Abstract
The mature Raillietina (Raillietina) baeri spermatozoon exhibits an apical cone of electron-dense material about 2.5 μm long and 0.5 μm wide and two helicoidal crest-like bodies roughly 100–125 nm thick. The latter are of different lengths, spiralized and stand in an angle of about 50° with the spermatozoon axis. The axoneme is of the 9 + “1” pattern and does not reach the posterior extremity of the gamete. The nucleus is an electron-dense cord coiled in a spiral around the axoneme. The cytoplasm exhibits a posterior densification and contains few small electron-dense granules in regions I, II and V of the spermatozoon. In regions III and IV, it is divided into irregular compartments by walls of electron-dense material. The cortical microtubules are spiralized and make an angle of 40–50° to the spermatozoon axis. In this work, we describe, for the first time, a spermatozoon of a davaineidaean cestode parasitic of mammals. This has enabled us to show a wide apical cone, which has never been described before in a cyclophyllidean species the spermatozoon of which has two crest-like bodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The order Cyclophyllidea comprises 15 families (Khalil et al. 1994) among which the Davaineidae which are parasites of birds and mammals at their adult stages (Yamaguti 1959; Schmidt 1986). To our knowledge, only two species of Davaineidae belonging to two genera, both parasitic of birds, have been up until now the subject of ultrastructural study of spermiogenesis and/or the spermatozoon, up till now. These are Cotugnia polyacantha (Bâ and Marchand 1994a) and Raillietina (Raillietina) tunetensis (Bâ and Marchand 1994b). In the present work, we describe the ultrastructure of the spermatozoon of Raillietina (Raillietina) baeri. The latter was previously reported in Burma and Africa from the rodents, Mus coucha and Rattus rattus (Meggitt and Subramanian 1927; Schmidt 1986).
Materials and methods
The specimens of Raillietina (Raillietina) baeri (Meggitt and Subramanian 1927) were gathered live from the small intestine of Mastomys huberti (Rodent) collected in Richard-Toll, North Senegal. Then, the worms were kept active in physiological saline solution (0.9 % NaCl). Portions of strobila 3–6 cm long, made up of mature proglottids, were quickly taken and then stretched out with a brush soaked in cold (4°C) 2.5% glutaraldehyde buffered with 0.1 M sodium cacodylate solution at pH 7.2. The male genitalia were removed under a binocular microscope, fixed for about 24 h in glutaraldehyde, rinsed for one night in a sodium cacodylate buffer, postfixed with cold 1% osmium tetroxide for 1 h, dehydrated with ethanol and propylene oxide, and then embedded in epon. Ultrathin sections (50–60 nm thick) were cut on a LKB Ultramicrotome with diamond knife, then stained with uranyl acetate and lead citrate. They were examined in a Hitachi H-600 electron microscope at 75 kV.
Results
The mature Raillietina (Raillietina) baeri spermatozoon has no mitochondria, is filiform and tapered at both ends (Figs. 1, 7 and 11). Five regions (I–V) could be distinguished from front to back exhibiting distinctive ultrastructural characters.
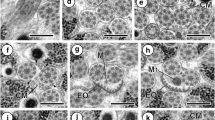
scale bars=0.5 μm
Longitudinal sections of regions I–V of the mature Raillietina (Raillietina) baeri spermatozoon
Region I showing the apical cone and the crest-like bodies of electron-dense material
Region I showing the crest-like bodies (Cb) wound in a spirale around the cortical microtubules (Cm). A axoneme; C centriole
Regions I and II. Cb crest-like body
Regions II and III. W intracytoplasmic wall of electron-dense material
Regions III and IV. A axoneme; Sh periaxonemal sheath of electron-dense material; W intracytoplasmic wall of electron-dense material
Regions IV and V. A axoneme; N nucleus
Region V. G electron-dense granule
Cross sections of regions I–III of the mature Raillietina (Raillietina) baeri spermatozoon
Region I showing one (Cb) and two crest-like bodies (arrowheads). Cm cortical microtubules
Region II. Sh periaxonemal sheath of electron-dense material
Region III. Sh periaxonemal sheath of electron-dense material; W intracytoplasmic wall of electron-dense material
Attempted reconstruction of the mature spermatozoon. Ax axoneme, Aae axonemal anterior extremity, Ac apical cone, Ape axonemal posterior extremity, Ase anterior spermatozoon extremity, C centriole; Cb crest-like body, Cm cortical microtubule, G electron-dense granule, N nucleus, Ps periaxonemal sheath of electron-dense material, Pse posterior spermatozoon extremity, W intracytoplasmic wall of electron-dense material
Region I (Figs. 1, 2, 3, 8, 11) varies in width from 0.2 to 0.5 μm. It corresponds to the anterior extremity of the spermatozoon. It exhibits an apical cone of slightly electron-dense material about 2.5 μm long and 0.5μm wide at its base, and two helicoidal crest-like bodies of about 100 nm thick. The latter lie outside the cortical microtubules and are of different lengths. Thus, in cross-sections, depending on the level of the section, their number varies between 1 and 2 (Fig. 8). The axoneme is of the 9 + “1” pattern and central. It is surrounded by a sheath of electron-dense material and a thin layer of slightly electron-dense cytoplasm that contains some scarce granules of electron-dense material (Fig. 8). The cortical microtubules are spiralized and appear in longitudinal and transverse sections in the form of a layer of continous dense material in close contact with the plasma membrane (Figs. 2–10).
