Abstract
This histopathological study was carried out in order to investigate the cellular response in the jejunum to Ascaridia galli during the first 7 weeks of infection. Fourty-two ISA Brown chickens (7 weeks old) were infected orally with 500 embryonated A. galli eggs each while 28 chickens were left as uninfected controls. Six infected and four control chickens were necropsied at each time point 3, 7, 10, 14, 21, 28 and 42 days post-infection (dpi). Samples for histopathology were taken from three sites of the jejunoileum. Significantly higher eosinophil counts were seen in infected chickens compared to uninfected at 3, 7, 10, 14 and 28 dpi (P < 0.01). In both groups, the initial number of mast cells was high, but this high level of mast cells remained for a longer period in the infected group compared to the control group. Significantly higher counts were thus found in the infected group at 21 (P < 0.001), 28 (P < 0.01) and 42 dpi (P < 0.05). A. galli infection induced changes in the mucosal thickness as reduced villi length at 7, 10, 14, 21 and 28 dpi and in the degree of general cellular infiltration in the lamina propria of the mucosal layer. No adult worms were seen during the experiment; therefore, A. galli larvae have elicited a moderate cellular response in the lamina propria, mainly consisting of eosinophils in the early phase and later of mast cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ascaridia galli (Schrank 1788) is a common parasitic roundworm of the small intestine of chickens with a worldwide distribution (Soulsby 1982). Prevalence of 64 % in free-range/organic systems and 42 % in deep-litter systems have been demonstrated (Permin et al. 1999). Infection with A. galli has been associated with substantial economic losses due to poorer feed conversion, weight loss and reduced egg production (Dänicke et al. 2009; Reid and Carmon 1958; Ackert and Herrick 1928). The life cycle is direct and the birds get infected by ingestion of embryonated eggs (Araujo and Bressan 1977; Herd and McNaught 1975; Ackert 1931). After ingestion of the embryonated eggs, these are transported until they reach the duodenum and/or jejunoileum, where they hatch and larvae are released within 24 h (Hansen et al. 1956; Tugwell and Ackert 1952; Ackert 1923) or even within 3 h (Ferdushy, Kyvsgaard and Roepstorff, unpublished data). This is supported by Ferdushy et al. (2012) found that the majority of the larvae were located in the anterior half of the jenunoileum at 3, 5 and 7 days post-infection (dpi). The majority of the larvae are found deeply in the mucosa, i.e. in the lumen of the crypts during the early stage of infection (Luna-Olivares et al. 2012). The embedded larvae may cause haemorrhage and extensive destruction of the epithelium, proliferation of mucus secreting cells and, thus, adhesion and atrophy of the villi (Ikeme 1971). Later, in the course of infection, the larvae migrate to the intestinal lumen (Ferdushy et al. 2013; Herd and McNaught 1975). There is only limited knowledge on the regulation of the parasite population at host level. Acquired immunity in ascarid infections seems to play a role in older birds (Ackert et al. 1935; Ackert and Herrick 1928). Immunity does, however, not provide complete protection as high worm burdens can regularly be seen in adults in the field, and furthermore, birds become re-infected after anthelminthic treatment when kept in heavily contaminated environments (Höglund and Jansson 2011). Limited information is available on acquisition of immunity and/or associated immunological reactions in chickens, e.g. few reports on the presence of eosinophils in poultry pathologic processes appear in the literature (Schwarz et al. 2011; Marcos-Atxutegi et al. 2009; Degen et al. 2005; Jeurissen et al. 1999). A description of the changes in the cellular population of the intestine during A. galli infection may contribute to a better understanding of immunity. Most studies have focused their examination on a single time-point after infection with A. galli. In the very early phase (3 dpi), Luna-Olivares et al. (2012) found a eosinophil response in the lamina propria of the jejunal mucosa. Two weeks after infection, histopathological lesions such as the formation of lymphoid aggregations and mild lymphocytic infiltration in the jejunal lamina propria of inoculated birds were seen (Schwarz et al. 2011). A typical Th2 type cytokine response with selectively augmented IL-4 and IL-13 expression in the ileal immune tissue could also be found at 14 dpi (Degen et al. 2005). Eight weeks post-infection, low and medium degree of eosinophil numbers were observed in the lamina propria of jejunal and ileal tissue in hematoxilin and eosin (HE)-stained slides, but the numbers were not closely related to infection intensity (Dänicke et al. 2009). To get a picture of the cellular reaction to infection with A. galli, the objective of this study was to provide a comprehensive description of the cellular reactions in the intestine at several time points during the first 7 weeks of infection, a period which is considered to be the prepatent period that generally is between 5 and 6 weeks (Ramadan and Aboud Znada 1992; Ackert 1931).
