Abstract
The shape of the urogenital papilla is used to visually determine the sexual dimorphism of Dormitator maculatus. However, it is occasionally not possible to determine this in practice. The objective of the present study was to propose and statistically validate other morphometric measures to fulfill this same purpose. The analysis was designed on the basis of a group of previously identified adults with urogenital papilla. It was found that the most useful morphometric measures are related to the size and weight of the species. The dorsal fins and the geometric structure of the head were also recommended as morphometric measures. The males are larger in size, they are robust with the fin 1DFL longer compared to the 2DFL fin and have a broad, elongated head. Females are smaller in size, slightly robust, with longer 2DFL fins compared to 1DFL fins, and have slender, not elongated heads. In the Alvarado lagoon, sexual differentiation in D. maculatus is important due to the selective exploitation of its gonads (mainly on females). For this reason, and to avoid errors in the identification of females for protection purposes, the results obtained are a viable alternative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The “Naca” fish Dormitator maculatus (Bloch, 1972) is a demersal species that inhabits the coastal ecosystems of the Western Atlantic, from the USA to Brazil (Aiken et al. 2015). The species is important for fisheries because it is a food resource for other fish with high commercial value in the Gulf of Mexico (Rodríguez-Varela et al. 1992). The gonad of the females of D. maculatus is of commercial interest (Ayala-Pérez et al. 2015), because it is used to extract the gonad or roe, which regionally is considered a dish with a pleasant flavor in its different preparations (Ré-Regis and Estrada-García 1992). The greatest fishing activity for D. maculatus takes place inside the Alvarado lagoon in the rainy season, between September and October, coinciding with its reproductive season (Schmitter-Soto 1996). The rest of the year, D. maculatus remains semi-sunk in the mud and sheltered between the roots of mangroves or grasses (Schmitter-Soto 1996). In the coastal zone of the state of Campeche, Mexico, the species is reported to be at risk of extinction (Ayala-Pérez et al. 2015) and with decreasing catch volumes in the Alvarado lagoon, Veracruz (Dávila-Camacho 2020).
Pezold and Cage (2001) described sexual dimorphism of the urogenital papilla and reported that the papilla is rounded in females and tapered in males. The shape of urogenital papilla is currently the only key to determine the sex of this species without dissection. However, it is often difficult to distinguish in the field or laboratory and dissection is sometimes necessary to observe the gonads. Therefore, an alternative morphological key for sex determination is required.
The objective of this study was to propose and statistically validate other morphometric measures for the determination of sex in adults of D. maculatus. The analysis was designed on the basis of a group of previously identified adults with the urogenital papilla. In the Alvarado lagoon, sexual determination of D. maculatus is important due to the selective exploitation of its gonads. This study can be applied toward the correct identification and protection of females, giving continuity to the fishery and as a viable alternative, in the development of culture technology.
Materials and methods
Field work
The present study was carried out at a sampling station strategically located at the mouth of Laguna Alvarado where the migration route of D. maculatus passes through (Fig. 1). During one of the reproductive seasons, in October 2017 (when the species is more accessible for fishing), approximately 0.5 ton was caught with a gillnet with a mesh size of 1.5 inches. Afterward, 52 live specimens without lacerations or external damage were selected. The specimens were transported to the Aquatic Resources Research Laboratory in the Tecnológico de Boca del Río, Veracruz., in a 25-L plastic container with drops of clove essence used as an anesthetic. The species was described taking into account the average weight and total length (Fischer et al. 1995; Schmitter-Soto 1996; Nelson 2006).
Morphometric character records
Males were distinguished from females by the copulatory organ called urogenital papill (Bacheler 2002; Pezold and Cage 2001). After differentiation between sexes, 52 specimens (24 males and 28 females) were measured for characters shown in Fig. 2. All fish were sexed and measured while alive and anesthetized. For recording the measures, an ichthyometer (Aquatic® biotechnology, Mod. iK2, 40 cm and 0.1 mm precision), an electronic digital scale (Core Adam CQT202, 200 g capacity and 0.001 g precision), and digital vernier (General®, Mod. H-7352, resolution 0.001 mm) were used. The morphometric characteristics of the species were identified with the taxonomic keys proposed by Cervigón et al. (1992), Fischer et al. (1995) and Schmitter-Soto (1996) and the morphometric measurements proposed by González-Martínez et al (2020). Subsequently, once recovered from anesthesia, the specimens were returned to the extraction site.
