Abstract
One of the main factors creating intraspecific morphological variation, sexual dimorphism (SD) could be expressed as adult male and female animals having different body sizes or shapes. Female-biased sexual dimorphism has been documented in the vast majority of amphibians and more than half of salamanders. In this study, 18 morphometric characters were used to analyze the size and shape dimorphism of the southern banded newt, Ommatotriton vittatus, a species whose congeners exhibit male-biased dimorphism. In this way, the hypothesis that species within the same genus would have similar sexual dimorphism (for example, male- or female-biased) was tested. Results of the current study confirmed the existence of male-biased sexual size and shape dimorphism in O. vittatus. For instance, snout-vent length and tail length were found to be significantly different between sexes, with males being larger. Moreover, males have larger forelimbs and hindlimbs than females. Data from the present study also indicated significant male-biased differences in five (head length and width, eye diameter, distance between the orbit and naris, and internarial distance) out of eight head characters. This result supports the assumption that species within the same genus will have a similar tendency for sexual dimorphism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In animals, sexual dimorphism (SD) refers to significant variations between sexes, such as in morphology (Fairbairn et al. 2007) and it is linked to unequal selective pressures operating on males and females to improve their fitness. The primary forces that induce SD are typically sexual selection, natural selection, and fecundity selection, as well as others (Andersson 1994; Liao et al. 2013). The first hypothesis proposes that sexual selection stems from the rivalry among males to obtain territory or mating opportunities with females. This theory claims that males with larger bodies have an advantage because they have better reproductive success (Andersson 1994). Most of the studies on the evolution of sexual size dimorphism (SSD) have tested the hypothesis of divergence between sexes due to sexual selection. Intrasex interactions, such as male-male competition for females, and intersex interactions, such as female partner choice, are two important mechanisms of sexual selection (Fairbairn et al. 2007; Liao et al. 2015). Individuals may, for example, pick their partner based on size, with males having larger bodies and a male-biased SSD. Second, natural selection might promote survival by driving SSD development in distinct ways, for example, through competition for food among individuals (Fairbairn 1997). Lastly, SSD may evolve in a path that favors larger females who can more effectively employ their resources to increase reproductive output than males (fecundity selection favors female-biased SSD as a result of this tendency). Apart from these major mechanisms, ecological (e.g. intersexual variation in size is caused by ecological niche divergence; Fairbairn et al. 2007) and behavioral characteristics (e.g. aggressive behavior; Xiong et al. 2016) can also play a role in causing sexual dimorphism (Baraquet et al. 2018).
In addition to SSD, the phrase sexual shape dimorphism (SShD) express body form distinctions between females and males. SShD patterns may also offer insight into the evolution of variations in life-history characteristics. Because various body regions are under different selection forces, studying the morphology of species might help us better understand how SSD evolved (Labus et al. 2013; Cruz-Elizalde et al. 2022).
The magnitude and extent of SSD can vary considerably across species (Liao et al. 2015). Male-biased dimorphism is widespread in lizards and mammals; however, amphibians and insects exhibit female-biased dimorphism more frequently (Fairbairn 1997; Monnet and Cherry 2002; Altunışık 2017, 2018a). Specifically, a sexual size dimorphism that favors females is present in 90% of anurans and 61% of urodeles (Kupfer 2007; Altunışık 2017). Considering that they have a life cycle that includes both terrestrial and aquatic environments (Mani et al. 2022; Tatlı et al. 2022) and varies in important life history traits, amphibians are interesting organisms to investigate SD patterns (Duellman and Trueb, 1994). Three models of SD have been described in mature amphibians: (1) female-biased SD; (2) male-biased SD; and (3) unbiased SD.
Ommatotriton vittatus (Gray, 1835) (southern banded newt) is one of the three species of the Ommatotriton genus, which belongs to the Salamandridae family and is found from the mid-south of Türkiye to Israel, passing via the western Syrian Arab Republic, Lebanon, and northern Jordan. The species’ western distribution ends in the middle south of Türkiye (van Riemsdijk et al. 2017).
