Abstract
Naive and immune specific-pathogen-free rabbits were inoculated in the duodenum with sporocysts of Eimeria coecicola or Eimeria intestinalis. Samples were taken from the following tissues: duodenum (site of penetration of sporozoites), ileum (specific target site of the endogenous development of E. intestinalis), vermiform appendix (target site of E. coecicola) and two extraintestinal sites, mesenteric lymph nodes (MLNs), and spleen. The presence of sporozoites was checked by immunohistochemistry. In rabbits primary-infected with E. coecicola, large numbers of sporozoites were detected in the duodenum, extraintestinal sites, and vermiform appendix. The abundance of sporozoites in the spleen, MLN, and appendix was significantly reduced in the immune rabbits, and the migration seemed impeded. In the rabbits infected with E. intestinalis, sporozoites were absent in the spleen and MLN, indicating that the route of migration is different from that of E. coecicola. The number of sporozoites in the crypts of the ileum was markedly reduced in the immune animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies (Drouet-Viard et al. 1994; Pakandl et al. 1993, 1995, 1996) showed that the sporozoites of three coccidian species developing in the gut of rabbits, Eimeria coecicola, Eimeria intestinalis, and Eimeria magna, first penetrate the mucosa of the small intestine, especially the duodenum. They are first observed in villous epithelial cells and subsequently in intraepithelial lymphocytes (IELs) and lamina propria. Later, the sporozoites reach the sites where they subsequently develop into meronts, i.e., the ileum for E. magna and E. intestinalis and gut-associated lymphoid tissue, including vermiform appendix, sacculus rotundus, and Peyer’s patches, for E. coecicola. The existence of an extraintestinal phase was demonstrated by Renaux et al. (2001) in E. coecicola.
In the present work, the invasion process of the rabbit intestine (penetration and migration of sporozoites) used by two coccidia, E. coecicola and E. intestinalis, is compared in naive and immunized animals to assess the influence of host immune response on this phase of the life cycle. Simultaneously, ultrastructural modifications of the parasite and the host cell that could be linked with the host immune status were determined.
Materials and methods
Animals
A total of 52 coccidia-free New Zealand white rabbits at 5–6 weeks of age reared under controlled conditions (Coudert et al. 1988) were used. The experiments were performed in INRA and were officially permitted.
Immunization process
The immunized rabbits were inoculated twice with 2.5×104 oocysts of E. coecicola or with 103 oocysts (first inoculation) and 5×103 oocysts (second inoculation) of E. intestinalis. In both cases, the interval between inoculations was 2 weeks, and the rabbits were euthanized in our experiments 2–3 weeks after the last inoculation.
Parasites
Freshly prepared inocula originated from pure strains of E. coecicola and E. intestinalis were obtained from field isolates.
Inoculation of rabbits and sampling
Both immune and naive animals were inoculated under anesthesia with 3×107 sporocysts of either E. coecicola or E. intestinalis directly into the duodenum as previously described (Pakandl et al. 1993). Some rabbits were orally inoculated with 7.5×106 oocysts of E. intestinalis. The animals were then killed at different time after inoculation.
Tissue samples were taken from the duodenum, ileum, spleen, and mesenteric lymph nodes (MLNs) of rabbits inoculated both with E. intestinalis and E. coecicola, and an additional sample was taken from the appendix of the rabbits inoculated with E. coecicola. The samples were processed for immunohistochemistry and transmission electron microscopy (TEM).
Immunohistochemistry
The samples were fixed overnight with a fixative composed of 5% (w/v) formaldehyde (prepared from paraformaldehyde before use) diluted in phosphate-buffered saline (PBS), washed in PBS, and dehydrated with two baths in 96% ethanol and two baths in 100% ethanol. All these steps were performed at 4°C. The samples were subsequently treated with three baths of xylene for 1 h at room temperature and with three baths of Paraplast (melting range 50–54°C; Sigma) for 2 h at a temperature not exceeding 57°C and then embedded in Paraplast.