Recorded morphometric measurements for Dormitator maculatus. First dorsal fin length (1DFL), second dorsal fin length (2DFL), caudal fin length (CFL), anal fin length (AFL), pelvic fin length (PFL), pectoral fin length (PCFL), caudal peduncle length (CPL), intra-orbital length (IOL), orbital length (OL), pre-pelvic length (PPL), pre-dorsal length (PDL), head length (HL), height maximum (HM), height minimum (Hm) and standard length (SL)
A preliminary statistic analysis on morphometric characters of male and females was implemented via the use of Statistica software version 7.0.
Dimorphism's analysis
A preliminary analysis on the numerical variation of each morphometric character was performed for males and females (Table 2). Thus, statistic normality at each morphometric characters was evaluated with the Shapiro–Wilk’s test (Zar 1999) implemented using Statistica software version 7.0. In this case, the following hypotheses were established:
-
1.
If for a morphometric character Ho: σ2 > \(\overline{\mathrm{X} }\), then, the morphometric character has a normal distribution. This mean that in records of the morphometric character the numerical variation is present (records are not similar and repetitive). In order to accept Ho, a P-level > 0.05 is necessary.
-
2.
If for a morphometric character Ha: σ2 < \(\overline{\mathrm{X} }\), then the morphometric character does not have a normal distribution. This mean that in records of the morphometric character the numerical variation is not present (records are similar and repetitive). To accept Ha, a P-level < 0.05 is necessary.
In the present study, the Ha hypothesis has higher probability to be accepted because caught adult specimens during the reproductive season were to very similar sizes. For this reason, from the beginning comparative analyses between morphometric characters of male and females were not necessary to perform because they were repetitive. About the recorded morphometric characters in the present study, it was concluded that with this information type the sexual dimorphism cannot be reliable to establish. Anticipating this situation, a classification neuronal model (Haykin 1994) was implemented to standardize all morphometric measurements within a probabilistic variation ranging between 0.00 and 1.00. The main objective of the mentioned analysis was to establish a specific probabilistic trend for an each morphometric characters of both sexes. Afterward, these estimated probabilistic trends were visually compared to establish the sexual dimorphism more reliable for this species. Structurally, a morphometric (SL) character was considered shorter in males or females when this morphometric character recorded a probabilistic descendent trend (from 1.00 to 0.00). The opposite case was considered when a morphometric (SL) character recorded a probabilistic ascendant trend (from 0.00 to 1.00).
The classification neuronal model was implemented using Statistica software version 7.0. In this case, the classification neuronal model was solved with an automatized routine where linear and nonlinear model combinations were established by the software, which afterward were optimized with the least squares method by a compiler dubbed “Intelligent Problem Solver (IPS)” (Haykin 1994). This option is a tool to create different alternatives to solve a problem, and one of these alternatives is marked by IPS as the better solution. This specific solution was chosen and interpreted in probabilistic terms. So, the use of particular references to explain implemented linear and nonlinear model combinations was not possible because these results were generated with a particular automatized process by the software. However, the use of linear and nonlinear models is completely justified to explain the length growth in different fish populations by Im et al. (2016) and Blasina et al. (2018).
Obtained standardized probabilistic trends with classification neuronal model were used to construct a second data matrix that was used to implement the correspondence multivariate analysis (Hair et al. 1999). Based on this, a correspondence graphic was structured to spatially represent morphometric characters in relation to males and females. Taking into account spatial approximation between the morphometric characters in relation to sex types, it was considered that one or more morphometric characters can be used to differentiate between males and females. Thus, when higher is the approximation between a specific morphometric characteristic and a type of sex, this morphometric characteristic will be distinctive for a sex type. On the other hand, spatial separation between the morphometric characters with reference to sex types indicates that morphometric characters are not distinctive for a sex type.
The correspondence multivariate analysis was statistically evaluated with the Total Inertia index (TI = eigen-value from principal component 1 + eigen-value from principal component 2) (Hair et al. 1999). The aforementioned was specifically interpreted as follows:
-
1.
If TI ~ 0.00, morphometric characters reached an important approximation in relation to male-to-sex information, and thus obtained results are reliable to explain the dimorphism between sexes.
-
2.
