Abstract
The present study provides the first ultrastructural data on the digenean Gonocerca phycidis belonging to the family Gonocercidae. Live adults digenean were collected from the stomach of the marine fish Gephyroberyx darwinii (Teleostei: Trachichthyidae) caught in the Atlantic Ocean, off Dakar (Senegal). This is also the first report of G. phycidis from Senegal and from this fish. The mature spermatozoon exhibits (i) two axonemes with the 9 + ‘1’ pattern of the Trepaxonemata, (ii) a lateral expansion associated with external ornamentations and without cortical microtubules, (iii) two types of external ornamentation of the plasma membrane located in the anterior region of the spermatozoon, (iv) two bundles of cortical microtubules in the mitochondrial and nuclear areas of the spermatozoon, (v) one mitochondrion and (vi) a nucleus. The maximum number of cortical microtubules is located in the median part of the spermatozoon. Moreover, the mature spermatozoon of G. phycidis presents some peculiarities. The presence of a lateral expansion without cortical microtubules associated with external ornamentations is described here, for the first time, in digenean spermatozoa. The presence of two elongated bundles of cortical microtubules is also reported for the first time in the superfamily of Hemiuroidea. However, the ultrastructure of the posterior spermatozoon extremity confirms that already described in other hemiuroidean species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The systematic position of the genus Gonocerca has been the subject of many controversies. It was considered as a member of the subfamily Gonocercinae Skrjabin and Guschanskaja 1955, within the family Derogenidae in the Superfamily of Hemiuroidea (Gibson and Bray 1979; Gibson 2002). However, based on molecular studies, Olson et al. (2003) and Panklov et al. (2006) were the first to propose the reconsideration of the Gonocercinae as an independent family. According to these authors, the family Derogenidae is polyphyletic. Likewise, Hemipera manteri, a member of the Gonocercinae, and Derogenes varicus, the type genus of the Derogenidae, are phylogenetically distant from others. This fact was also supported posteriorly by Sokolov et al. (2016). More recently, based on molecular studies carried out on four gonocercids including the type species G. phycidis, Sokolov et al. (2018) have considered the Gonocercidae as a separate family from the Derogenidae. Molecular studies show also that this family is basal to the remaining families of the Hemiuroidea (Sokolov et al. 2019). To date, the Gonorcercidae includes only two genera namely Gonocerca (the type genus) and Hemipera. However, according to Sokolov et al. (2018) the generic composition of this new family requires further clarification as the molecular data do not support the inclusion of the genus Hemipera in the Gonocercidae.
In this context, the ultrastructural studies of species belonging to the family Gonocercidae are of great importance to bring additional information to complement the existing molecular data. Gonocerca phycidis is the type species of the genus Gonocerca and the first member of the Gonocercidae family to be studied by transmission electron microscopy.
To our knowledge ultrastructural data of the mature spermatozoon exist for only fourteen species belonging to five of the fourteen families of the Hemiuroidea. These are three Didymozoidae: Didymocystis wedli, Didymozoon sp. and Gonapodasmius sp. (Justine and Mattei 1982, 1983, 1984; Pamplona-Basilio et al. 2001), seven Hemiuridae: Aphanurus stossichii, Ectenurus lepidus, Hemiurus appendiculatus, Lecithochirium microstomum, L. musculus, Lecithocladium excisum and Parahemiurus merus (Ndiaye et al. 2012a, 2013a, 2014; Dione et al. 2016; Kacem et al. 2020), one Lecithasteridae: Aponurus laguncula (Quilichini et al. 2010a), two Sclerodistomidae: Prosorchis palinurichthi and Sclerodistomum italicum (Ndiaye et al. 2013b, 2017) and one Sclerodistomoididae: Sclerodistomoides pacificus (Bâ et al. 2020).
To increase the data on spermatological studies in the Hemiuroidea, the present study provides, for the first time, the spermatological characteristics of the genus Gonocerca and the family Gonocercidae. We compare our results with those obtained in other hemiuroideans previously described.
Material and methods
Adult specimens of Gonocerca phycidis Manter, 1925 were collected from the stomach of Gephyroberyx darwinii (Trachichtyidae) caught off the coast of Dakar (Atlantic Ocean). The adult specimens were washed in 0.9% NaCl solution and fixed in cold (4 °C) 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at pH 7.2, post-fixed in cold (4 °C) 1% osmium tetroxide in the same buffer for 1 h, dehydrated in ethanol and propylene oxide, embedded in Spurr’s resin and finally polymerized at 60 °C for 48 h.