The sexual dimorphism of O. vittatus has not been widely investigated (Bülbül and Kutrup 2013), although the morphology of other salamandrid species has been extensively studied (Labus et al. 2013; Balogová and Uhrin 2015; Alarcón-Ríos et al. 2017; Altunışık 2017; Najbar et al. 2019; Pogoda and Kupfer 2020). It is hypothesized that species within the same genus will have a similar tendency for SD (e.g. male-biased or female-biased; Malmgren and Thollesson 1999; Bülbül and Kutrup 2013; Reinhard and Kupfer 2015). Since Ommatotriton nesterovi (Litvinchuk, Zuiderwijk, Borkin and Rosanov, 2005) and Ommatotriton ophryticus (Berthold, 1846), the other two species of the genus Ommatotriton, show male-biased sexual dimorphism (Çiçek et al. 2011; Bülbül and Kutrup 2013); the main goal of this study is to evaluate whether there may be a widespread male-biased pattern of SD in the southern banded newt.
Materials and methods
The study site (25 m above sea level) is in Tarsus, Mersin (36°54’N, 34°53’E) in Türkiye’s middle south, with a Mediterranean and fairly continental climate (Altunışık 2018b). The average summer temperature was 27.16 °C, and the average winter temperature was 10.9 °C, based on climatic information gathered from a meteorological station near the research area (Meteorological Station of Tarsus, Türkiye) for the years 1950–2017 (www.mgm.gov.tr).
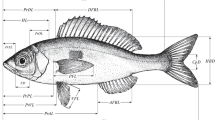
Throughout the 2017–2018 breeding season, a total of 68 (38 males, 30 females) O. vittatus specimens were captured by hand or using a dip net during the day. Eighteen variables (Table 1; Online resource: Fig. S1) associated with their body measurements were taken using a digital vernier caliper to the nearest 0.01 mm accuracy after anesthesia in MS-222. All tests were carried out in compliance with Turkish legislation and with the authorization of Recep Tayyip Erdogan University’s local ethics committee for animal experimentation (approval reference number: 2015/71). Externally apparent secondary sexual traits were used to determine the individuals’ sexes (prominent cloaca and dorsal crest in males) (Altunışık 2018b). All analyzed specimens were adults with fully developed gonads and were at least 40 mm in SVL (Bülbül and Kutrup 2013; Altunışık 2018b).
SPSS 21 (IBM, Statistics for Windows) was used for the statistical analyses. The Sexual Dimorphism Index (SDI) was calculated using Ranta et al. (1994)’s formula which is defined as “(size of larger sex/size of smaller sex) ± 1”, with + 1 if males are larger or -1 if females are larger, and arbitrarily defined as positive when females are larger than males. Given that the data were distributed normally (Shapiro-Wilk test, p > 0.05) and variances were homogenous (Levene’s test, p > 0.05) for all variables, the t-test was conducted to determine the morphometric differences between sexes. In addition, a principal component analysis (PCA) was used to investigate general size and shape differences between females and males. The first principal component (PC1), derived from a set of morphometric measurements, is mostly considered as an axis of overall body size variation when all traits load largely and in the same direction, with the remaining variance describing relative shape differences expressed in consecutive PCs (Schäuble 2004; Zhang et al. 2014; Xiong et al. 2016). Then, a multivariate analysis of covariance (MANCOVA) was carried out to identify which characteristics were different between the sexes using sex as a factor and SVL value as a covariate (Romano et al. 2009; Altunışık 2017).
Results
Results of the present study indicate that Ommatotriton vittatus males have a significantly larger body size than females (Independent sample t-test for SVL: t = 2.57, df = 66, p < 0.05; Fig. 1). SDI was positive (0.05), representing a male-biased size dimorphism. PCA yielded three major components, which together account for 64.49% of the total variance. The first two components (PC1 and PC2) explained 56.89% of the total variance (Fig. 2; Table 2). The t-test results revealed significant differences in the body form for 15 of the 18 morphological characteristics, with males having greater scores than females for each of these characters: TL (t = 2.65, df = 66, p < 0.05), TH (t = 13.50, df = 66, p < 0.001), HL (t = 13.55, df = 66, p < 0.001), HW (t = 7.68, df = 66, p < 0.001), AxG (t = 8.89, df = 66, p < 0.001), HLL (t = 8.89, df = 66, p < 0.001), FLL (t = 8.89, df = 66, p < 0.001), HAW (t = 8.89, df = 66, p < 0.001), FW (t = 8.89, df = 66, p < 0.001), LTOE (t = 8.89, df = 66, p < 0.001), CW (t = 8.89, df = 66, p < 0.001), DE (t = 8.19, df = 66, p < 0.001), IN (t = 8.89, df = 66, p < 0.001) and IC (t = 8.89, df = 66, p < 0.001) (Online resource: Figs. S2-S3).
When the effect of SVL was controlled, the differences in body size and shape between sexes were significant (MANCOVA: Wilks’ ƛ = 0.529, F17,48 = 2.517, p < 0.01).