If TI = 1.00 or > 1.00, morphometric characters did not reach an important approximation in relation to sex information, and thus obtained results are not reliable to explain the dimorphism between sexes.
The correspondence multivariate analysis was performed by Statistica software version 7.0
Results
Morphometric character records
Preliminary statistic analysis of morphometric characters is shown in Table 1.
Dimorphism's analysis
In the present study, the Shapiro–Wilks test corroborated that all morphometric characters of male and female specimens had similar and repetitive records (Table 2). For this reason, for these morphometric characters it was corroborated that they do not have a normal distribution. As was mentioned, this lack of statistic normality was generated because caught adult specimens during the reproductive season had very similar sizes. Due to the aforementioned, the classification neuronal model was implemented.
The best solution for the classification neuronal model was composed of three learning layers to process observed records of 1DFL, 2DFL, CFL, AFL, PFL, PCFL, CPL, IOL, OL, PPL, PDL, HL, HM, Hm, and SL (Table 2). The first learning layer has 15 neurons, the second learning layer is composed by 13 neurons, and the third learning layer has one neuron. Fifteen different linear models were used to activate the first learning layer [ai = (b‧Xi)-c], where a is i activated neuron with estimated linear parameters b and c and X is the i morphometric character. For each ai, fifteen synaptic signals were estimated using the following equation [di = (∑ai∙Wi)-fi], where d is the i synaptic signal, Wi is the statistical weight of each ai, and f is the estimated thresh each di. A hyperbolic model was used to activate the 13 neurons in the second learning layer [gi = edi–e−di/edi + e−di], where g is the i activated neuron (− 1, + 1). For each gi, thirteen synaptic signals were estimated using the following equation [hi = (∑gi∙Wi)-k], where h is the i synaptic signal, Wi is the statistical weight of each gi, and k is the estimated thresh for hi. Afterward, a logistic model was used to activate the last neuron in the third learning layer [li = 1/(1–e−hi)], where l is the activated neuron (0, + 1). With the latter equation, the probabilistic tendencies of each morphometric character were estimated for males and females.
Standardize probabilistic values are listed in Table 3, and their trends are shown in Fig. 3. The results of probabilistic trends indicated that, in comparison with females, males recorded shorter the following morphometric characters: 2DFL, PFL, PDL, AFL, and CPL. On the other hand, males recorded longer the following morphometric characters: PCFL, 1DFL, HM, Hm, PPL, IOL, SL, OL, CFL, and HL (Fig. 3). In comparison with males, females recorded shorter the following morphometric characters: PCFL, 1DFL, HM, Hm, PPL, IOL, SL, OL HL and CFL (Fig. 3). On the other hand, females recorded longer the following morphometric characters: 2DFL, PFL, PDL, AFL, and CPL.
Standardize probabilistic trends for morphometric measure in males and females of D. maculatus. In this figure, a morphometric character is shorter in males or females when its probabilistic trend is descendent (from 1.00 to 0.00). The opposite case was considered when a morphometric character shows a probabilistic ascendant trend (from 0.00 to 1.00)
For the correspondence multivariate analysis, the TI at 0.41 was estimated (Fig. 4). Obtained results indicated that specific morphometric characters reached an important spatial approximation in relation to sex information. Then, the following morphometric characters were validated to explain the dimorphism in the D. maculatus:
-
1.
The morphometric characters that should characterize females better were 2DFL, IOL and OL.
-
2.
The morphometric characters that should characterize males better were 1DFL, SL and HL. With the SL morphometric measure, it was evidenced that males are larger than females. This result was consistent with that listed in Table 1 because for males, weight averages were recorded in 10.31 ± 4.23 g and the total length in 9.11 ± 1.78 cm. For females, weight averages were recorded in 9.5 ± 3.81 g and the total length in 8.51 ± 1.04 cm. With these results, it was confirmed that, persistently, males are larger in size compared to females (Fig. 4).
-
3.
The PDL, AFL, CFL, HM, PCFL, PPL, Hm, and PFL morphometric characters were not helpful in differentiating males from females. For these cases, it was concluded that these morphometric characters are reliable similar between males and females.
Figure 4 shows the analysis of the trend probability values of the neural network and shows the spatial approximation between the morphometric characters in relation to sex types means that one or more morphometric characters can be used to differentiate between males and females. Principal components PC1 and PC2 (Total Inertia index = eigenvalue from PC1 + eigenvalue from PC2) (Hair et al. 1999).