The blocks were cut into 5-μm-thick sections, which were deparaffinized with toluene, then with 100 and 96% ethanol and finally placed in distilled water. The sporozoites in sections were labeled by an immunoperoxidase technique using Vectastain Elite ABC and Vector VIP substrate kits (Vector Laboratories). Primary antibodies to both E. coecicola and E. intestinalis were prepared in chickens (Renaux 2001). The working dilution of the antibody was 1:6,000, and biotinylated antibody against chicken immunoglobulin G (Vector Laboratories) was used as a secondary antibody. The labeling was performed according to manufacturer’s instructions, and the sections were counterstained with methyl green.
Semiquantitative evaluation of the number of sporozoites in host tissues
In all Eimeria species, the distribution of the parasites along the intestine is discontinuous. Therefore, quantification cannot be precise enough to perform statistics, and we made a semiquantitative assessment of the abundance of sporozoites. The sporozoites were counted in six nonserial sections (the distance between them was at least 30 μm to avoid counting the same parasites), and the number of sporozoites per section was assessed using a scoring system.
Each average number per section was obtained from pairs of rabbits which had the same immune status and treatment. The amount of sporozoites was expressed using the following score: 0, none; 1, 1–5; 2, 5–10; 3, 10–20; 4, 20–50; 5, 50–100; 6, >100.
Transmission electron microscopy
For both coccidian species, samples from the duodenum and the target organ (appendix for E. coecicola and ileum for E. intestinalis) were taken for TEM. The samples were fixed with 3% glutaraldehyde in 0.1 M cacodylate buffer, postfixed with 1% osmium tetroxide in the same buffer, dehydrated in graded ethanol series, and embedded in Spurr. Semithin sections were stained with Warmke’s polychrome for light microscopy, and ultrathin sections were contrasted with uranyl acetate and lead citrate and examined using the Jeol 1010 electron microscope.
Experimental design
The intervals and sites of sampling in our experiments were settled according to previous results (Licois et al. 1992; Pakandl et al. 1993; Drouet-Viard et al. 1994).
The experiments were repeated three times with E. coecicola and twice with E. intestinalis.
Eimeria coecicola
Both naive and immune animals were inoculated and then killed at 6, 16, 32, 48, and 64 h postinoculation (p.i.). Two naive and two immune animals were used at each interval.
Eimeria intestinalis
Both naive and immune animals were inoculated and then killed 1, 4, 8, 14, 20, and 48 h p.i. Two naive and two immunized rabbits were used at each interval as well. Besides animals inoculated with sporocysts into the duodenum, some animals (two naive and two immune) were orally infected with oocysts and euthanized 8 and 48 h p.i.
Results
The occurrence of sporozoites in the intestine and lymphoid organs after inoculation of rabbits
Eimeria coecicola
The abundance of sporozoites in the sections is summarized in Fig. 1a (intestine) and Fig. 1b (MLN and spleen).
Changes in the numbers of sporozoites detected in intestine and extraintestinal sites in primary-infected or immune rabbits infected with either E. coecicola or E. intestinalis. P primary-infected, I immune, h p.i. hours postinoculation. The abundance (average number per section) of parasites is expressed as a score: 0, none; 1, 1–5; 2, 5–10; 3, 10–20; 4, 20–50; 5, 50–100; 6, >100. a Intestine of rabbits infected with E. coecicola. b Extraintestinal sites in rabbits infected with E. coecicola. MLN mesenteric lymph nodes. c Intestine of rabbits infected with E. intestinalis. i.d. intraduodenal inoculation, p.o. inoculation per os
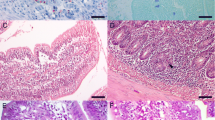
After infection of both naive and immune rabbits, the sporozoites were seen almost exclusively in the epithelial zone of the duodenum at 6 h p.i. They were found in enterocytes as well as in IEL. Later, they were also detected in the lamina propria (Fig. 2a). The sporozoites were detected in MLN and spleen (Fig. 2b,c) from 32 h p.i. They were selectively located in the white pulp of the spleen, and in MLN, they predominantly occurred in deep cortex. In the vermiform appendix, most of the sporozoites were seen in lymphoid cells at the base of the domes and, sometimes, in M cells in the epithelial zone. The number of sporozoites in spleen and MLN was markedly lower in the immune animals than in the primary-infected rabbits at 32, 48, and 64 h p.i. From 48 h p.i., the sporozoites were seen in the appendix (Fig. 2d), and the difference in the amount of sporozoites between both groups of animals was remarkable in this organ as well. Only sporozoites were observed in the organs of both naive and immune animals up to 48 h p.i. At 64 h p.i., numerous meronts were found in the appendix of the primary-infected rabbits, whereas in the immune animals, the parasite development seemed to be arrested at sporozoite stage.
