic cases and 1.2% in non-diarrhea cases in children from Kolkata, India (Das et al. 2006). Thus, the studies in general indicate higher number of Cryptosporidium positive findings in patients with diarrhea as compared to those without diarrhea.
In the samples examined here, Cryptosporidium sp. infections in cattle were 13% by AFS. This is higher than the prevalence (0.6%) of Cryptosporidium found among dairy cows in the Nong Pho region of central Thailand by AFS reported by Jittapalapong et al. (2006) and lower than the prevalence of Cryptosporidium in Tanzania and Zambia (Swai et al. 2007; Geurden et al. 2006). However, Cryptosporidium infection correlated with host age, which is an important factor characterizing the distribution of Cryptosporidium infections and suggesting a degree of resistance conferred to individuals infected early in life (Arslan and Vicki 1999; Castro-Hermida et al. 2002; Tanriverdi et al. 2006; Kvac et al. 2006; Lefay et al. 2000; Geurden et al. 2006; Azami 2007; Uga et al. 2000; Nguyen et al. 2007).
In recent years, molecular techniques for species genotyping identification have been developed and evaluated (Abe et al. 2006; Nichols et al. 2003, 2006). It is advisable to use both methods (microscopy and PCR) in combination. According to other authors, C. parvum is the predominant species infecting humans in Thailand (Tiangtip and Jongwutiwes 2002; Gatei et al. 2002). Xiao et al. (2001) reported five types of Cryptosporidium in Peruvian children. However, in some countries, C. parvum is slightly more commonly identified than C. hominis in both immunocompetent and immunocompromised individuals (Caccio et al. 2000; Ong et al. 2002; Meamar et al. 2007; Cama et al. 2007; Llorente et al. 2007), while other authors reporting Cryptosporidium isolates obtained from HIV-infected patients have identified at least five species: C. hominis, C. parvum, C. felis, C. meleagridis, and C. muris (Xiao et al. 2004).
By sequencing DNA of Cryptosporidium from cattle samples, we identified the genotype of the Cryptosporidium from five samples, all of which were C. parvum. Other Cryptosporidium species have been reported to infect cattle including C. bovis, C. andersoni, and the Cryptosporidium deer-like genotype (Santín et al. 2004; Fayer et al. 2006; Abdul Halim et al. 2008), but none of these were found here in our few PCR positive samples. The most common zoonotic species is C. parvum and it is also the most common species infecting cattle. It has been reported elsewhere as the most prevalent in pre-weaned calves, infecting 35% of all pre-weaned calves examined but diminishing in post-weaned calves, heifers, and mature cows to 0.2%, 0.7%, and 0.4%, respectively, of all animals examined (Fayer et al. 2006). In Vietnam, the prevalence of C. parvum type (bovine genotype) infections was 33.5%, those of the C. andersoni type were 5.6%, and mixed infection of both types was 3.4% (Nguyen et al. 2007). Both C. bovis and the deer-like genotype found in cattle are host specific for these animals and of no known health risk to humans or other animals. One heifer infected with C. suis, a rare infection in humans, has been reported (Fayer et al. 2006). C. andersoni has been found in post-weaned calves, heifers, and cows. No C. andersoni oocysts have been reported in cattle less than 3 months old, supporting other reports that chronic C. andersoni infection usually occurs in adult cattle with no clinical symptoms.
The results described here suggest that C. parvum is the most common species of Cryptosporidium in humans and animals in Thailand. The HIV positive patients attending Prabat Nampu Temple come from several almost exclusively rural and agricultural provinces. Cattle in Chon Buri Province appear typical of those in similar rural agricultural provinces. They may serve as the reservoir of C. parvum for transmission to humans through food and water contamination or from livestock directly. HIV positive patients may have been infected by Cryptosporidium oocysts from direct contact with cattle or other environmental sources.