Discussion
When identification problems are recurrent to analyze sexual dimorphism based on a single morphological structure (as is the case of the genital papilla in D. maculatus), other morphological measures should be available and statistically validated. However, the drawback that arises when proposing other alternative morphological measurements is the little difference in length that exists between different morphological measurements (Table 1) and additionally, the little variation in their records when specimens with approximately the same dominant are analyzed. According to Armbruster (2012), the standardization of morphological records is essential to highlight the details in the information. In the present study, standardization was performed based on MNC probability trends and details, such as the difference between lengths for different morphological measurements obtained. Additionally, specific measures were recommended to identify males from females of D. maculatus, provided that the urogenital papilla method cannot be implemented. On the contrary, the morphometric measurements that were partially useful should not be used in future studies.
The morphological measurements that were considered in the present work were based in part on the reference points for the fish geometry recommended by Strauss and Bookstein (1982) and Hubbs and Lagler (1964), and others were added to complement the analysis of sexual dimorphism (Fischer et al. 1995). For this case, it was taken into account that the morphometric measurements were consistent up to the family level, since the details by species were analyzed separately.
Based on the probabilistic trend of the SL (Fig. 3) and additionally with the direct measurement of the TL, it was corroborated persistently for D. maculatus, that males are larger in size compared to females (Fig. 4). On the other hand, with the total weight records it was concluded that males are persistently more robust compared to females (Table 1). These same trends were documented for Dormitator latifrons (Richardson 1844) by González-Martínez et al. (2020). The species presented sexual dimorphism, and the main distinctive features were greater height and weight in males than in females. As an additional data point, the authors recorded that there was no significant difference in the morphometric measurements of the fins. This information is not consistent with the present work, because the aforementioned authors did not make the comparison with probability trends. In the present analysis, it was observed that probabilistically the 1DFL fin is longer in males and the 2DFL fin is longer in females (Fig. 3).
Another sexual dimorphism in males is HL, which, being longer, is consistently related to increases in TL and SL. In males, the head geometry was persistently very elongated and wider (IOL), compared to females (Figs. 3, 4). According to the TL, SL, and W(g) measurements, it is observed that males are longer and heavier than females, which has been reported by Franco-López et al. (2021) and Dávila-Camacho and Galaviz-Villa (2021) for D. maculatus. This sexual dimorphism has been documented by Gonzalez-Martínez et al. (2020) for Dormitator latifrons, and by Segura-Guevara et al. (2011) for Gobiomorus dormitor, both species of the Eleotridae family.
Consistent with the present work, Gonzalez-Martínez et al. (2020) report that HL is longer in males Dormitator latifrons. Franco-López et al. (2021) reported the OL size is not significantly different between males and females of D. maculatus. In the present study, the OL size in males and females of D. maculatus was probabilistically and directly correlated with the morphometric measurements HL and IOL (Fig. 3). Thus, the head of D. maculatus grows proportionally in HL, IOL and OL. On the other hand, the PDL and PPL morphometric measurements maintained a concordance with the geometric growth of the head, but when registering variation in the correlation effects with the LH, they were not recommended to be used for the analysis of sexual dimorphism.
The probabilistic trends of PFL-PCFL (Fig. 3) establish that both morphometric measurements are not significantly different between males and females of D. maculatus (Fig. 4). For this reason, they were not recommended for use in the analysis of sexual dimorphism. 2DFL and CPL morphometric measurements are distinctive of the Eleotridae family (Fischer et al. 1995). At the species level, the above was confirmed for the 2DFL fin. However, it was found CPL has a direct correlation with the 2DFL fin (R = 0.99 P < 0.05). In accordance with Fig. 2, these two morphometric measurements are adjacent (the same with CPL and AFL), which explains the result of the direct correlation and its high approximation in Fig. 4. Unlike that indicated by the aforementioned authors, it is advisable to extend the 2DFL fin measurement to include CPL. For this reason, CPL is not recommended in the analysis of sexual dimorphism.
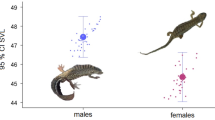
For the CFL and AFL morphometric measures, no discussion material was found, but like PDL, PPL, AFL, CFL, CPL, PCFL, PFL, HM, and Hm, they were not recommended in the determination of sexual dimorphism. On the other hand, it was concluded that HM probabilistically does not vary between males and females (F = 0.70, F0.05 = 4.04, P = 0.40). However, according to Fig. 4, in HM females tend to be of lower amplitude.