Region II (Figs. 3, 4, 9, 11) is roughly 0.5 μm wide. It lacks crest-like bodies. As the preceeding region it exhibits spiralized cortical microtubules and a central axoneme surrounded by a sheath of electron-dense material (Fig. 9). The cytoplasm contains few small granules of electron-dense material.
Region III (Figs. 4, 5, 10, 11) is roughly 0.5 μm in width. The axoneme is central and surrounded by a sheath of electron-dense material and a lucent cytoplasm (Fig. 5).The latter is divided into compartments by irregularly spaced partitions of electron-dense material which join the periaxonemal sheath of electron-dense material to the cortical microtubules (Figs. 5 , 10).
Region IV (Figs. 5, 6 , 11) is 0.6 μm wide at the most. It is characterized by the presence of a nucleus. This is a fine compact cord of elecron-dense material about 50–100 nm thick, coiled in a helix around the axoneme (Figs. 5, 6). In the cross section, depending on the level where the section is cut, it envelops partially or entirely the axoneme. The cytoplasm is slightly electron-dense and contains numerous walls of electron-dense material between the peri-axonemal sheath and the spiralized cortical microtubules (Fig. 5).
Region V (Figs. 6, 7, 11) is between 0.1 μm and 0.3 μm wide. It corresponds to the posterior end of the gamete. It lacks an axoneme and crest-like bodies. Nevertheless, the cytoplasm exhibits numerous and small granules of electron-dense material and a posterior densification (Figs. 7, 11). The cortical microtubules are still spiralized.
Discussion
The crest-like body (or bodies), if it exists, always indicates the anterior extremity of the Eucestodes spermatozoon (Bâ et al. 1991). Consequently, the extremity with crest-like bodies of the Raillietina (Raillietina) baeri spermatozoon corresponds to its anterior extremity, and the extremity without crest-like bodies to its posterior extremity.
One, 2, 5, 6–8 and 12 crest-like bodies of different thicknesses have been described in the spermatozoa of 25 cyclophyllidean species spread over 21 genera and 7 families (Table 1).
The angle of spiralization of crest-like bodies in the cestodes spermatozoon varies according to the species (Table 1). It has been estimated at 20° in Mesocestoides litteratus (Miquel et al. 1999), 35° in Avitellina centripunctata (Bâ and Marchand 1994a), 40° in Moniezia expansa and M. benedeni (Bâ and Marchand 1992b), Dipylidium caninum (Miquel et al. 1998), Catenotaenia pusilla (Hidalgo et al. 2000) and Echinocotyle dolosa (Bâ et al. 2002), and between 40° and 50° in Thysaniezia ovilla (Bâ et al. 1991). It is about 50° in Raillietina (Raillietina) baeri as well as in Stilesia globipuntata (Bâ and Marchand 1992c).