The results represent the first genetic identification of Cryptosporidium species in cattle in Thailand and in cattle in Chon Buri Province. Finding Cryptosporidium spp. present in domestic animal populations is not surprising based on its virtually ubiquitous distribution according to the literature. The presence of Cryptosporidium infections in the human population in this rural area of Thailand is also not surprising and easily accounted for by direct contact with other infected hosts, either human or animal, or through other environmental sources including food and water. Further, the results described here suggest that dairy farms should be considered as a potential source of water contamination and C. parvum is an important zoonotic species in dairy calf population in Thailand.
References
Abdul Halim N, Plutzer J, Bakheit MA, Karanis P (2008) First report of Cryptosporidium deer-like genotype in Malaysian cattle. Vet Parasitol 152:325–329
Abe N, Matsubayashi M, Kimata I, Iseki M (2006) Subgenotype analysis of Cryptosporidium parvum isolates from humans and animals in Japan using the 60 kDa glycoprotein gene sequences. Parasitol Res 99:303–305
Arslan M, Vicki Y (1999) Prevalence of Cryptosporidium spp. oocysts in diarrhoeic calves in Kars province, Turkey. Turk J Vet Anim Sci 25:161–164
Azami M (2007) Prevalence of Cryptosporidium infection in cattle in Isfahan, Iran. J Eukaryot Microbiol 54:100–102
Ballal P, Tukaram A, Chandran P, Shivanda G (1999) Cryptosporidium and Isospora belli diarrhoea in immunocompromised hosts. Indian J Cancer 36:38–42
Brandonisio O, Maggi P, Panaro MA, Lisi S, Andriola A, Acquafredda A, Angarano G (1999) Intestinal protozoa in HIV-infected patients in Apulia, South Italy. Epidemiol Infect 123:457–462
Caccio S, Homan W, Camilli R, Traldi G, Kortbeek T, Pozio E (2000) A microsatellite marker reveals population heterogeneity within human and animal genotypes of Cryptosporidium parvum. Parasitology 120:237–244
Cama VA, Ross JM, Crawford S, Kawai V, Chavez-Valdez R, Vargas D, Vivar A, Ticona E, Navincopa M, Williamson J, Ortega Y, Gilman RH, Bern C, Xiao L (2007) Differences in clinical manifestations among Cryptosporidium species and subtypes in HIV-infected persons. J Infect Dis 196:684–691
Casemore DP (1991) Laboratory methods for diagnosing cryptosporidiosis. J Clin Pathol 44:445–451
Castro-Hermida JA, Gonzalez-Posada A, Ares-Mazas E (2002) Prevalence of and risk factors involved in the spread neonatal bovine cryptosporidiosis in Galicia (NW Spain). Vet Parasitol 106:1–10
Chintu C, Luo C, Baboo S, Med M, Khumalo-Ngwenya B, Mathewson J, DuPont HL, Zumla A (1995) Intestinal parasites in HIV-seropositive Zambian children with diarrhoea. J Trop Pediatr 41:149–152
Cotte L, Rabodonirina M, Piens MA, Perreard M, Mojon M, Trepo C (1993) Prevalence of intestinal protozoans in French patients infected with HIV. J Acquir Immune Defic Syndr 6:1024–1029
Das P, Roy SS, MitraDhar K, Dutta P, Bhattacharya MK, Sen A, Ganguly S, Bhattacharya SK, Lal AA, Xiao L (2006) Molecular characterization of Cryptosporidium spp. from children in Kolkata, India. J Clin Microbiol 44:4246–4249
Esteban JG, Aguirre C, Flores A, Strauss W, Angles R, Mas-Coma S (1998) High Cryptosporidium prevalences in healthy Aymara children from the northern Bolivian Altiplano. Am J Trop Med Hyg 58:50–55