Conclusions
-
1.
Alternative morphometric measures were identified for the determination of sexual dimorphism in D. maculatus. The most useful morphometric measurements were related to the size and weight of the species. The dorsal fins and the geometric structure of the head were also recommended.
-
2.
Males are larger in size, robust with a longer 1DFL fin compared to 2DFL fin, and have a broad, elongated head.
-
3.
Females are smaller in size, not very robust with a longer 2DFL fin compared to 1DFL fin, and have a slender, not elongated head.
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability (software application or custom code)
Not applicable.
References
Aiken KA, Van Tassell J, Pezold F, Tornabene L, Bouchereau JL (2015) Dormitator maculatus. The IUCN Red List of Threatened Species. https://www.iucnredlist.org/es/species/185972/69415589 Accessed 24 January 2016
Armbruster JW (2012) Standardized measurements, landmarks, and meristic counts for cypriniform fishes. Zootaxa 3586:8–16
Ayala-Pérez LA, Ramos Miranda J, Flores Hernández D, Sosa López A, Martínez Romero GE (2015) Ictiofauna marina y costera de Campeche. Universidad Autónoma de Campeche, Universidad Autónoma Metropolitana-Xochimilco. p 502 ISBN 978-607-7887-95-9
Bacheler NM (2002) Ecology of bigmouth sleepers (Eleotridae: Gobiomorus dormitor) in a Puerto Rico reservoir. Master’s thesis. North Carolina State University, Raleigh, North Carolina, USA, p 110
Blasina GE, Izzo L, Figueroa D (2018) Sexual dimorphism and length-weight relationship of the hairy Conger Eel Bassanagoalbescens(Anguilliformes: Congridae). J Ichthyol 58(3):396–400. https://doi.org/10.4194/1303-2712-v-20_01_05
Cervigón F, Cipriani R, Fischer W, Garibaldi L, Hendrickx M, Lemus AJ, Márquez R, Poutiers JM, Robaina G, Rodriguez B (1992) Fichas FAO de identificación de especies para los fines de la pesca. Guía de campo de las especies comerciales marinas y de las aguas salobres de la costa norte de Sur América. FAO, Roma. Comunidades Europeas y de NORAD, p 513
Dávila-Camacho CA, Galaviz-Villa I (2021) Basic Biological Aspects of Dormitator maculatus “Naca” (Bloch, 1792) from the Alvarado Lagoon in Veracruz, Mexico. IOP Conf. Series Earth and Environmental Science. 690:1–10. Print ISSN: 1755–1307. Online ISSN: 1755–1315. https://doi.org/10.1088/1755-1315/690/1/012063
Dávila-Camacho CA (2020) Parámetros reproductivos de Dormitator maculatus (Bloch, 1792) y su relación con factores ambientales de la laguna de Alvarado Ver., para proponer aspectos básicos de cultivo. Tesis Doctoral. Tecnológico Nacional de México/Instituto Tecnológico de Boca del Río, Veracruz, México, p 19 http://posgrado.bdelrio.tecnm.mx/images/MaestriaAcuacultura/REPOSITORIO%20TESIS/Tesis%20MCACUA%202016-2019/Edna%20Fabiola%20Castillo%20Marquez.pdf Accessed 24 September 2019
Fischer W, Krupp F, Schneider W, Sommer C, Carpenter KE, Niem VH (1995) Guía FAO para la identificación de peces para los fines de pesca. Pacífico Central-Oriental. Roma. Vol. II. Vertebrados parte 1, Roma. pp 647–1200 ISBN:92-5-303409-2
Franco-López J, Abarca-Arenas LG, Rodríguez-Pelaez E, Viveros-Legorreta JL, González-Acosta AF (2021) Biometric relationships of the fat sleeper Dormitator maculatus (Blonch, 1792) (Teleostei: Eleotridae) from Alvarado lagoon, Veracruz. México Ecosistemas y Recursos Agropecuarios 8(1):e2679. https://doi.org/10.19136/era.a8n1.2679
González-Martínez A, López M, Mario Molero H, Rodríguez J, González M, Barba C, García A (2020) Morphometric and meristic characterization of native Chame Fish (Dormitator latifrons) in Ecuador using multivariate analysis. J Animals 10(1805):1–16. https://doi.org/10.3390/ani10101805