Distribution of sporozoites in paraffin sections of tissues from rabbits primary-infected with E. coecicola. The sporozoites were labeled with chicken polyclonal antibodies visualized with the immunoperoxidase technique. a Two sporozoites (arrows) in a duodenal villus, left in lamina propria, right in the epithelium (×400), 16 h p.i. b Sporozoites in the MLN (×640), 32 h p.i. c Sporozoites in the spleen (×330), 48 h p.i. d Sporozoites in the appendix (×280), 48 h p.i. D domes, M mushrooms
Eimeria intestinalis
The amount of sporozoites in the sections is summarized in the Fig. 1c. No correlation between host immune status and the number of sporozoites in the duodenum was noted during the whole experiment. The same observation is valid for the ileum, except at 48 h p.i.
Since no sporozoites were found in the spleens and MLN, these data are not shown. From 1 to 20 h p.i., the sporozoites were found almost exclusively in upper parts of the villi of both the duodenum and ileum (Fig. 3a,b) and in the epithelial cells as well as in the lamina propria. It was impossible to find any dependence of sporozoite distribution on the host immune status at this time. In contrast, the sporozoites mostly occurred in the epithelium of crypts both in the duodenum and ileum 48 h p.i. (Fig. 3c). The crypts of the ileum are the specific sites of parasite multiplication, and the number of sporozoites in this location was strongly reduced in the immune animals. No substantial difference was found between the rabbits inoculated per os vs those inoculated into the duodenum.
Distribution of sporozoites in paraffin sections of intestine from rabbits primary-infected with E. intestinalis. a Sporozoites (arrows) in a duodenal villus close to basal membrane of the epithelium (×400), 4 h p.i. b Sporozoites near the top of the ileal villus (×400), 14 h p.i. c Sporozoites in the epithelium of ileal crypts (×340), 48 h p.i. C crypts, LP lamina propria, TS tela submucosa
In both species, very few sporozoites were occasionally observed in nonspecific sites, such as the ileum for E. coecicola and crypts of the duodenum for E. intestinalis. This result may be caused by the very large inoculum, which is, however, necessary to perform this study focusing on the migration of sporozoites.
Ultrastructural findings in immune rabbits
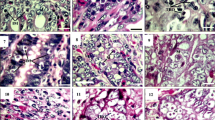
Electron microscopy was used to examine ultrastructural changes of the host cell and parasite, possibly related to the host immune status. In the duodenum, the sporozoites of both parasite species were found in villous epithelial cells and IEL, close to the surface, as well as at the base of the epithelium and within lamina propria lymphocytes (LPL). No ultrastructural changes related to the immune reaction of the host cells were noted in the duodenum of both naive and immune hosts infected with E. coecicola or E. intestinalis, respectively. On the contrary, the observations were different with E. coecicola and E. intestinalis in the target sites. The sporozoites of E. coecicola were found within leukocytes in the vermiform appendix at 48 and 64 h p.i., usually at the base of the domes. Unlike the duodenum, an ultrastructural peculiarity was found: small vesicles limited by a single membrane were seen in the cytoplasm of host cells harboring sporozoites in both naive and immune rabbits. However, these vesicles sometimes fused with the parasitophorous vacuole (Figs. 4a,b) in the immune but not the naive animals. First generation meronts were seen 64 h p.i. in the lymphocytes or M cells in the follicle-associated epithelium covering the domes in the primary-infected animals. However, meronts were only rarely found in their immune counterparts.