Materials and methods
Experimental design and animals
Seventy ISA Brown chickens (7 weeks old) were bought from a commercial pullet rearing farm with no history of A. galli infection. The chickens were randomly allocated into three pens, one housing 28 chickens (control group) and two pens housing a total of 42 infected chickens. The control group was included to confirm freedom of uncontrolled A. galli infection and to compare the jejunal cellular response of the two groups. The three pens had beddings of wood shavings and were separated from each other by at least 2 m. The diet consisted of a commercial pullet feed provided three times per day and the birds had access to water ad libitum. After 1 week of acclimatization, the 8 weeks old chickens were assigned randomly to seven groups each comprising two subgroups of six infected and four controls to be examined post-mortem at 3, 7, 10, 14, 21, 28 and 42 dpi. Eight-week-old birds were used, as this age is representative of organic poultry production, where the pullets have access to a potentially infected outdoor area from 6 weeks of age in summer and 9 weeks in winter.
Infective material and inoculation
Chicken faeces from a farm with a high prevalence of A. galli were used to collect eggs as described previously by Luna-Olivares et al. (2012). Prior to infection, the eggs were embryonated in 0.05 M H2SO4 (pH 1) according to Ferdushy et al. (2012). In brief, the eggs were kept in culture flasks for 6 weeks at 22 °C in the dark and the rate of embryonation was measured every week. After embryonation, eggs were stored at 5 °C for 2 weeks until use. The concentration of eggs was estimated and experimental inoculation doses were made by re-suspension in 1 mL of tap water. The infected groups were inoculated orally with 500 embryonated A. galli eggs per bird. The infection was carried out using a plastic Pasteur pipette inserted via the oesophagus to the level of the crop. The pipette was refilled twice with 1 mL tap water and introduced into the oesophagus (Luna-Olivares et al. 2012; Ferdushy et al. 2012). The birds used in this experiment were housed at the experimental animal Unit of the Faculty of Health and Medical Sciences, University of Copenhagen, Denmark and were treated according to Danish legislation (License: A. Roepstorff, 2010/561-1914, 13 October 2000).
Necropsy
At each time point (3, 7, 10, 14, 21, 28 and 42 dpi), six infected and four control chickens were euthanized. The gastrointestinal tract was removed from the proventriculus to the cloaca. The large intestine was removed and the small intestine was divided into two parts, duodenum and jejunoileum. The duodenum was defined as the duodenal loop, whereas the posterior part of the small intestine was determined as jejunoileum (Schummer et al. 1992). The jejunoileum was furthermore divided into four segments of equal length (J1, J2, J3 and J4). The focus of this study was the segments J1 and J2 as a previous study had shown that more than 95 % of the larvae were located in the anterior half of the jejunoileum (Ferdushy et al. 2012).
Histopathology
Immediately after dividing the jejunoileum, the tissue samples for histological examination were collected from the middle part of segments J1 and J2 as well as from the border area between the two segments called JX (Luna-Olivares et al. 2012). From each sampling site, a 1–1.5-cm-long sample was taken and opened along the mesenteric border. The tissue was divided transversally in two pieces. Each piece was placed on the serosal side in tissue cassettes with wet filter papers to avoid curling and folding. One cassette was immersed in Carnoy’s fixative (43.3 % chloroform, 46.5 % ethanol and 10.2 % acetic acid). The second cassette was immersed in 10 % neutral buffered formalin (4 % formaldehyde). After 24 h, the cassettes were transferred to 70 % ethanol, processed conventionally, trimmed after processing and embedded in paraffin so two transverse sections of the intestinal sample was positioned in each paraffin block. Slides, 3 μm thick, were made from each block and stained with HE for overall evaluation, Luna’s stain for eosinophils (Luna 1968), methyl green-pyronin for plasma cells (Pearse 1985) and with 1 % alcian blue, pH 2.5, followed by periodic acid-Schiff (PAS; Ab 2.5/PAS; Bancroft and Stevens 1996) to allow neutral (magenta), acidic (blue) and mixed (purple) mucins to be differentiated. Furthermore, slides from tissue fixed in Carnoy’s solution were stained with toluidine blue, pH 0.5, to detect mast cells (Xu et al. 1993). Prior to evaluation, all slides were blinded to prevent bias at the histological assessment. Light microscopy was used to evaluate the slides.