Discussion
SSD and SShD have been documented in several vertebrate species (Cox et al. 2003). The female-biased SSD is more common in Classis Amphibia (Shine 1979; Kupfer 2007), and only about 19% of salamanders exhibit male-biased SSD (Kupfer 2007; Amat 2019). For example, male-biased SDD was shown in Phaeognathus hubrichti Highton, 1961 (Bakkegard and Guyer 2004), Onychodactylus zhangyapingi Che, Poyarkov and Yan, 2012 (Xiong et al. 2016) and Pachyhynobius shangchengensis Fei, Qu, and Wu, 1983 (Xiong et al. 2019). Ommatotriton vittatus exhibits male-biased sexual dimorphism in terms of many morphometric characters, including SVL. Ommatotriton nesterovi and O. ophryticus, which are the other two species belonging to the genus Ommatotriton, showed male-biased sexual dimorphism (Bülbül and Kutrup 2013), which supports the hypothesis that the species in the same genus show a similar pattern in terms of SSD.
In addition to having larger SVL, O. vittatus males have larger forelimbs and hindlimbs than females. In salamander species that mate in an amplexus, it is common that male individuals have larger forelimbs and forelimb muscles than female individuals (Malmgren and Thollesson 1999; Wells 2007; Çiçek et al. 2011; Reinhard et al. 2015; Altunışık 2017). Since the larger male forelimbs of salamanders may provide an advantage in male-male competition (e.g. aggressive behavior and male fighting; Zhang et al. 2014), mating success may be attributed to sexual selection (Bruce 1993; Bakkegard and Guyer 2004; Fairbairn et al. 2007; Liao et al. 2013). The success of mating is increased by sexual selection favoring bigger males with more aggressive behavior and superior fighting skills (Shine 1979; Xiong et al. 2019). Accordingly, it may be claimed that sexual selection explains male-biased SSD in O. vittatus since males’ aggressive behavior has been witnessed several times in this and previous studies (Altunışık 2018b).
The southern banded newt was observed to exhibit sexual dimorphism of the tail (males’ tails are longer and wider than females’), which was also reported in other Ommatotriton species (Çiçek et al. 2011; Bülbül and Kutrup 2013). In taxa other than Ommatotriton, for example in Salamandra salamandra (Labus et al. 2013), Pachyhynobius shangchengensis (Xiong et al. 2019), and Hynobius maoershanensis (Chen et al. 2022), it has been shown that males have longer tails than females. The longer and wider tail in males may be attributed to energy storage and reproductive success (Xiong et al. 2016; Kakegawa et al. 2017).
The analyzes showed that five (HL, HW, DE, IN and IC) out of eight head characters were male-biased, although the results obtained in previous research in O. nesterovi and O. ophyrticus showed that head measurements were not sexually dimorphic (Çiçek et al. 2011; Bülbül and Kutrup 2013). The longer and wider head in males may contribute to male-male competition, which has been explained in many urodele species, e.g., Euprocuts platycephalus (Gravenhorst, 1829) (Bovero et al. 2003), Phaeognathus hubrichti (Bakkegard and Guyer 2004) Salamandrella keyserlingii Dybowski, 1870 (Hasumi 2010), Liua shihi (Liu, 1950) (Zhang et al. 2014), Pachyhynobius shangchengensis (Xiong et al. 2019) and Hynobius maoershanensis Zhou, Jiang and Jiang, 2006 (Chen et al. 2022). It is assumed that larger-headed males can win more easily in male competition (aggressive behavior such as biting females, pers.comm.) and have more mating opportunities. Hence, the sexual selection hypothesis may be used to explain the SShD of the head characters of O. vittatus.
In other salamandrids like Ichthyosaura (Ivanović et al. 2009), Lissotriton (Ivanović and Kalezić 2012), Salamandrina (Romano et al. 2009; Pogoda and Kupfer 2020), and Salamandra (Alarcón-Ríos et al. 2017; Altunışık 2017), morphological variations between populations from male to female have already been identified. As a general rule, it should be noted that if selection favors size in one sex, this may result in shape disparities as a result of allometric shifts during growth (Ivanović and Kalezić 2012; Pogoda and Kupfer 2020). It is challenging to identify specific selecting mechanisms because of the intricate interplay of various allometric trajectories between species and sexes. The results of this study show that sexual dimorphism of the O.vittatus occurs not only in body size but also in body shape. Intriguingly, conflicting findings regarding SD within the family Salamandridae have been recorded. Even in different populations of the same species, the direction of SD was different. In the case of Salamandra salamandra (Linnaeus, 1758), Kalezić et al. (2000) reported that the tail length, forelimb length, and head width were all male-biased SD. However, other populations of S. salamandra have been found to have female-biased head size, inter-limb distances, and parotid gland characteristics in contrast to male-biased tails, forelimbs, hindlimbs, forefoot, and hind foot length (Labus et al. 2013). On the other hand, males are reported to be of equal size to females in the species Salamandra atra (Laurenti, 1768) (Kalezić et al. 2000) and Salamandra algira Bedriaga, 1883 (Reinhard et al. 2015).