In E. intestinalis, the sporozoites were seen in both the ileum and duodenum in absorptive cells but also in IEL and LPL. Like in E. coecicola, young meronts were found in the primary-infected animals but only rarely in the immune rabbits. However, no other ultrastructural peculiarity linked with the immune status of the host was seen in the ileum of these rabbits.
Discussion
The present experiments were designed to compare early events in the life cycles of two Eimeria species in naive animals and determine the effect of the immune response on these events. With regard to the invasion of the host by sporozoites, it has been already stated that there is a substantial difference between chicken and rabbit coccidia (Pakandl et al. 1995). While the sporozoites of chicken coccidia penetrate the same part of the intestine in which they subsequently develop (Lawn and Rose 1982; Fernando et al. 1987), the sporozoites of rabbit species first enter the duodenum, i.e. another part of the intestine and, only later, are found in their specific site of multiplication. The present results confirm our previous findings that in rabbit-infecting species, unlike chicken coccidia, the site of penetration of sporozoites is not species-specific (Pakandl et al. 1993, 1995, 1996; Drouet-Viard et al. 1994). However, our results have shown that the penetration of sporozoites is not limited to the duodenum in E. intestinalis. The sporozoites enter epithelial cells at the tops of the villi, probably along the whole length of the small intestine, and subsequently migrate to the epithelium of crypts in the ileum, the specific site of the first merogony in this species.
The most important difference between the two rabbit coccidia studied is the massive presence of E. coecicola sporozoites in extraintestinal sites, MLN, and spleen, while the E. intestinalis sporozoites were virtually absent in these organs. This observation suggests that the route of migration is different in these two species. The extraintestinal migration of E. coecicola seems to be an integral part of its life cycle involving a majority, if not all, of the sporozoites. In contrast, only a few sporozoites of E. intestinalis seem to undergo extraintestinal migration; thus, the early events in the endogenous development of both species substantially differ.
We also sought to assess the effect of the immune status on the parasite invasion process. At the site of penetration, when E. intestinalis sporozoites occurred in nonspecific sites (duodenum and tops of the villi), their distribution was related with neither the time after inoculation nor host immune status. They were present in their target site (crypts of the ileum) at 48 h p.i., where the abundance of sporozoites markedly differed between immune and primary-infected animals.
We have previously established that the total oocyst output after administration of 3×107 sporocysts into the duodenum, or the equivalent amount of oocysts (7.5×106) given per os, to the animals immunized against either species is usually undetectable or very low (<100 oocysts; data not shown). However, in the immune animals, some sporozoites were observed in the target sites of both species in immune animals. This observation suggests that further stages of parasite development, which were not examined in this study, are also affected by host immune response.
The number of sporozoites of E. coecicola in MLN, spleen, and vermiform appendix was markedly reduced in the immune rabbits, whereas no conspicuous difference between primary-infected and immune animals was noted in the duodenum. Thus, the migration of sporozoites seems to be impeded, and most of the parasites and/or parasitized cells are probably already eliminated at this site. Moreover, the very rare occurrence of meronts in the immune animals suggests that their formation is also suppressed by the host immune response. Similarly, a reduced number of sporozoites and meronts were found in the specific site (crypts of the ileum) in rabbits immunized against and infected with E. intestinalis; therefore, similar conclusions may be drawn for this species.
We also looked for ultrastructural changes that might be related to immune response. In E. coecicola, we noted fusion of small vesicles (possibly lysosomes) with the parasitophorous vacuole within host lymphoid cells harboring sporozoites. Since this occurrence has been observed only in immune animals, this process is probably related to the immune reaction of the host cells. It should be pointed out that lymphoid cells carrying sporozoites can play the double role of vectors for the parasites and effector cells of the immune response.
Two steps should be distinguished in the endogenous development of coccidia: penetration of sporozoites, i.e., their first entry into host cells, and their migration within host tissues to the species-specific site. The first step was not conspicuously influenced by the host immune response to either E. coecicola or E. intestinalis, whereas the migration was affected and the number of sporozoites in the target sites was markedly reduced.