In the HE slides from J1, JX and J2, the overall inflammatory response in all intestinal layers, foci of eosinophils and mucosal thickness was evaluated. Foci of aggregated eosinophils were graded into small foci (consisting of 3–9 eosinophils), medium-sized foci (10–15 eosinophils) and large foci (more than 16 eosinophils). In one transverse section from each of the three sampling sites of each animal, the number of foci was counted within an intestinal segment of 1 cm. To measure the mucosal thickness, 3–5 perfectly cut villus–crypt complexes (villus with visible lacteal and smooth muscles centrally and visible crypt lumen all the way to the lamina muscularis mucosae) were identified in each intestinal segment (J1, JX and J2), i.e. a total of 9–15 per bird, and measured at ×40 magnification using a lens graticule calibrated with a stage micrometer.
The number of eosinophils, mast cells and plasma cells in the lamina propria were counted on special stained slides from J1, JX and J2 of each animal using an eyepiece grid calibrated with a stage micrometer. Counting was done at ×400 magnification in a systematic random fashion beginning at the left side of the slide and moving the eyepiece three fields of visions. Counting of eosinophils and mast cells in the villus and crypt zone for each intestinal segment was done by alternating the reading between villi and crypts until 20 squares, representing a total area of 0.0125 mm2, had been read. Ten squares were counted for plasma cells in each intestinal segment representing a total area of 0.00625 mm2. The number of goblet cells in the epithelium of the mucosal layer containing either neutral, acid or mixed mucins were counted in a systematic random fashion by moving the eyepiece three fields of visions, choosing a villus and a crypt and counting each type of mucins in all the epithelium of the entire villus and crypt. Goblet cells from a total of 10 villi and 10 crypts were counted from each intestinal segment.
Statistical analyses
Multivariate linear models were formulated to analyse the response of the numbers of eosinophils, mast cells, plasma cells or goblet cells per slide for the effect of intestinal segment (J1, JX or J2), dpi (3, 7, 10, 14, 21, 28, or 42) and infection (infected or control). All analyses were performed using the lm function of the statistical program R (version 2.11.1).
Results
Clinical and parasitological findings
During the entire study, no animals showed signs of clinical disease of A. galli. For details about the parasitological results and larval localization, please refer to Ferdushy et al. (2013); in brief, the study determined a reduction in larval counts during the course of the infection. The reduction was consistent with an exponential function showing an initial larval recovery of approximately 100 larvae, and a constant rate of reduction of the number of larvae. The half-life of the larvae was of 37.8 days.
Histopathological findings
No macroscopic lesions were found in A. galli-inoculated chickens. At the histological examination, a number of changes were seen mainly in the lamina propria. Only two larvae of A. galli were seen in the lumen of crypts in one chicken in the intestinal segment JX.
Overall evaluation
General cellular infiltration
Microscopic examination showed that chickens infected with A. galli had mild to moderate general cellular infiltration at 7, 10, 14, 21, 28 and 42 dpi. A mild to moderate increase of cellular infiltration was seen in different intestinal segments of some animals at 7 dpi. At 10 and 14 dpi, chickens showed a moderate cellular infiltration in the lamina propria. However, only few animals had a moderate cellular infiltration at 21 dpi and most of the chickens showed a mild cellular infiltration at 28 and 42 dpi. In the control group, no or mild cellular infiltrations were observed.