Hair FJ, Anderson RE, Tatham L, Black WC (1999) Multivariate data analysis. Prentice Hall, New Jersey (491). p 799 ISBN: 0-13894858-5
Haykin S (1994) Neural networks: a comprehensive foundation. MacMillan College, New York, Publishing Company, Englewood Cliffs, p 696 ISBN-10:0023527617
Hubbs CL, Lagler KF (1964) Fishes of the Great Lakes Region. University of Michigan Press, Ann Arbor, MI, USA
Im JH, Gil HW, Lee TH, Kong HJ, Ahn CM, Kim BS, Kim DS, Zhang CI, Park IS (2016) Morphometric characteristics and Fin Dimorphism between male and female on the Marine Medaka, Oryziasdancena. Dev Reprod 20(4):331–347. https://doi.org/10.12717/DR.2016.20.4.331
Nelson JS (2006) Fishes of the World. 4ª Edit. John Wiley & Sons, Inc., Canada, p 421
Pezold F, Cage B (2001) A review of the spiny-cheek sleepers, genus Eleotris (Teleostei: Eleotridae), of the Western Hemisphere, with comparison to the west African species. Tulane Stud Zool Bot 31(2):1–45
Ré-Regis MC, Estrada-García J (1992). Determinación de las fases de desarrollo gonádico de la "Naca" Dormitator maculatus. III Congreso Nacional de Ictiología, Oaxtepec, Morelos. p 24
Richardson J (1844) Dormitator latifrons (Richardson 1844) in GBIF Secretariat (2021). GBIF Backbone Taxonomy. Checklist dataset. https://doi.org/10.15468/39omei accessed via GBIF.org. Accessed 11 Sept 2022
Rodríguez-Varela A, Cruz-Gómez A, Torres-Rodríguez MA (1992) Análisis de la abundancia del ictioplancton de las familias Gobiidae y Eleotridae en seis Sistemas Estuarinos del Estado de Veracruz. III Congreso Nacional de Ictiología, Oaxtepec. pp 41–42
Schmitter-Soto J (1996) Catálogo de lospecesContinentales de Quintana Roo, Museum of Zoology, division of Fisher. pp 150–151
Segura-Guevara F, Olaya-Nieto CW, Torralvo Fajardo W (2011) Relación longitud-peso del guabino, Gobiomorus dormitor (pisces: Eleotridae) en el Río Sinú. Colombia Rev Asoc Colomb Ictiol 11:61–74
Strauss RE, Bookstein FL (1982) The truss: body form reconstruction in morphometrics. Syst Zool 31:113–135
Zar JH (1999) Bioestatistical Analysis, 4th edn. Prentice-Hall, Upper Saddle River, New Jeersey, U.S.A
Acknowledgements
We thank the National Technological Institute of Mexico (TecNM) for financial support to the project “Biological and biochemical characterization of Dormitator maculatus (Bloch, 1792) of the Alvarado lagoon, Veracruz” directed by Prof. Itzel Galaviz. We also thank the Universidad del Mar (UMAR) for statistical support, the Technological Institute of Boca del Rio, and the National Commission of Science and Technology of Mexico (CONACYT) for the doctoral grant 269154. We thank Derek J. Brockett (UMAR) and anonymous reviewers for comments that significantly improved the final version, and we also thank Jorge Alberto Dávila Montaño and Luis José Ortiz-Martinez, for the illustration of D. maculatus and the area study map, respectively.
Funding
This work was support for National Technological of Mexico (TecNM), by the Scientific and Technological Research granting funding for the research project “Biological and biochemical characterization of Dormitator maculatus (Bloch, 1792) of the Alvarado lagoon, Veracruz” (Code 6033.17-P).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by CADC, IGV, and PC-H. The first draft of the manuscript was written by CADC, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflicts of interest exist.
Ethics approval/declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dávila-Camacho, C.A., Galaviz-Villa, I., Cervantes-Hernández, P. et al. Sexual dimorphism in the fish Dormitator maculatus (Teleosti: Eleotridae) from Alvarado Lagoon, Veracruz, Mexico. Zoomorphology 142, 77–86 (2023). https://doi.org/10.1007/s00435-022-00582-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-022-00582-4