In chickens, the migration of sporozoites is also influenced by the host immune response. In Eimeria tenella, Rose et al. (1984) noted a marked reduction in the numbers of developing parasites in immune chickens compared to their nonimmune counterparts. This effect was due, at least partially, to failure of sporozoites transported by IEL to transfer to crypt enterocytes. Riley and Fernando (1988) found that in immune, compared to nonimmune birds, a significantly greater number of IEL harboring sporozoites of Eimeria maxima tended to remain in the lamina propria rather than migrate to the target site, i.e., crypts.
Taken together, the early events in the life cycle of chicken and rabbit coccidia are similar, but the rabbit species have at least two peculiarities: (a) nonspecific site of penetration and (b) massive extraintestinal migration of sporozoites demonstrated presently in E. coecicola. This extraintestinal migration is also most probably the rule in Eimeria stiedai (Horton 1967; Fitzgerald 1972, 1974).
References
Coudert P, Licois D, Besnard J (1988) Establishment of a SPF breeding colony without hysterectomy and handrearing procedures. In: Proceedings of the 4th congress of the world rabbit science association, Budapest, 10–14 October, p 480
Drouet-Viard F, Licois D, Provôt F, Coudert P (1994) The invasion of the rabbit intestinal tract by Eimeria intestinalis sporozoites. Parasitol Res 80:118
Fernando MA, Rose ME, Millard BJ (1987) Eimeria spp. of the domestic fowl: the migration of sporozoites intra- and extraenterically. J Parasitol 73:561–567
Fitzgerald PR (1972) Transmission of Eimeria stiedai by blood transfusion. J Parasitol 58:62
Fitzgerald PR (1974) Results of blood transmission from donor rabbits infected with Eimeria stiedai to recipient coccidia-free rabbits. J Protozool 21:336–338
Horton RJ (1967) The route of migration of Eimeria stiedai sporozoites between the duodenum and bile ducts of the rabbit. Parasitology 57:9–17
Lawn AM, Rose ME (1982) Mucosal transport of Eimeria tenella in the cecum of the chicken. J Parasitol 68:1117–1123
Licois D, Coudert P, Bahagia S, Rossi GL (1992) Characterisation of Eimeria species in rabbits (Oryctolagus cuniculus): endogenous development of Eimeria intestinalis Cheissin, 1948. J Parasitol 78:1041–1046
Pakandl M, Coudert P, Licois D (1993) Migration of sporozoites and merogony of Eimeria coecicola in the gut-associated lymphoid tissue. Parasitol Res 79:593–598
Pakandl M, Drouet-Viard F, Coudert P (1995) How do sporozoites of rabbit Eimeria species reach their target cells? C R Acad Sci III 318:1213–1217
Pakandl M, Gaca K, Drouet-Viard F, Coudert P (1996) Eimeria coecicola: endogenous development in gut-associated lymphoid tissue. Parasitol Res 82:347–351
Renaux S (2001) Eimeria du lapin: étude de la migration extra-intestinale du sporozoïte et du développement de l’immunité protectrice. Thèse pour obtenir le grade de docteur de l’Université de Tours
Renaux S, Drouet-Viard F, Chanteloup NK, Le Vern Y, Kerboeuf D, Pakandl M, Coudert P (2001) Tissue and cells involved in the invasion of the rabbit intestinal tract by sporozoites of Eimeria coecicola. Parasitol Res 87:98–106
Riley D, Fernando MA (1988) Eimeria maxima (Apicomplexa): a comparison of sporozoite transport in naive and immune chickens. J Parasitol 74:103–110
Rose ME, Lawn AM, Millard BJ (1984) The effects of immunity on the early events in the life-cycle of Eimeria tenella in cecal mucosa of the chickens. Parasitology 88:199–210
Acknowledgements
The present work was supported by a grant of the Grant Agency of the Academy of Sciences of the Czech Republic No. S6022002 and research project of the Institute of Parasitology, AS CR (Z60220518). The experiments performed on the animals complied with the current laws of France and the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pakandl, M., Sewald, B. & Drouet-Viard, F. Invasion of the intestinal tract by sporozoites of Eimeria coecicola and Eimeria intestinalis in naive and immune rabbits. Parasitol Res 98, 310–316 (2006). https://doi.org/10.1007/s00436-005-0071-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-0071-1