Fayer R, Santin M, Jame TJ, Greiner E (2006) Prevalence of species and genotypes of Cryptosporidium found in 1–2 year-old dairy cattle in the eastern United States. Vet Parasitol 135:105–112
Gatei W, Suputtamongkol Y, Waywa D, Ashford RW, Bailey JW, Greensill J, Beeching NJ, Hart CA (2002) Zoonotic species of Cryptosporidium are as prevalent as the anthroponotic in HIV-infected patients in Thailand. Ann Trop Med Parasitol 96:797–802
Geurden T, Goma FY, Siwila J, Phiri IG, Mwanza AM, Gabriel S, Claerebout E, Vercruyss J (2006) Prevalence and genotyping of Cryptosporidium in three cattle husbandry systems in Zambia. Vet Parasitol 138:217–222
Guk SM, Seo M, Park YK, Oh MD, Choe KW, Kim JL, Choi MH, Hong ST, Chai JY (2005) Parasitic infections in HIV-infected patients who visited Seoul National University Hospital during the period 1995–2003. Korean J Parasitol 43:1–5
Houpt ER, Bushen OY, Noel ES, Anita K, Amon A, Cherie TN, David PC, Richard LG, Venance M, Sendui ON, John FS (2005) Asymptomatic Cryptosporidium hominis infection among human immunodeficiency virus-infected patients in Tanzania. Am J Trop Med Hyg 73:520–522
Hunter PR, Thompson RCA (2005) The zoonotic transmission of Giardia and Cryptosporidium. Int J Parasitol 35:1181–1190
Janoff EN, Mead PS, Mead JR, Echeverria P, Bodhidatta L, Bhaibulaya M, Sterling CR, Taylor DN (1990) Endemic Cryptosporidium and Giardia lamblia infections in a Thai orphanage. Trop Med Hyg 43:248–256
Jirapinyo P, Ruangsiri K, Tesjaroen S, Limsathayourat N, Sripiangjan J, Yoolek A, Junnoo V (2002) High prevalence of Cryptosporidium in young children with prolonged diarrhoea. Southeast Asian J Trop Med Public Health 24:730–733
Jittapalapong S, Pinyopanuwat N, Chimnoi W, Siripanth C, Stich RW (2006) Prevalence of Cryptosporidium among dairy cows in Thailand. Ann N Y Acad Sci 1081:328–335
Jongwutiwes S, Kraivichian P, Kulkumthorn M, Sitthichareonchai P, Jaroenkorn M (1990) Cryptosporidiosis among orphanage children in Thailand: a one year prospective study. Southeast Asian J Trop Med Public Health 21:458–464
Karanis P, Kourenti C, Smith HW (2007a) Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health 5:1–38
Karanis P, Plutzer J, Halim NA, Igori K, Nagasawa H, Ongerth J, Liqing M (2007b) Molecular characterization of Cryptosporidium from animal sources in Qinghai province of China. Parasitol Res 101:1575–1580
Kaushik K, Khurana S, Wanchu A, Malla N (2008) Evaluation of staining techniques, antigen detection and nested PCR for the diagnosis of cryptosporidiosis in HIV seropositive and seronegative patients. Acta Trop 107:1–7
Kumar SS, Ananthan S, Lakshmi P (2002a) Intestinal parasitic infection in HIV infected patients with diarrhoea in Chennai. Indian J Med Microbiol 20:88–91
Kumar SS, Ananthan S, Sarvanan P (2002b) Role of coccidian parasites in causation of diarrhoea in HIV infected patients in Chennai. Indian J Med Res 116:85–89
Kvac M, Kouba M, Vitovec J (2006) Age-related and housing-dependence of Cryptosporidium infection of calves from dairy and beef herds in South Bohemia, Czech Republic. Vet Parasitol 137:202–209
Lefay D, Naciri M, Poirier P, Chermette R (2000) Prevalence of Cryptosporidium infection in calves in France. Vet Parasitol 89:1–9
Leoni F, Amar C, Nichols G, Pedraza-Diaz S, McLauchlin J (2006) Genetic analysis of Cryptosporidium from 2414 humans with diarrhoea in England between 1985 and 2000. J Med Microbiol 55:703–707