Ultrathin Sects. (60–90 nm thick) were cut on an ultramicrotome (Power tome PC, RMC Boeckeler) with a diamond knife, placed on copper and gold grids. Sections on copper grids were double-stained with uranyl acetate and lead citrate. The ultrathin sections were examined using a Hitachi H-7650 electron microscope operated at 80 kV, in the ‘’Service d’Étude et de Recherche en Microscopie Électronique’’ of the University of Corsica (Corte, France).
Results
The interpretation of several cross and longitudinal sections of the mature spermatozoon of G. phycidis allows us to establish three distinctive regions (I-III) from the anterior to the posterior spermatozoon extremity.
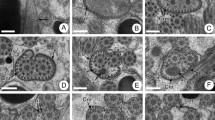
Region I (Figs. 1A–H and 3I) corresponds to the anterior extremity of the spermatozoon. Cross-sections of the anterior tip show axonemal microtubules surrounded by a continuous and submembranous layer of parallel cortical microtubules with a maximum number around 24 (Fig. 1A, B). Then, the two centrioles appear almost simultaneously and they are covered entirely by filamentous ornamentation associated with the layer of cortical microtubules (Fig. 1C, D). A well-developed lateral expansion associated only with external ornamentation of the plasma membrane appears before the complete formation of the second axoneme (Fig. 1E). At this level the layer of cortical microtubules becomes discontinuous and their number decreases progressively (Fig. 2E, H). The transition toward region II is characterized by the disappearance of lateral expansion.
Mature spermatozoon of Gonocerca phycidis, region I. A Cross-section in the anterior tip shows only cortical microtubules. B Cross-section shows the axonemal microtubules corresponding to the centrioles surrounded by a continuous layer of submembranous cortical microtubules of about 24. C, D, Consecutive cross-sections show the presence of filamentous ornamentation associated with cortical microtubules, E cross-section in the middle part of region I characterized by the appearance of the lateral expansion before the complete formation of the second axoneme. Note the decrease of cortical microtubules. F–H Consecutive cross-sections of the posterior part of region I show the progressive decrease in the number of cortical microtubules. Az Attachment zone, C1 centriole of the first axoneme, C2 centriole of the second axoneme, Cm cortical microtubules, Feo filamentous external ornamentation, Le lateral expansion. Scale bars 0.2 µm
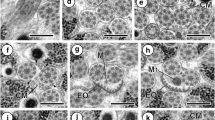
Mature spermatozoon of Gonocerca phycidis, regions II and III. A–C Consecutive cross-sections in the anterior part of region II show only two axonemes and an increasing number of cortical microtubules from 4 to 32. D–F Consecutive cross-sections in the posterior part of region II show the appearance of the mitochondrion and the arrangement of cortical microtubules in two fields. G Cross-section in the anterior part of region III shows the appearance of the nucleus and cortical microtubules (about 17). H Cross-section shows the enlarging of the nucleus and seven cortical microtubules. I Cross-section shows the disappearance of the mitochondrion and only five cortical microtubules. J Cross-section shows the nucleus and the first axoneme without a central element. K-M Consecutive cross-sections are characterized by the progressive disorganization of both axonemes, the disappearance of cortical microtubules then the nucleus. M Posterior extremity of the mature spermatozoon with only doublets of the second axoneme. Az attachment zones, Cm cortical microtubules, D doublets of the second axoneme, M mitochondrion, N nucleus, Pae1 posterior extremity of the first axoneme, Pae2 posterior extremity of the second axoneme. Scale bars 0.2 µm
Region II (Figs. 2A–F and 3II) corresponds to the middle region of the spermatozoon. In the anterior part of this region, cross-sections show only two axonemes, their attachment zones, and four cortical microtubules (Fig. 2A). The number of cortical microtubules progressively increases in this region by 4 (Fig. 2A) to 8 (Fig. 2B), then 32–36 (Fig. 2C) which corresponds to the maximum number. Several cross-sections in the distal part of region II show the progressive decrease of cortical microtubules (Fig. 2D–F), the appearance of the mitochondrion (Fig. 2E), and the arrangement of cortical microtubules into two fields (Fig. 2F).