Subepithelial oedema
The intestinal villi of jejunoileum of the majority of infected chickens had a mild to severe degree of vascular subepithelial oedema at 3, 7, 10, 14, 21, 28 and 42 dpi. Large areas of subepithelial oedema in several villi were seen in only one of the chicken at 3, 7 and 21 dpi. However, in 10, 14, 28 and 42 dpi, the majority of the chickens had moderate and severe subepithelial oedema consisting of small and large focal oedematous areas in most of the villi (Fig. 1c). In the control group, no or mild subepithelial oedema was seen.
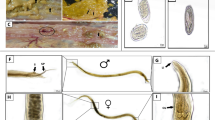
Cellular reactions in the intestinal lamina propria and subepithelial oedema in the jejunoileum. a Eosinophils (red cytoplasm) around intestinal crypts at 3 dpi, JX; Luna stain. Bar = 75 μm). b Mast cells (blue cytoplasm) in an intestinal villus at 3 dpi, J2; toludine blue stain. Bar = 75 μm). c Subepithelial oedema in a villus at 14 dpi, JX; haematoxylin–eosin stain (HE). Bar = 150 μm). d Small eosinophil foci (3–9 eosinophils in a clump; arrow head) in the LP near cross-sectioned crypts at 3 dpi, JX; HE. Bar = 150 μm. e Medium-sized eosinophil foci (10–15) eosinophils (arrow head) in a villus at 3 dpi, JX. Note also the scattered infiltration with eosinophils and additional small eosinophil foci; HE. Bar = 75 μm. f On long coherent large eosinophil foci (+16 eosinophils; arrow head) in a villus at 10 dpi, J2; HE. Bar = 75 μm
Number of foci of eosinophilic staining cells in H&E
The eosinophils were diffusely distributed in the lamina propria and showed a tendency to aggregate. The mean number of small, medium-sized and large eosinophil foci increased in the infected group at 3, 7, 10, 14, 21 and 28 dpi comparing to the control group (Fig. 1d, e, f). Furthermore, the number of eosinophil foci was higher at 28 dpi than 21 dpi. Only few small aggregated eosinophil foci were found at 42 dpi. Whereas, in the control group, a few number of small foci were seen at 3, 7, 10, 14, 21 and 28 dpi. Medium-sized foci were seen at 3, 7 and 10 dpi. However, no eosinophil foci were seen at 42 dpi (Fig. 2).
The mean number of foci of eosinophils identified in jejunoileum of chickens inoculated with Ascaridia galli. Eosinophils in lamina propria were clustered into small foci (3–9 eosinophils), medium-sized foci (10–15 eosinophils) and large foci (more than 16 eosinophils). All the foci of the eosinophils were counted in control (4) and infected groups (6) at 3, 7, 10, 14, 21, 28 and 42 dpi. In each bird, all foci found in an area covering a length of 1 cm in one of the transverse sections (HE stained) from three different sampling sites (J1, JX and J2) were counted
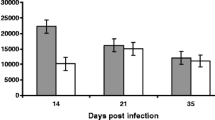
Mucosal thickness of jejunum
Evidence of change in the length of the villi was observed in the infected group. No difference was observed at 3 dpi but the length of the villi tended to decrease from 7 dpi, and a significant shortening was noted at 10, 14, 21 and 28 dpi. The villi at these time points also appeared plumper than in the control chickens. No difference was found at 42 dpi between groups. There were statistical significant differences in mean length between intestinal segments (more posterior sections have shorter villi) and between groups (P < 0.001), mainly in the intestinal segments J2 (Fig. 3).
Mean mucosal thickness (length of villi in μm) in different segments of the jejunoileum (J1, JX and J2; represented in left, centre and right bars, respectively) of chickens in the control group (C); n = 4 per day of necropsy) and the A. galli-infected group (I; n = 6) at different days post-infection (dpi)
Number of eosinophilic staining cell in Luna
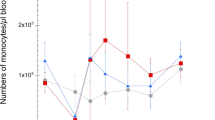
Counting individual eosinophils in the Luna-stained sections confirmed what was seen in HE. The eosinophils were diffusely distributed in the lamina propria and often in aggregates of various sizes located in both villi and crypt areas (Fig. 1a). Infected birds showed consistently higher levels of eosinophils when compared to controls at 3, 10, 14 and 28 (P < 0.001) and 7 dpi (P < 0.01; Fig. 4a). No significant differences were found between intestinal segments (J1, JX and J2).