Llorente MT, Clavel A, Goni MP, Varea M, Seral C, Becerril R, Suarez L, Gómez-Lus R (2007) Genetic characterization of Cryptosporidium species form humans in Spain. Parasitol Int 497:1–5
Meamar AR, Guyot K, Certad G, Dei-Cas E, Mohraz M, Mehdi M, Kazem M, Amir AM, Sasan R, Mostafa R (2007) Molecular characterization of Cryptosporidium isolates from humans and animals in Iran. Appl Environ Microbiol 73:1033–1035
Mohandas K, Sehgal R, Sud A, Malla N (2002) Prevalence of intestinal parasitic pathogens in HIV-seropositive individuals in northern India. Jpn J Infect Dis 55:83–84
Moolasart P, Eampokalap B, Ratanasrithong M, Kanthasing P, Tansupaswaskul S, Tanchanpong C (1995) Cryptosporidiosis in HIV infected patients in Thailand. Southeast Asian J Trop Med Public Health 26:335–338
Mukhopadhya A, Ramakrishna BS, Kang G, Pulimood AB, Mathan AZ, Mathai DC (1999) enteric pathogens in Southern Indian HIV infected patients with and without diarrhoea. Indian J Med Res 109:85–89
Muthusamy D, Rao SR, Ramani S, Monica B, Banerjee I, Abraham OC, Matahi DC, Primrose B, Muliyil J, Wanke CA, Ward HD, Kang G (2006) Multilocus genotyping of Cryptosporidium sp. isolates from human immunodeficiency virus-infected individuals in South India. J Clin Microbiol 44:632–634
Nguyen ST, Nguyen DT, Le DQ, Hua LV, Nguyen T, Honma H, Nakai Y (2007) Prevalence and first genetic identification of Cryptosporidium spp. in cattle in central Viet Nam. Vet Parasitol 150:357–361
Nichols RA, Campbell BM, Smith HV (2003) Identification of Cryptosporidium spp. oocysts in United Kingdom noncarbonated natural mineral waters and drinking waters by using a modified nested PCR-restriction fragment length polymorphism assay. Appl Environ Microbiol 69:4183–4189
Nichols RA, Campbell BM, Smith HV (2006) Molecular fingerprinting of Cryptosporidium oocysts isolated during water monitoring. Appl Environ Microbiol. 72:5428–5435
Oguntibeju OO (2006) Prevalence of intestinal parasites in HIV-positive/AIDS patients, Malaysia. J Med Sci 13:68–73
Ong CSL, Eisler DL, Alikhani A, Fung VWK, Tomblin J, Bowie WR, Isaak-Renton JL (2002) Novel Cryptosporidium genotypes in sporadic cryptosporidiosis cases: first report of human infections with a cervine genotype. Emerg Infect Dis 8:263–268
Ongerth JE, Stibbs HE (1987) Identification of Cryptosporidium in river water. Appl Environ Microbiol 53:672–676
Plutzer J, Karanis P (2007) Genotype and subtype analyses of Cryptosporidium isolates from cattle in Hungary. Vet Parasitol 146:357–362
Ramakrishnan K, Shenbarathai R, Uma A, Kavitha K, Rajendran R, Thirumalaikolundusubramanian P (2007) Prevalence of intestinal parasitic infestation in HIV/AIDS patients with diarrhoea in Madurai City, South India. J Infect Dis 60:209–210
Read CM, Monis PT, Thompson RCA (2004) Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR–RFLP. Infect Genet Evol 4:125–130
Saksirisampant W, Eampokalap B, Rattanasrithong M, Likanonsakul S, Wiwanitkit V, Nasingkarn A, Denmasae N (2002) A prevalence of Cryptosporidium infections among Thai HIV-infected patients. Parasitol 85:424–428
Santín M, Trout JM, Xiao L, Zhou L, Greiner E, Fayer R (2004) Prevalence and age related variation of Cryptosporidium species and genotypes in dairy calves. Vet Parasitol 122:103–117
Slapeta J (2006) Cryptosporidium species found in cattle: a proposal for a new species. Trends Parasitol 10:469–474