The thickness of crest-like body or bodies also varies according to the cestode species (Table 1). It has been evaluated at 15–40 nm in Aporina delafondi (Bâ and Marchand 1994b), 30–40 nm and 30–60 nm in Moniezia expansa and M. benedeni respectively (Bâ and Marchand 1992b), 75 nm in Catenotaenia pusilla (Hidalgo et al. 2000) and Taenia mustelae (Miquel et al. 2000), 80 nm in Thysaniezia ovilla (Bâ et al. 1991), 50–100 nm in Sandonella sandoni (Bâ and Marchand 1994c), Sudarikovina taterae (Bâ et al. 2000) and Hymenolepis straminea (Bâ and Marchand 1996), 140 nm in Anoplocephaloides dentata (Miquel and Marchand 1998a), 150 nm in Dipylidium caninum (Miquel et al. 1998), 180 nm in Paranoplocephala omphalodes (Miquel and Marchand 1998b), 100 to 150 nm in Mesocestoides litteratus (Miquel et al. 1999), 100–200 nm in Raillietina (Raillietina) tunetensis (Bâ and Marchand 1994d) and Vampirolepis microstoma (Bâ and Marchand 998), 150–200 nm in Avitellina centripunctata (Bâ and Marchand 1994a). It is between 100–125 nm in Raillietina (Raillietina) baeri
An apical cone has been described in the front of the spermatozoon of 25 cyclophyllidean cestodes (Table 1). Its length varies between 0.1 and 2.5 μm and its width is from 0.1 μm to 0.5 μm (Bâ et al. 2002). In Raillietina (Raillietina) baeri, the apical cone is about 2.5 μm long and 0.5 μm wide at its base. To our knowledge, an apical cone of such a width has never been described before in a cyclophyllidean species the spermatozoon of which has two crested-like bodies (Table 1).
The width of the nucleus varies according to the cestode species. It has been estimated between 10 nm to 75 nm in Inermicasifer guineensis (Bâ and Marchand 1995), 30–150 nm in I. madagascariensis (Bâ and Marchand 1995) and 300–700 nm in Echinocotyle dolosa (Bâ et al. 2002). In Raillietina (Raillietina) baeri, it measures between 50 nm to 100 nm width.
References
Bâ CT, Marchand B (1992a) Reinvestigation of the ultrastructure of spermiogenesis and the spermatozoon of Hymenolepis nana (Cestoda, Cyclophyllidea) parasite of the small intestine of Rattus rattus. Mol Reprod Dev 33:39–45
Bâ CT, Marchand B (1992b) Ultrastuctural study of the spermatozoa of Moniezia expansa Rudolphi, 1810 and M. benedeni Moniez, 1879 (Cestoda, Cyclophyllidea, Anoplocephalidae). Ann Parasitol Hum Comp 67:111–115
Bâ CT, Marchand B (1992c) Ultrastructural particularities of the spermatozoon of Stilesia globipunctata (Cestoda) parasite of the small intestine of sheep and goats in Senegal. J Submicrosc Cytol Pathol 24:29–34
Bâ CT, Marchand B (1993) Ultrastructure of the Retinometra serrata spermatozoon (Cestoda) intestinal parasite of the turtle-doves in Senegal. J Submicrosc Cytol Pathol 25:233–238
Bâ CT, Marchand B (1994a) Ultrastructure of spermatozoon of Avitellina centripunctata (Cestoda, Cyclophyllidea) a parasite of the small intestine of cattle in Senegal. Acta Zool 7:161–166
Bâ CT, Marchand B (1994b) Ultrastructure of the spermiogenesis and the spermatozoon of Aporina delafondi (Cyclophyllidea, Anoplocephalidae) intestinal parasite of turtle-doves in Senegal. Int J Parasitol 24:225–235
Bâ CT, Marchand B (1994c) Ultrastructure of the spermatozoon of Sandonella sandoni (Cestoda, Proteocephalidea, Sandonellinae) intestinal parasite of Heterotis niloticus (Fish, Teleost.). Inv Reprod Dev 25:9–17
Bâ CT, Marchand B (1994d) Ultrastructure of spermiogenesis and the spermatozoon of Raillietina (Raillietina) tunetensis (Cyclophyllidea, Davaineidae) intestinal parasite of turtle-doves in Senegal. Int J Parasitol 24:237–248
Bâ CT, Marchand B (1994e) Ultrastructure of spermiogenesis and the spermatozoon of Mathevotaenia herpestis (Cestoda) intestinal parasite of Atelerix albiventris in Senegal. Acta Zool 75:167–175