Schematic reconstruction of the mature spermatozoon of Gonocerca phycidis. Ase anterior spermatozoon extremity, Ax1 first axoneme, Ax2 second axoneme, Az attachment zones, C1 centriole of the first axoneme, C2 centriole of the second axoneme, Cm cortical microtubules, Eo external ornamentation of the plasma membrane, Feo, filamentous external ornamentation, Le lateral expansion, M mitochondrion, N nucleus, Pm plasma membrane, Pae1 posterior extremity of the first axoneme, Pae2 posterior extremity of the second axoneme, Pse posterior spermatozoon extremity
Region III (Figs. 2G–M and 3III) corresponds to the nuclear region and posterior spermatozoon extremity. In its proximal part, we notice the appearance of the nucleus with the simultaneous presence of the posterior part of the mitochondrion, two axonemes, and cortical microtubules disposed in two fields (Fig. 2G–H). Consecutive cross-sections show the disappearance of the mitochondrion (Fig. 2H–I), the gradual increase of the nucleus size (Fig. 2H–J) and the progressive disorganization of the first and the second axoneme (Fig. 2J–K). When cortical microtubules disappear completely, the cross-section show only the posterior extremity of the second axoneme and the nucleus with a reduced diameter (Fig. 2L). The posterior spermatozoon tip is characterized by the disappearance of the nucleus and the presence of only the doublets of the second axoneme (Figs. 2M and 3III).
Discussion
The mature spermatozoon of G. phycidis exhibits the general pattern described in most hemiuroideans: two axonemes of the 9 + ‘1’ pattern of Trepaxonemata (Ehlers 1984), nucleus, mitochondria, external ornamentation of the plasma membrane, and parallel cortical microtubules (Kacem et al. 2020). However, compared with hemiuroideans studied to date, the mature spermatozoon of G. phycidis presents some unusual characteristics (Table 1).
Anterior spermatozoon morphology
The mature spermatozoon of G. phycidis exhibits in its anterior tip two centrioles corresponding to the two axonemes of 9 + ‘1’ trepaxonematan pattern (Ehlers 1984). In the Hemiuroidea, mature spermatozoa exhibiting two axonemes in their anterior extremity are reported only in two species namely the Didymozoidae Gonapodasmius sp. (Justine and Mattei 1982) and the Sclerodistomoididae Sclerodistomoides pacificus (Bâ et al. 2020), whereas in the remaining studied species there is only one axoneme in their anterior tip (see Table 1).
In this anterior tip, there is also the presence of a continuous and submembranous layer of cortical microtubules. Within the Hemiuroidea, this feature was previously observed only in the didymozoid Gonapodasmius sp. by Justine and Mattei (1982). However, some cortical microtubules were also observed in the anterior spermatozoon extremity of other hemiuroideans namely the Hemiuridae Hemiurus appendiculatus and Lecithochirium microstomum (Ndiaye et al. 2014; Dione et al. 2016) and the Sclerodistomidae Prosorchis palinurichthi and Sclerodistomum italicum (Ndiaye et al. 2013b, 2017) whereas in the remaining hemiuroideans, cortical microtubules are absent in the anterior tip of the spermatozoon (Table 1). Nevertheless, their appearance is noted only when both axonemes are already formed. However, it is important to remark that in the didymozoids Didymocystis wedli and Didymozoon sp. (Justine and Mattei 1983; Pamplona-Basilio et al. 2001), the sperm cell lacks cortical microtubules.
Another peculiarity observed in the anterior spermatozoon region is the presence of a well-developed lateral expansion that distinguishes the mature spermatozoon of G. phycidis from those of the remaining hemiuroids studied until now. A lateral expansion has been described at the anterior extremity of the spermatozoon of digeneans belonging to five superfamilies: the Echinostomatoidea, the Microscaphidioidea, the Paramphistomoidea, the Pronocephaloidea and the Bucephaloidea (Bakhoum et al. 2017a; Kacem and Miquel 2018; Ndiaye et al. 2019). In the present work, we describe for the first time its presence in a Hemiuroidea. Its morphology is variable according to the species and it is generally associated with external ornamentation, cortical microtubules, and sometimes with spine-like bodies. In G. phycidis the lateral expansion is associated with only external ornamentation. This type of lateral expansion is described here for the first time in the anterior extremity of the digenean spermatozoa.