Life-history characteristics (e.g., growth, longevity, survival) and ecology (niche distribution between the sexes) have also been reported in some studies as hypotheses to explain SD (Kalezić et al. 2000; Cadeddu et al. 2012). The mean age of male and female individuals in an O. vittatus population did not differ significantly in a previous study (Altunışık 2018b), therefore, it is thought that a conclusion can be reached as a result of studying other life-history traits such as the number, size and sex ratio of offspring, the timing of reproduction, and growth pattern. On the other hand, we think that natural selection will be insufficient to account for male-biased SD, given that food is abundant in the studied habitat and male and female individuals in this population do not compete for food (Altunışık 2018b).
In conclusion, in this study sexual dimorphism in size and shape is described for the first time in O. vittatus, with males being larger. This result supports the assumption that species within the same genus will have a similar tendency for SD. This variation can be explained by the sexual selection hypothesis, as shown in studies of other salamander species with male-biased SD (Zhang et al. 2014; Xiong et al. 2016, 2019; Chen et al. 2022). Therefore, the reproductive system of the southern banded newt needs to be investigated to understand whether there is competition between males associated with sexual selection. The size and shape dimorphism may be the outcome of ecological and behavioral variances, future research should therefore concentrate on comprehending these discrepancies to elucidate the observed SD.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alarcón-Ríos L, Velo-Antón G, Kaliontzopoulou A (2017) A non-invasive geometric morphometrics method for exploring variation in dorsal head shape in urodeles: sexual dimorphism and geographic variation in Salamandra salamandra. J Morphol 278:475–485. https://doi.org/10.1002/jmor.20643
Altunışık A (2017) Sexual size and shape dimorphism in the Near Eastern fire salamander, Salamandra infraimmaculata (Caudata: Salamandridae). Anim Biol 67:29–40. https://doi.org/10.1163/15707563-00002519
Altunışık A (2018a) Age, survivorship and life expectancy in near eastern fire salamander, Salamandra infraimmaculata (Caudata: Salamandridae). Russ J Ecol 49:166–171. https://doi.org/10.1134/S1067413618020029
Altunışık A (2018b) The first demographic data and body size of the southern banded newt, Ommatotriton vittatus (Caudata: Salamandridae). Acta Herpetol 13:13–19. https://doi.org/10.13128/Acta_Herpetol-21171
Amat F (2019) Patterns and allometries of sexual size dimorphism in salamanders and the rejection of rensch’s rule. Salamandra 55:145–150
Andersson M (1994) Sexual Selection. Princeton University Press, Princeton
Bakkegard KA, Guyer C (2004) Sexual size dimorphism in the red hills salamander, Phaeognathus hubrichti (Caudata: Plethodontidae: Desmognathinae). J Herpetol 38:8–15
Balogová M, Uhrin M (2015) Sex-biased dorsal spot patterns in the fire salamander (Salamandra salamandra). Salamandra 51:12–18
Baraquet M, Otero MA, Grenat PR, Babini MS, Martino AL (2018) Effect to age on the geographic variation in morphometric traits among populations of Boana cordobae (Anura: Hylidae). Rev Biol Trop 66:1401–1411. https://doi.org/10.15517/rbt.v66i4.32365
Bovero S, Sotgiu G, Castellano S, Giacoma C (2003) Age and sexual dimorphism in a population of Euproctus platycephalus (Caudata: Salamandridae) from Sardinia. Copeia 2003:149–154. https://doi.org/10.1643/0045-8511(2003)003[0149:AASDIA]2.0.CO;2
Bruce RC (1993) Sexual size dimorphism in desmognathine salamanders. Copeia 1993:313–318. https://doi.org/10.2307/1447131