Mean number of cells counted per mm2 per intestinal segments (J1, JX and J2), per bird in the mucosa of the jejunoileum from six chickens inoculated with 500 A. galli eggs and four non-inoculated (control group), slaughtered at 3, 7, 10, 14, 21, 28 and 42 dpi (a eosinophils, b mast cells, c plasma cells)
Mast cell response
In both control and infected animals, the initial number of mast cells was high but declined later in the study period. The mast cells were distributed diffusely in the lamina propria of the mucosal layer, but a few mast cells could also be found in muscular and sub mucosa layers (Fig. 1b). The number of mast cells declined faster in the control group than in the infected group and a significant difference between groups was found at 21 (P < 0.001), 28 (P < 0.01) and 42 dpi (P < 0.05; Fig. 4b). No difference between intestinal segments JI, JX and J2 was observed.
Plasma cells
The cells were seen diffusely in the lamina propria. No significant differences were seen between groups, dpi, or intestinal segments (Fig. 4c)
Goblet cells
The distribution of goblet cells (neutral, acidic or mixed mucins) was similar in both groups. In the villi, the majority of goblet cells contained neutral mucins and mixed mucins; whereas, acidic mucin-containing cells were abundant in the crypts. No significant changes were observed between intestinal segments and groups in goblet cell counts due to A. galli infection (Fig. 5).
Mean number of goblet cells (acidic mucin, neutral mucin, and mixed mucin) counted in 10 villi and 10 crypts per intestinal segment per animal in the epithelium of the mucosa of the jejunum in six chickens inoculated with A. galli (I) and four uninoculated chickens in the control group (C), slaughtered at 3, 7, 10, 14, 21, 28 and 42 days post-infection (dpi)
Discussion
The present histological time course study demonstrated mucosal immunocellular changes in the lamina propria of the jejunum during 7 weeks post-infection of A. galli infection in chickens. The time course of the infection induced cellular population changes in form of increased number of eosinophils initially and a possible delayed decline of mast cells. Furthermore, the infection induced an increase in the general cellular infiltration of the lamina propria mainly at days 10 to 14 post-infection (p.i.) with a concurrent slight reduction of the mucosal thickness. At the same time points, the villi appeared shorter and broader in the infected group, which coincides with increases in both general cellular infiltration and eosinophil number in the lamina propria. Some of the infected chickens also showed a moderate to severe subepithelial oedema at 10, 14, 28 and 42 dpi that could indicate vascular alteration as a part of the inflammatory response.
Immunopathological studies of A. galli-induced reactions are relatively scarce. An increase of moderate cellular infiltration in the lamina propria mostly from 10 to 14 dpi was observed in our study. This is in accordance with (Schwarz et al. 2011) who detected histological changes at 2 weeks p.i. in chickens infected with 250 A. galli eggs, characterized as mild and moderate lymphocyte infiltration in the jejunal lamina propria and formation of lymphoid aggregations.
Several changes in the mucosal layer were observed by Marcos-Atxutegi et al. (2009), who noted that the mucous layer was completely altered at 105 dpi; the villi disappeared and these authors also found haemorrhagic areas indicating vascular lesions. Moreover, an inflammatory cellular infiltration was seen in the basal zone of some villi. The differences between our study and the one of Marcos-Atxutegi et al. (2009) can be related to the presence of adult worms in their study and differences in sampling time points as the latest samples were taken at 42 dpi in our study.
In our study, a slight but statistically significant reduction in the length of the villi was observed in the infected group compared to the control group from 7 throughout 28 dpi, but not at 42 dpi (Fig. 3). Changes in the thickness of the tunica muscularis of the jejunum and the ileum were also observed by Dänicke et al. (2009).
The main changes in cellular counts were an increase in eosinophil counts at 3, 7, 10 and 14 dpi and a higher level of mast cells at 21, 28 and 42 dpi in infected chickens. The number of eosinophils was highest at 3 dpi, which confirms the findings by Löscher and Saathoff (2008) describing that eosinophils frequently accumulate in tissue soon after helminth invasion and play a role in innate defense against this type of pathogen. Eosinophil aggregation in the lamina propria around A. galli larvae in a high infection at 3 dpi in birds was also reported by Luna-Olivares et al. (2012). Our findings is also supported by Uribe (1922) who observed small areas of hemorrhage and foci of infiltration with eosinophils in the caeca of the birds infected with Heterakis gallinarum.