An external ornamentation of the plasma membrane is described in the anterior region of the spermatozoon from most species of Digenea in general and in particular from all Hemiuroidea studied to date except in the Didymozoids Didymocystis wedli and Didymozoon sp. (Pamplona-Basilio et al. 2001; Justine and Mattei 1983) which lack external ornamentation (see Table 1). The mature spermatozoon of G. phycidis displays two types of external ornamentation in its anterior region. The first type is filamentous and appears as a continuous layer associated with cortical microtubules while the second type is the classic one found in the majority of digeneans and usually is associated with cortical microtubules. However, in the Hemiuroidea this classical external ornamentation, when present, is not associated with cortical microtubules. To date, the presence of filamentous ornamentation is reported only in species of the superfamily Hemiuroidea, namely Aphanurus stossichii, H. appendiculatus, L. microstomum, P. palinurichthi, S. italicum and Gonapodasmius sp. (Justine and Mattei 1982; Ndiaye et al. 2013b, 2014, 2017; Dione et al. 2016; Kacem et al. 2020).
Cortical microtubules
In the Digenea, the number of cortical microtubules in the mature spermatozoon varies according to the species from zero in the Didymozoidae Didymozoon sp. and Didymocystis wedli (Justine and Mattei 1983; Pamplona-Basilio et al. 2001) to 73 in Diplodiscus subclavatus (Bakhoum et al. 2011). In most hemiuroids this number is between 5 to 11 as described in the Hemiuridae and the Lecithasteridae (Quilichini et al. 2010a; Kacem et al. 2020). However, about 24 to 36 cortical microtubules have been reported in Sclerodistomidae (Ndiaye et al. 2013b, 2017), Sclerodistomoididae (Bâ et al. 2020), Didymozoidae (Justine and Mattei, 1982), and Gonocercidae (present study).
The location of the maximum number of cortical microtubules is also variable according to the species. Quilichini et al. (2007) proposed for the first time two groups of digeneans according to the location of the maximum number of cortical microtubules in the spermatozoa: type 1 with the maximum number of cortical microtubules in the anterior part and type 2 in the median part of the spermatozoon. In G. phycidis, the maximum number of cortical microtubules is located in the median part of the spermatozoon and corresponds to the type 2 sensu Quilichini et al. (2007). This type 2 is also present in all hemiuroideans studied until now, except Gonapodasmius sp. which exhibits the maximum number in the anterior region and S. pacificus in the posterior one (see Table 1).
Most digeneans show an arrangement of cortical microtubules into two fields in the mitochondrial and nuclear regions of the spermatozoon as occurs in G. phycidis (present study) and in the didymozoid Gonapodasmius sp. (see Table 1). In contrast, in the remaining hemiuroideans studied to date, only one field of cortical microtubules has been described (see Table 1).
Mitochondria
In the digenean spermatozoa, the number of mitochondria varies from one to three, depending on the species (Bakhoum et al. 2017a). The mature spermatozoon of G. phycidis exhibits one mitochondrion as described in the remaining hemiuroideans studied up to now (see Table 1). On the other hand, the morphology of the mitochondrion is variable according to the species. Recently, a mitochondrial matrix granule has been observed in the hemiurid Aphanurus stossichii (Kacem et al. 2020). Moreover, a moniliform mitochondrion has been described in the sclerodistomoidid S. pacificus (Bâ et al. 2020). The morphological particularity of this type of mitochondrion has been described by the authors as a succession of bulges and cords. This type was also described in the male gamete of some digeneans such as the acanthocolpid Stephanostomoides tenuis (Bakhoum et al. 2015), the aephnidiogenids Holorchis micracanthum and H. pycnoporus (Bâ et al. 2011; Kacem and Miquel 2020a), the cryptogonimids Aphallus tubarium and Timoniella imbutiformis (Foata et al. 2012; Kacem et al. 2017a), the lepocreadids Opechona bacilliaris and Prodistomum polonii (Ndiaye et al. 2015; Kacem and Miquel 2020b), the opecoelids Allopodocotyle pedicellata and Macvicaria obovata (Bakhoum et al. 2017b; Kacem et al. 2017b) and the plagiorchiid Enodiotrema reductum (Ndiaye et al. 2012b). Another morphology of mitochondrion with a U-shaped posterior extremity has been described in the opecoelid Allopodocotyle tunisiensis by Kacem et al. (2019).