Bülbül U, Kutrup B (2013) Morphological and genetic variations of Ommatotriton in Turkey. Anim Biol 63:297–312. https://doi.org/10.1163/15707563-00002413
Cadeddu G, Giacoma C, Castellano S (2012) Sexual size dimorphism in the Tyrrhenian tree frog: a life-history perspective. J Zool 286:285–292. https://doi.org/10.1111/j.1469-7998.2011.00878.x
Chen H, Bu R, Ning M, Yang B, Wu Z, Huang H (2022) Sexual dimorphism in the Chinese endemic species Hynobius maoershanensis (Urodela: Hynobiidae). Animals 12(13):1712. https://doi.org/10.3390/ani12131712
Çiçek K, Ayaz D, Bayrakci Y (2011) Morphology of the Northern Banded newt, Ommatotriton ophryticus (Berthold, 1846) (Caudata: Salamandridae) in Uludaǧ (Bursa, Turkey). Herpetol Notes 4:161–165
Cox RM, Skelly SL, John-Alder HB (2003) A comparative test of adaptive hypotheses for sexual size dimorphism in lizards. Evolution 57:1653–1669. https://doi.org/10.1111/j.0014-3820.2003.tb00371.x
Cruz-Elizalde R, Ramírez-Bautista A, Lozano A, Velasco JA, Octavio-Aguilar P, Berriozabal-Islas C (2022) Variation in size and shape sexual dimorphism in the Sceloporus scalaris species group (Squamata: Phrynosomatidae) from the Transvolcanic Belt of Mexico. Biol J Linn Soc 135:499–517. https://doi.org/10.1093/biolinnean/blab169
Duellman WE, Trueb L (1994) Biology of Amphibians. The Johns Hopkins University Press, Baltimore
Fairbairn DJ (1997) Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annu Rev Ecol Syst 28:659–687. https://doi.org/10.1146/annurev.ecolsys.28.1.659
Fairbairn DJ, Blanckenhorn WU, Székely T (eds) (2007) Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford. https://doi.org/10.1093/acprof:oso/9780199208784.001.0001
Hasumi M (2010) Age, body size, and sexual dimorphism in size and shape in Salamandrella keyserlingii (Caudata: Hynobiidae). Evol Biol 37:38–48. https://doi.org/10.1007/s11692-010-9080-9
Ivanović A, Kalezić ML (2012) Sexual dimorphism in the skull geometry of newt species of Ichthyosaura, Triturus and Lissotriton (Salamandridae, Caudata, Amphibia). Zoomorphology 131:69–78. https://doi.org/10.1007/s00435-011-0143-y
Ivanović A, Sotiropoulos K, Džukić G, Kalezić ML (2009) Skull size and shape variation versus molecular phylogeny: a case study of alpine newts (Mesotriton alpestris, Salamandridae) from the Balkan Peninsula. Zoomorphology 128:157–167. https://doi.org/10.1007/s00435-009-0085-9
Kakegawa M, Kishi F, Saikawa Y, Hasumi M (2017) Seasonal changes in body shape and mass in a lotic-breeding and externally fertilizing salamander Hynobius kimurae. Zool Anz 268:55–63. https://doi.org/10.1016/j.jcz.2017.04.003
Kalezić M, Džukić G, Ivanović A, Aleksić I (2000) Body size, age and sexual dimorphism in the genus Salamandra: a study case of the Balkan species. Spixiana 23: 283–292. https://biore.bio.bg.ac.rs/handle/123456789/3520. Accessed 10 Dec 2022
Kupfer A (2007) Chap. 5. Sexual size dimorphism in amphibians: an overview. In: Fairbairn DJ, Blanckenhorn WU, Székely T (eds) Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford Academic, Oxford, pp 50–59. https://doi.org/10.1093/acprof:oso/9780199208784.003.0006
Labus N, Cvijanović M, Vukov T (2013) Sexual size and shape dimorphism in Salamandra salamandra (Amphibia, Caudata, Salamandridae) from the Central Balkans. Arch Biol Sci 65:969–976. https://doi.org/10.2298/ABS1303969L
Liao WB, Zeng Y, Yang JD (2013) Sexual size dimorphism in anurans: roles of mating system and habitat types. Front Zool 10:1–5. https://doi.org/10.1186/1742-9994-10-65
Liao WB, Liu WC, Merilä J (2015) Andrew meets Rensch: sexual size dimorphism and the inverse of Rensch’s rule in Andrew’s toad (Bufo andrewsi). Oecologia 177:389–399. https://doi.org/10.1007/s00442-014-3147-8
Malmgren JC, Thollesson M (1999) Sexual size and shape dimorphism in two species of newts, Triturus cristatus and T. vulgaris (Caudata: Salamandridae). J Zool 249:127–136. https://doi.org/10.1111/j.1469-7998.1999.tb00750.x