In the present study, a general decline in eosinophil count was noted after 3 dpi, with some fluctuations over time, and no significant difference between groups at 42 dpi. Although the role of eosinophils in chickens is not fully understood, the magnitude of eosinophils tends to correlate with the extent of tissue invasion by larvae or adult helminths. It seems to be that an increase of eosinophil can play a role in the host protection against A. galli, which is confirmed with the parasitological findings by Ferdushy et al. (2013) who found a moderate rate of decline of A. galli larvae during the course of the infection and the larvae were displaced more aborally with time. The role of eosinophils is well described in mammals, where, e.g. Miquel (2005) reported an eosinophilic response in the mucosal layer of the small intestine at 12 and 17 dpi in Ascaris suum-infected pigs. Kaiser (2007) stated that birds lack neutrophils and functional eosinophils, although the presence of eosinophils has been confirmed in healthy chickens using Luna´s stain (Andreasen and Latimer 1990). The Luna’s stain is more specific to eosinophils than the H&E staining, which may also stain granules of heterophils (Andreasen and Latimer 1990). The present study has confirmed an increase in these Luna-positive cells in A. galli-infected chickens and is, thus, suggesting a role of eosinophils in the response to helminth infections (Fig. 1a).
Studies of mast cell responses to A. galli infection in poultry are limited. Jeurissen et al. (1996) indicated that macrophages, granulocytes and other leukocyte sub-populations are attracted to the lamina propria during primary infections, suggesting that these cells play a regulatory role on the intensity of infection. Galli et al. (1999) observed that mast cells have an important role in both natural and acquired immune response to different types of pathogens in chickens. Mast cells play a role in immunity against A. galli infection. This is confirmed by Darmawi et al. (2013) who found a significant increase of mast cells in the mucosa of jejunum in chickens infected with A. galli at 14 dpi. In our study mast cells were seen in high numbers in the intestinal mucosa at 3, 7, 10 and 14 dpi in both control and infected groups, indicating that a permanent population of mast cells are normally present as a part of the innate mucosal defence system in young animals. This may be supported by the findings of Karaca et al. (2006) who demonstrated higher levels of mast cells in the bursa of Fabricius of 7-day-old birds compared to 21, 30 and 120-day-old chickens. Also, they observed a significant increase in mast cells in the thymus and spleen of 21-day-old birds compared to the 7, 30 and 120-day-old. These findings demonstrate that the young lymphoid organs contain a low number of mast cells that may increase and in turn decrease with age. Yong (1997) observed that mast cells arose from caudal mesenchyme about the 15th to 16th day of embryonic life in young rats. Subsequently, there was proliferation and maturation of mast cells reaching the adult population in two months. During the first month of neonatal life, many immature mast cells were present in the peripheral blood. Furthermore, in our study, we observed significantly higher mast cell counts at 21, 28 and 42 dpi in the lamina propria in the infected group compared to the control group. This could indicate that the mast cells responded to the A. galli infection later than the eosinophils. This later response also suggests that mast cells depend on stimulation from the Th2 cytokine environment in order to respond to the parasite infection. According Schwarz et al. (2011) and Degen et al. (2005), such a response develops 2 weeks after infection, whereas the eosinophils might react more acute to the danger signals from distressed intestinal epithelial cells (Stenfeldt and Wennerås 2004).
Goblet cells have been shown to play a protective role in other parasite–host reactions. Thus, Wells (1963) and Ishikawa et al. (1993) observed a protective role of goblet cell and enhanced mucus secretion in the elimination of the parasitic nematode Nippostrongylus brasiliensis in rats. Miquel (2005) observed increased goblet cell number in the villous region of A. suum-infected pigs at 12 dpi and a significant shift from mixed mucins to acidic and neutral was most significant at 17 dpi. However, this shift was not seen in the present study in chickens and no significant differences in the goblet cell population were observed between groups, intestinal segments and dpi.