Posterior spermatozoon morphology
The posterior spermatozoon extremity is morphologically variable within digeneans. Quilichini et al. (2010b) were the first authors to propose three types of posterior spermatozoon extremities according to the disappearance of characters toward the posterior tip. Type 1 or opecoelidean type with the sequence “second axoneme, nucleus and cortical microtubules”, type 2 or fasciolidean type with the sequence “cortical microtubules, second axoneme and nucleus” and type 3 or cryptogonimidean type with the sequence “cortical microtubules, nucleus and second axoneme”. Following this criterion, the mature spermatozoon of G. phycidis exhibits type 3 of posterior spermatozoon extremity. This type has been observed in nearly all digenean species belonging to the Hemiuroidea (see Table 1).
Spermatozoa models in the Hemiuroidea
The ultrastructural study of species belonging to the Hemiuroidea reveals similarities in their mature spermatozoa. According to Bakhoum et al. (2017a), the hemiuroideans described until now show the type II sperm model. The latter is characterized by the presence of two 9 + ‘1’ axonemes, external ornamentation of the plasma membrane not associated with cortical microtubules and located in the anterior part of the spermatozoon, one bundle of cortical microtubules of which the maximum number is located in a middle part of the spermatozoon and one mitochondrion. However, the mature spermatozoon described in G. phycidis seems not to follow this type II sperm model. Several characters present in the male gamete of G. phycidis are absent in the mature spermatozoa of other hemiuroideans. Those are:
-
The lateral expansion associated with external ornamentation of the plasma membrane and without cortical microtubules,
-
The two types of external ornamentation of the plasma membrane located in the anterior extremity of the spermatozoon,
-
The two bundles of cortical microtubules in the mitochondrial and nuclear areas of the spermatozoon.
The position of the Gonocercidae within the Hemiuroidea seems to be confirmed. Our study confirms the previous molecular studies regarding the position of Gonocercidae compared with the rest of the hemiuroidean species (Sokolov et al. 2016, 2018, 2019). According to these authors, Gonocerca spp. are phylogenetically distant from other hemiuroid trematodes, including Derogenes varicus, representative of the type genus of the family Derogenidae. Unfortunately, the family Derogenidae remains unexplored from the spermatological point of view. In the future, it would be important to perform ultrastructural studies of spermatozoon not only in representatives of the family Derogenidae but also in the remaining genera of Gonocercidae.
Conclusion
The present study enlarges the data on ultrastructural studies in the Digenea providing, for the first time, spermatological characteristics in the Gonocercidae. The most interesting findings are the presence of a lateral expansion associated only with external ornamentation and the presence of two fields of cortical microtubules in the mitochondrial and nuclear regions of the male gamete of G. phycidis. These features distinguish the mature spermatozoon of G. phycidis from those reported in other hemiuroideans. However, additional spermatological studies are needed on this family, but especially on the Derogenidae from which the Gonocercidae has been separated to confirm and complete the results of the molecular studies.
References
Bâ CT, Ndiaye PI, Dione A, Quilichini Y, Marchand B (2011) Ultrastructure of the spermatozoon of Holorchis micracanthum (Digenea: Lepocreadiidae), an intestinal parasite of Plectorhinchus mediterraneus (Pisces, Teleostei) in Senegal. Parasitol Res 109:1099–1106. https://doi.org/10.1007/s00436-011-2352-1