Stark D, Fotedar R, Hal SV, Beebe N, Marriot D, Ells JT, Harkness J (2007) Prevalence of enteric protozoa in human immunodeficiency virus (HIV)-positive and HIV-negative men who have sex with men from Sydney, Australia. Am J Trop Med Hyg 76:549–552
Swai ES, French NP, Karimuribo ED, Fitzpatrick JL, Bryant MJ, Kambarage DM, Ogden NH (2007) Prevalence and determinants of Cryptosporidium spp. infection in smallholder dairy cattle in Iringa and Tanga regions of Tanzania. Onderstepoort J Vet Res 74:23–29
Tanriverdi S, Markovics A, Arslan MO, Itik A, Shklap V, Widmer G (2006) Emergence of distinct genotypes of Cryptosporidium parvum in structured host populations. Appl Environ Microbiol 72:2507–2513
Thamlikitkul V, Tepmongkol M, Lamon C, Sripochang S, Rungnapawate W, Suvajeejarun T (1987) Cryptosporidiosis in Siriraj Hospital, Bangkok, Thailand. Southeast Asian J Trop Med Public Health 18:229–232
Tiangtip R, Jongwutiwes S (2002) Molecular analysis of Cryptosporidium species isolated from HIV-infected patients in Thailand. Trop Med Int Health 7:357–364
Uga S, Kunaruk N, Rai SK, Watanabe M (1998) Cryptosporidium infection in HIV-seropositive and seronegative populations in southern Thailand. Trop Med Public Health 29:100–104
Uga S, Kawamura T, Hotta H, Endo T, Masuda K, Yamamoto A, Shiba Kumar R, Tsuji H, Ono K (2000) Prevalence of Cryptosporidium parvum infection and pattern of oocyst shedding in calves in Japan. Appl Environ Microbiol 67:3832–3836
Waywa D, Kongkriengdaj S, Chaidatch S, Tiengrim S, Kowadisaiburana B, Chaikachonpat S, Suwanagool S, Chaiprasert A, Curry A, Bailey W, Suputtamongkol Y, Beeching NJ (2001) Protozoan enteric infection in AIDS related diarrhoea in Thailand. Southeast Asian J Trop Med Public Health 32:151–155
Xiao L, Bern C, Limor J, Sulaiman IM, Roberts J, Checkley W, Cabrera L, Gilman RH, Lal AA (2001) Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J Infect Dis 183:492–497
Xiao L, Morgan UM, Limor J, Escalante A, Arrowood M, Shulaw W, Thompson RCA, Fayer R, Lal AA (2004) Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Am Soc Microbiol 65:3386–3391
Yu JR, Lee JK, Seo M, Kim SI, Sohn WM, Huh S, Choi HY, Kim TS (2004) Prevalence of cryptosporidiosis among the villagers and domestic animals in several rural areas of Korea. Korean J Parasitol 42:1–6
Zaidah AR, Chan YY, Asma HS, Abdullah S, Nurhaslindawati AR, Salleh M, Zeehaida M, Lalitha P, Mustafa M, Ravichandran M (2008) Detection of Cryptosporidium parvum in HIV-infected patients in Malaysia using a molecular approach. Southeast Asian J Trop Med Public Health 39:511–516
Acknowledgments
This study was supported by Grant-in-Aid for Young Scientists, Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and from the 21st Century COE Program (A-1), Ministry of Education, Sports, Science and Technology of Japan and by the Commission on Higher Education (CHE), Ministry of Education, Thailand (the University Mobility in Asia and the Pacific-UMAP; The Faculty Exchange Program Between Thailand and Neighboring Countries for the Year 2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nuchjangreed, C., Boonrod, K., Ongerth, J. et al. Prevalence and molecular characterization of human and bovine Cryptosporidium isolates in Thailand. Parasitol Res 103, 1347–1353 (2008). https://doi.org/10.1007/s00436-008-1139-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1139-5