In summary, our findings demonstrated that the A. galli infection elicited a moderate cellular response; mainly in form of increased numbers of eosinophils in the early phase and a possible delayed decline of mast cells in the lamina propria of the jejunum. The pattern of the immunological response seems to be compatible with the relatively constant and moderate rate of decline in larval number observed over time in the parasitological examination of the chickens (see Ferdushy et al. 2013), which is contrary to what is seen for, e.g. A. suum infections in pigs, where larval counts drop dramatically between 14 and 21 dpi accompanied by a marked eosinophilic cellular response (Miquel 2005). In our experimental only a limited expulsion of larger larvae may have taken place between day 28 and 42 (Ferdushy et al. 2013). Thus, the regulation of A. galli in chickens seems to be fundamentally different from the regulation of A. suum in pigs.
References
Ackert JE (1923) On the habitat of Ascaridia perspicillum (Rud.). J Parasitol 10:101–103
Ackert JE (1931) The morphology and life history of the fowl nematode Ascaridia lineate (Schneider). Parasitol 23:360–379
Ackert JE, Herrick CA (1928) Effects of the nematode Ascaridia lineata (Schneider) on growing chickens. J Parasitol 15:1–13
Ackert J, Porter D, Beach T (1935) Age resistance of chickens to the nematode Ascaridia lineate (Schneider). J Parasitol 21:205–213
Andreasen CB, Latimer KS (1990) Cytochemical staining characteristics of chicken heterophils and eosinophils. Vet Clin Pathol 19:51–54
Araujo P, Bressan C (1976) Observations on the second moult of the larvae of Ascaridia galli (author’s transl)]. Ann Parasitol Hum Comp 52:531–537
Araujo P, Bressan CR (1977) Observations on the second moult of the larvae of Ascaridia galli. Annales de Parasitologie Humaine et Comparee 52:531–537
Bancroft JD, Stevens A (1996) Theory and practice of histological techniques, 4th edn. Churchill Livingstone, New York, p 776
Dänicke S, Moors E, Beineke A, Gauly M (2009) Ascaridia galli infection of pullets and intestinal viscosity: consequences for nutrient retention and gut morphology. Br Poult Sci 50:512–520
Darmawi BU, Hambal PBP (2013) Mucosal mast cells response in the jejunum of Ascaridia galli-infected laying hens. Media-Peternakan J Animal Sci Tech 36(2):113–119
Degen WG, Nv D, Rothwell L, Kaiser P, Schijns VE (2005) Th1/Th2 polarization by viral and helminth infection in birds. Vet Microbiol 105:163–167
Ferdushy T, Nejsum P, Roepstorff A, Thamsborg SM, Kyvsgaard NC (2012) Ascaridia galli in chickens: intestinal localization and comparison of methods to isolate the larvae within the first week of infection. Parasitol Res 111:2273–2279
Ferdushy T, Luna-Olivares LA, Nejsum P, Roepstorff A, Thamsborg SM, Kyvsgaard NC (2013) Population dynamics of Ascaridia galli following single infection in young chickens. Parasitol 140:1078–1084
Galli SJ, Maurer M, Lantz CS (1999) Mast cells as sentinels of innate immunity. Curr Opin Immunol 11:53–59
Hansen M, Terhaar C, Turner D (1956) Importance of the egg shell of Ascaridia galli to the infectivity of its larva. J Parasitol 42:122–125
Herd RP, McNaught DJ (1975) Arrested development and the histotrophic phase of Ascaridia galli in the chicken. Int J Parasitol 5:401–406
Höglund J, Jansson DS (2011) Infection dynamics of Ascaridia galli in non-caged laying hens. Vet Parasitol 180:267–273
Ikeme M (1971) Observations on the pathogenicity and pathology of Ascaridia galli. Parasitol 63:169–179
Ishikawa N, Horii Y, Nawa Y (1993) Immune-mediated alteration of the terminal sugars of goblet cell mucins in the small intestine of Nippostrongylus brasiliensis-infected rats. Immunol 78:303
Jeurissen S, Janse E, Vermeulen A, Vervelde L (1996) Eimeria tenella infections in chickens: aspects of host-parasite: interaction. Vet Immunol Immunopathol 54:231–238