Bâ A, Bakhoum AJS, Ndiaye PI, Bâ CT, Marchand B, Quilichini Y (2020) Spermatological characteristics of Sclerodistomoides pacificus (Digenea, Sclerodistomoididae) a parasite of the flying fish Cheilopogon pinnatibarbatus (Teleostei, Exocoetidae). Tissue Cell 62:101314. https://doi.org/10.1016/j.tice.2019.101314
Bakhoum AJS, Torres J, Shimalov VV, Bâ CT, Miquel J (2011) Spermiogenesis and spermatozoon ultrastructure of Diplodiscus subclavatus (Pallas, 1760) (Paramphistomoidea, Diplodiscidae), an intestinal fluke of the pool frog Rana lessonae (Amphibia, Anura). Parasitol Int 60:60–74. https://doi.org/10.1016/j.parint.2010.10.006
Bakhoum AJS, Quilichini Y, Justine J-L, Bray RA, Bâ CT, Marchand B (2015) Ultrastructural study of sperm cells in Acanthocolpidae: the case of Stephanostomum murielae and Stephanostomoides tenuis (Digenea). PeerJ. https://doi.org/10.7717/peerj.744
Bakhoum AJS, Miquel J, Ndiaye PI, Justine J-L, Falchi A, Bâ CT, Marchand B, Quilichini Y (2017a) Advances in spermatological characters in the Digenea: review and proposal of spermatozoa models and their phylogenetic importance. Adv Parasitol 98:111–165. https://doi.org/10.1016/bs.apar.2017.04.001
Bakhoum AJS, Kacem H, Neifar L, Miquel J (2017b) The Opecoelidae sperm model and its contribution to phylogeny: spermatozoon ultrastructural particularities of Allopodocotyle pedicellata (Plagioporinae, Digenea, Platyhelminthes). Zool Anz 266:28–34. https://doi.org/10.1016/j.jcz.2016.10.006
Dione A, Quilichini Y, Bâ CT, Diagne PM, Ndiaye PI, Marchand B (2016) Ultrastructural study of the spermatozoon of Hemiurus appendiculatus (Digenea, Hemiuroidea, Hemiuridae), a parasite of Boops boops (Pisces, Teleostei, Sparidae) off Senegal. Tissue Cell 48:96–103. https://doi.org/10.1016/j.tice.2016.01.002
Ehlers U (1984) Phylogenetisches system der Plathelminthes. Verh Naturwiss Ver Hambg (NF) 27:291–294
Foata J, Quilichini Y, Greani S, Marchand B (2012) Sperm ultrastructure of the digenean Aphallus tubarium (Rudolphi, 1819) Poche, 1926 (Platyhelminthes, Cryptogonimidae) intestinal parasite of Dentex dentex (Pisces, Teleostei). Tissue Cell 44:15–21. https://doi.org/10.1016/j.tice.2011.10.001
Gibson DI (2002) Superfamily Hemiuroidea Looss, 1899. In: Gibson DI, Jones A, Bray RA (eds) Keys to the Trematoda, vol 1. CABI Publishing and The Natural History Museum, Wallingford, pp 299–304
Gibson DI, Bray RA (1979) The Hemiuroidea: terminology, systematics and evolution. Bull Br Mus Nat Hist (zool) 36:35–146
Justine J-L, Mattei X (1982) Étude ultrastructurale de la spermiogenèse et du spermatozoïde d’un Plathelminthe: Gonapodasmius (Trematoda: Didymozoidae). J Ultrastruct Res 79:350–365. https://doi.org/10.1016/S0022-5320(82)90010-7
Justine J-L, Mattei X (1983) A spermatozoon with two 9 + 0 axonemes in a parasitic flatworm, Didymozoon (Digenea: Didymozoidae). J Submicrosc Cytol 15:1101–1105
Justine J-L, Mattei X (1984) Ultrastructural observations on the spermatozoon, ovocyte and fertilization process in Gonapodasmius, a gonochoristic Trematode (Trematoda: Digenea: Didymozoidae). Acta Zool (stockh) 65:171–177. https://doi.org/10.1111/j.1463-6395.1984.tb00822.x
Kacem H, Miquel J (2018) Sperm characters of the Bucephalid digenean Prosorhynchoides arcuatus and their phylogenetic significance. Zool Anz 274:6–13. https://doi.org/10.1016/j.jcz.2018.03.003
Kacem H, Miquel J (2020a) Spermatological characters in the Lepocreadioidea, with first data on Holorchis pycnoporus (Aephnidiogenidae), a parasite of the striped seabream Lithognathus mormyrus (Sparidae) from the Gulf Gabes (Tunisia). Tissue Cell 67:101409. https://doi.org/10.1016/j.tice.2020a.101409