Bâ CT, Marchand B (1994f) Similitude ultrastructurale des spermatozoïdes de quelques Cyclophyllidea. Parasite 1:51–55
Bâ CT, Marchand B (1995) Comparative ultrastructure of the spermatozoa of Inermicapsifer guineensis and I. madagascariensis (Cestoda, Anoplocephalidae, Inermicapsiferinae) intestinal parasites of rodents in Senegal. Can J Zool 72:1633–1638
Bâ CT, Marchand B (1996) Hymenolepis straminea (Cyclophyllidea, Hymenolepididae) intestinal parasite of Arvicanthis niloticus in Senegal. Inv Reprod Dev 9:243–247
Bâ CT, Marchand B (1998) ltrastructure of spermiogenesis and the spermatozoon of Vampirolepis microstoma (Cestoda, Hymenolepididae) intestinal parasite of Rattus rattus. Microsc Res Technique 42:218–225
Bâ CT, Marchand B, Mattei X (1991) Demonstration of the orientation of the Cestodes spermatozoon illustrated by ultrastructural study of spermiogenesis and the spermatozoon of a Cyclophyllidea: Thysaniezia ovilla, Rivolta, 1874. J Submicrosc Cytol Pathol 23:605–612
Bâ A, Bâ CT, Marchand B (2000) Ultrastructure of spermiogenesis and the spermatozoon of Sudarikovina taterae (Cestoda, Cyclophyllidea, Anoplocephalidae), intestinal parasite of Tatera gambiana (Rodentia, Gerbillidae) in Senegal. J Submicrosc Cytol Pathol 32:137–144
Bâ A, Bâ CT, Marchand B (2002) Ultrastructural study of the spermatozoon of Echinocotyle dolosa (Cestoda, Cyclophyllidea, Hymenolepididae). Acta Parasitol 47:131–136
Hidalgo C, Miquel J, Torres J, Marchand B (2000) Ultrastructural study of spermiogenesis and the spermatozoon of Catenotaenia pusilla, an intestinal parasite of Mus musculus. J Helminthol 74:73–81
Khalil LF, Jones A, Bray RA (1994) Keys to the cestode parasites of vertebrates. CAB International, Wallingford
Miquel J, Marchand B (1998a) Ultrastructure of spermiogenesis and the spermatozoon of Anoplocephaloides dentata, an intestinal parasite of Mus musculus. J parasitol 84:1128–1136
Miquel J, Marchand B (1998b) Utrastructure of the spermatozoon of the Bank Vole tapeworm, Paranoplocephala omphalodes (Cestoda, Cyclophyllidea, Anoplocephalidae). Parasitol Res 84:239–245
Miquel J, Bâ CT, Marchand B (1997) Ultrastructure of the spermatozoon of Skrjabinotaenia lobata (Cyclophyllidea, Catenotaeniidae), intestinal parasite of Apodemus sylvaticus. (Rodentia, Muridae). J Submicrosc Cytol Pathol 29:521–526
Miquel J, Bâ CT, Marchand B (1998) Ultrastructure of spermiogenesis of Dipylidium caninum (Cestoda, Cyclophyllidea, Dipylidiidae), an intestinal parasite of Canis familiaris. Int J Parasitol 28:1453–1458
Miquel J, Feliu C, Marchand B (1999) Ultrastructure of spermiogenesis and the spermatozoon of Mesocestoides litteratus (Cestoda, Mesocestoidae). Int J Parasitol 29:499–510
Miquel J, Hidalgo C, Feliu C, Marchand B (2000) Sperm ultrastructure of Taenia mustelae (Cestoda, Taeniidae), an intestinal parasite of the weasel, Mustela nivalis (Carnivora). Inv Reprod Dev 38:43–51
Ndiaye PI, Miquel J, Marchand B (2003) Ultrastructure of spermiogenesis and the spermatozoon of Taenia parva Baer, 1926 (Cestoda, Cyclophyllidea, Taeniidae), a parasite of the common genet (Genetta genetta). Parasitol Res 89:34–43
Schmidt GD (1986) Handbook of tapeworn identification. CRC Press, Boca Raton, Florida
Yamaguti S (1959) Systema helminthum, vol. 2. The cestodes of vertebrates. Interscience Publishers, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bâ, C.T., Bâ, A. & Marchand, B. Ultrastructure of the spermatozoon of Raillietina (Raillietina) baeri (Cyclophyllidea, Davaineidae) an intestinal parasite of the multimammate rat, Mastomys huberti (Rodentia, Muridae). Parasitol Res 97, 173–178 (2005). https://doi.org/10.1007/s00436-005-1395-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-1395-6