Jeurissen S, Wagenaar F, Janse EM (1999) Further characterization of M cells in gut-associated lymphoid tissues of the chicken. Poult Sci 78:965–972
Kaiser P (2007) The avian immune genome a glass half-full or half-empty. Cyt Gen Res 117:221–230
Karaca T, Yörük M, Uslu S (2006) Age‐related changes in the number of mast cells in the avian lymphoid organs. Anat Histol Embryol 35(6):375–379
Löscher T, Saathoff E (2008) Eosinophilia during intestinal infection. Best Prac Res Clin Gastr 22:511–536
Luna LG (1968) Manual of histological staining methods of the Armed Forces Institute of Pathology, 3rd edn. McGraw-Hill Book Company, New York, p 251
Luna-Olivares LA, Ferdushy T, Kyvsgaard NC, Nejsum P, Thamsborg SM, Roepstorff A, Iburg TM (2012) Localization of Ascaridia galli larvae in the jejunum of chickens 3 days post infection. Vet Parasitol 185:186–193
Marcos-Atxutegi C, Gandolfi B, Arangüena T, Sepulveda R, Arévalo M, Simon F (2009) Antibody and inflammatory responses in laying hens with experimental primary infections of Ascaridia galli. Vet Parasitol 161:69–75
Miquel N (2005) Immune responses in experimental Ascaris suum single infections in pigs. Ph.D. Thesis. The Royal Veterinary and Agricultural University. Copenhagen. Denmark
Pearse AGE (1985) Histochemistry. Theorical and applied. Analytical Technology. 4th ed. vol 2
Permin A, Bisgaard M, Frandsen F, Pearman M, Kold J, Nansen P (1999) Prevalence of gastrointestinal helminths in different poultry production systems. Br Poult Sci 40:439–443
Ramadan HH, Aboud znada NY (1992) Morphology and life history of Ascaridia galli in the domestic fowl that are raised in Jeddah. J KAU Sci 4:87–89
Reid WM, Carmon JL (1958) Effects of numbers of Ascaridia galli in depressing weight gains in chicks. J Parasitol 44:183–186
Schrank P Von F (1788) Verzeichniss der bisher hinlänglich bekannten Eingeweidewürmer, nebs einer Abhandlung über ihre Anverwandschaften. München, pp 116
Schummer A, Vollmerhaus B, Sinowatz F, Frewein J Waibl H (1992) Anatomie der Vögel. In Nickel, R., Schummer, A., Seiferle, E. (2.ed) Lehrbuch der Anatomie der Haustiere. Paul Parey, Berlin and Hamburg, Germany pp 203-204
Schwarz A, Gauly M, Abel H, Daş G, Humburg J, Rohn K, Breves G, Rautenschlein S (2011) Immunopathogenesis of Ascaridia galli infection in layer chicken. Dev Comparativ Immunol 35:774–784
Soulsby EJL (1982) Helminths, arthropods and protozoa of domesticated animals, 7th ed. Balliere Tindall. The English Language Book Society, London
Stenfeldt A, Wennerås C (2004) Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunol 112:605–614
Tugwell RL, Ackert JE (1952) On the tissue phase of the life cycle of the fowl nematode Ascaridia galli (Schrank). J Parasitol 38:277–288
Uribe C (1922) Observations on the development of Heterakis papillosa Bloch in the chicken. J Parasitol 8:167–176
Wells PD (1963) Mucin-secreting cells in rats infected with Nippostrongylus brasiliensis. Exp Parasitol 14:15–22
Xu L, Carr M, Bland A, Hall G (1993) Histochemistry and morphology of porcine mast cells. Histochem J 25:516–522
Yong L (1997) The mast cell: origin, morphology, distribution, and function. Exp Tox Path 49:409–424
Acknowledgements
DANIDA is thanked for financial support through grant DFC 09-054 LIFE. Thanks are given for the technical assistance from the Section of Pathology (Hanne Hornemann Møller, Betina Andersen and Lisbeth Kiørboe) and the Danish Centre for Experimental Parasitology (Lise-Lotte Christiansen), University of Copenhagen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luna-Olivares, L.A., Kyvsgaard, N.C., Ferdushy, T. et al. The jejunal cellular responses in chickens infected with a single dose of Ascaridia galli eggs. Parasitol Res 114, 2507–2515 (2015). https://doi.org/10.1007/s00436-015-4450-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4450-y