Kacem H, Miquel J (2020b) Sperm ultrastructure of Prodistomum polonii (Digenea, Lepocreadioidea), an intestinal parasite of horse mackerel, Trachurus trachurus (Teleostei, Carangidae), from the Gulf of Gabes, Mediterranean Sea. Zool Anz 286:100–107. https://doi.org/10.1016/j.jcz.2020.04.004
Kacem H, Blasco S, Foronda P, Miquel J (2017a) Sperm characters of Timoniella imbutiforme (Digenea, Opisthorchioidea, Cryptogonimidae), a parasite of the European seabass Dicentrarchus labrax. Zool Anz 271:49–56. https://doi.org/10.1016/j.jcz.2017.11.005
Kacem H, Quilichini Y, Neifar L, Torres J, Miquel J (2017b) Ultrastructure of the spermatozoon of Macvicaria obovata (Digenea: Opecoelidae), a parasite of Sparus aurata (Pisces: Teleostei) from the Gulf of Gabes, Mediterranean Sea. Acta Parasitol 62:520–528. https://doi.org/10.1515/ap-2017-0062
Kacem H, Diagne PM, Miquel J (2019) Ultrastructural organization of the spermatozoon of Allopodocotyle tunisiensis Derbel and Neifar, 2009 (Digenea, Opecoelidae), an intestinal parasite of Solea aegyptiaca Chabanaud, 1927 (Teleostei, Soleidae). Tissue Cell 57:1–7. https://doi.org/10.1016/j.tice.2019.01.008
Kacem H, Giese EG, Miquel J (2020) Sperm characters in the Hemiuridae (Digenea), first data on Aphanurus stosschii (Aphanuridae) and Ectenurus lepidus (Dinuridae). Parasitol Res 119:991–999. https://doi.org/10.1007/s00436-020-06609-3
Ndiaye PI, Diagne PMB, Sène A, Bakhoum AJS, Miquel J (2012a) Ultrastructure of the spermatozoon of the digenean Lecithocladium excisum (Rudolphi, 1819) (Hemiuroidea, Hemiuridae), a parasite of marine teleost in Senegal. Folia Parasitol 59:173–178. https://doi.org/10.14411/fp.2012a.024
Ndiaye PI, Quilichini Y, Sène A, Tkach VV, Bâ CT, Marchand B (2012b) Ultrastructural study of the spermatozoon of the digenean Enodiotrema reductum Looss, 1901 (Platyhelminthes, Plagiorchioidea, Plagiorchiidae), a parasite of the green turtle Chelonia mydas (Linnaeus, 1758) in Senegal. Parasitol Res 111:859–864. https://doi.org/10.1007/s00436-012-2911-0
Ndiaye PI, Bakhoum AJS, Sène A, Miquel J (2013a) Ultrastructure of the spermatozoon of Parahemiurus merus (Linton, 1910) (Digenea: Hemiuroidea: Hemiuridae), a parasite of Sardinella aurita Valenciennes, 1847 and S. maderensis (Lowe, 1838) (Teleostei: Clupeidae) in the Senegalese coast. Zool Anz 252:572–578. https://doi.org/10.1016/j.jcz.2012.11.005
Ndiaye PI, Quilichini Y, Sène A, Bray RA, Bâ CT, Marchand B (2013b) Prosorchis palinurichthi Kurochkin and Korotaeva, 1971 (Digenea, Sclerodistomidae): ultrastructure of the mature spermatozoon. Zool Anz 252:404–409. https://doi.org/10.1016/j.jcz.2012.11.001
Ndiaye PI, Quilichini Y, Sène A, Tkach VV, Bâ CT, Marchand B (2014) Ultrastructural characters of the spermatozoa in Digeneans of the genus Lecithochirium Lühe, 1901 (Digenea, Hemiuridae), parasites of fishes: a comparative study of L. microstomum and L. musculus. Parasite 21:49. https://doi.org/10.1051/parasite/2014050
Ndiaye PI, Bakhoum AJS, Sène A, Diagne PM, Miquel J (2015) The ultrastructural characters of the mature spermatozoon of Opechona bacillaris (Molin, 1859) (Digenea, Lepocreadiidae) a parasite of Scomber colias Gmelin, 1789 (Scombridae) off the coast of Dakar (Senegal). Acta Zool (stockh) 96:91–98. https://doi.org/10.1111/azo.12054
Ndiaye PI, Quilichini Y, Marigo AM, Bâ CT, Tkach VV, Marchand B (2017) Ultrastructural characteristics of the mature spermatozoon of the digenean Sclerodistomum italicum (Stossich, 1893) (Hemiuroidea, Sclerodistomidae) intestinal parasite of Hypocanthus amia (Teleostei, Carangidae). Tissue Cell 49:15–21. https://doi.org/10.1016/j.tice.2016.12.007
Ndiaye PI, Marchand B, Bâ CT, Justine J-L, Bray RA, Quilichini Y (2019) Ultrastructure of the mature spermatozoa of three Bucephalidae (Prosorhynchus longisaccatus, Rhipidocotyle khalili and Bucephalus margaritae) and phylogenetic implications. Parasite 25:65. https://doi.org/10.1051/parasite/2018065
Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DTJ (2003) Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int J Parasitol 33:733–755. https://doi.org/10.1016/S0020-7519(03)00049-3
Pamplona-Basilio MC, Baptista-Farias MFD, Kohn A (2001) Spermatogenesis and spermiogenesis in Didymocystis wedli Ariola, 1902 (Didymozoidae, Digenea). Mem Inst Oswaldo Cruz 96:1153–1159
Pankov P, Webster BL, Blasco-Costa I, Gibson DI, Littlewood DTJ, Balbuena JA, Kostadinova A (2006) Robinia aurata n. g., n. sp. (Digenea: Hemiuridae) from the mugilid Liza aurata with molecular confirmation of its position within the Hemiuroidea. Parasitology 133:217–227. https://doi.org/10.1017/S0031182006000126
Quilichini Y, Foata J, Marchand B (2007) Ultrastructural study of the spermatozoon of Pronoprymna ventricosa (Digenea, Baccigerinae), a parasite of the twaite shad Alosa fallax Lacepede (Pisces, Teleostei). Parasitol Res 101:1125–1130. https://doi.org/10.1007/s00436-007-0599-3
Quilichini Y, Foata J, Justine J-L, Bray RA, Marchand B (2010a) Spermatozoon ultrastructure of Aponurus laguncula (Digenea: Lecithasteridae), a parasite of Aluterus monoceros (Pisces, Teleostei). Parasitol Int 59:22–28. https://doi.org/10.1016/j.parint.2009.06.007
Quilichini Y, Foata J, Justine J-L, Bray RA, Marchand B (2010b) Ultrastructural study of the spermatozoon of Heterolebes maculosus (Digenea, Opistholebetidae), a parasite of the porcupinefish Diodon hystrix (Pisces, Teleostei). Parasitol Int 59:427–434. https://doi.org/10.1016/j.parint.2010b.06.002
Skryabin KI, Guschanskaja LKH (1955) Suborder Hemiurata (Markevitsch, 1951) Skrjabin et Gushanskaja, 1954. Part 3. In: Skryabin KI (ed) Trematody zhivotnykh i cheloveka. Publishing House of the USSR Academic Science, Moscow, pp 465–748 (in Russian)
Sokolov SG, Gordeev II, Atopkin DM (2016) Redescription of trematode Gonocerca muraenolepisi Paruchin et Ljadov, 1979 (Hemiuroidea: Derogenidae), a body cavity parasite of Antarctic fishes, with a discussion of its phylogenetic position. Invert Zool. 13:191–202. https://doi.org/10.15298/invertzool.13.2.02
Sokolov SG, Atopkin DM, Gordeev II, Shedko MB (2018) Phylogenetic position of the genus Gonocerca Manter, 1925 (Trematoda, Hemiuroidea), based on partial sequences of 28S rRNA gene and a reconsideration of taxonomic status of Gonocercinae Skrjabin et Guschanskaja, 1955. Parasitol Int 67:74–78. https://doi.org/10.1016/j.parint.2017.03.007
Sokolov SG, Atopkin DM, Urabe M, Gordeev II (2019) Phylogenetic analysis of the superfamily Hemiuroidea (Platyhelminthes, Neodermata: Trematoda) based on partial 28S rDNA sequences. Parasitol 146:596–603. https://doi.org/10.1017/S0031182018001841
Acknowledgements
The authors are grateful of the “Collectivité Teritorriale de Corse”. This study was also partly supported by the financial assistance of “Projet d’appui pour la Promotion des Enseignantes-chercheures du Sénégal (PAPES) du Ministère de l’Enseignement supérieur et de la Recherche du Sénégal”.
Author information
Authors and Affiliations
Contributions
All authors contributed to and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Collection of specimens was governed by national guidelines and permits.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bâ, A., Bakhoum, A.J.S., Ndiaye, P.I. et al. New spermatological characteristics in the Hemiuroidea inferred from the ultrastructure of the spermatozoon of Gonocerca phycidis a stomach parasite of Gephyroberyx darwinii (Teleostei: Trachichthyidae) off the Senegal coast. Zoomorphology 141, 223–231 (2022). https://doi.org/10.1007/s00435-022-00569-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-022-00569-1