Mani M, Altunışık A, Gedik K (2022) Bioaccumulation of trace elements and health risk predictions in edible tissues of the marsh frog. Biol Trace Elem Res 200:4493–4504. https://doi.org/10.1007/s12011-021-03017-1
Monnet JM, Cherry MI (2002) Sexual size dimorphism in anurans. Proc Biol Sci 269:2301–2307. https://doi.org/10.1098/rspb.2002.2170
Najbar A, Konowalik A, Halupka K, Najbar B, Ogielska M (2019) Body size and life history traits of the fire salamander Salamandra salamandra from Poland. Amphibia Reptilia 41:63–74. https://doi.org/10.1163/15685381-20191135
Pogoda P, Kupfer A (2020) Sexual shape dimorphism in the cranium and pelvic girdle of northern spectacled salamanders, Salamandrina perspicillata, investigated via 3d geometric morphometrics. Salamandra 56:113–122
Ranta P, Laurila A, Elmberg J (1994) Reinventing the wheel. Analysis of sexual dimorphism in body size. Oikos 70:313–321
Reinhard RS, Kupfer A (2015) Sexual dimorphism in a french population of the marbled newt, Triturus marmoratus (Urodela: Salamandridae). Salamandra 51:121–128
Reinhard S, Renner S, Kupfer A (2015) Sexual dimorphism and age of Mediterranean salamanders. Zoology 118:19–26. https://doi.org/10.1016/j.zool.2014.08.002
Romano A, Bruni G, Paoletti C (2009) Sexual dimorphism in the italian endemic species Salamandrina perspicillata (Savi, 1821) and testing of a field method for sexing salamanders. Amphibia Reptilia 30:425–434. https://doi.org/10.1163/156853809788795128
Schäuble CS (2004) Variation in body size and sexual dimorphism across geographical and environmental space in the frogs Limnodynastes tasmaniensis and L. peronii. Biol J Linn Soc 82:39–56. https://doi.org/10.1111/j.1095-8312.2004.00315.x
Shine R (1979) Sexual selection and sexual dimorphism in the Amphibia. Copeia 1979: 297–306. https://doi.org/10.2307/1443418
Tatlı H, Altunışık A, Gedik K (2022) Trace element bioaccumulation and health risk assessment derived from leg consumption of the marsh frog, Pelophylax ridibundus (Pallas, 1771). Ege J Fish Aquat Sci 39:182–190. https://doi.org/10.12714/egejfas.39.3.02
van Riemsdijk I, Arntzen JW, Bogaerts S, Franzen M, Litvinchuk SN, Olgun K, Wielstra B (2017) The Near East as a cradle of biodiversity: a phylogeography of banded newts (genus Ommatotriton) reveals extensive inter- and intraspecific genetic differentiation. Mol Phylogen Evol 114:73–81. https://doi.org/10.1016/j.ympev.2017.05.028
Wells KD (2007) The Ecology and Behavior of Amphibians. University of Chicago Press, Chicago
Xiong J, Liu X, Zhang X, Li M, Min Y (2016) Sexual dimorphism of the Jilin clawed salamander, Onychodactylus zhangyapingi, (Urodela: Hynobiidae: Onychodactylinae) from Jilin Province, China. Asian Herpetol Res 7:220–226. https://doi.org/10.16373/j.cnki.ahr.150057
Xiong J, Zhang B, Liu Q, Pan T, Gou J (2019) Sexual dimorphism in the Chinese endemic species Pachyhynobius shangchengensis Fei, Qu and Wu, 1983 (Urodela: Hynobiidae). PeerJ 7:e6408. https://doi.org/10.7717/peerj.6408
Zhang X, Xiong JL, Lv YY, Zhang L, Sun YY (2014) Sexual size and shape dimorphism in the Wushan salamander, Liua shihi (Liu, 1950) (Urodela: Hynobiidae). Ital J Zool 81: 368–373. https://doi.org/10.1080/11250003.2014.920927
Acknowledgements
This work was supported by grants from the Recep Tayyip Erdoğan University (2016.53007.102.03.01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No potential conflict of interest was reported by the author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Altunışık, A. Variation in size and shape: sexual dimorphism in the southern banded newt, Ommatotriton vittatus (Caudata: Salamandridae). Biologia 78, 2849–2854 (2023). https://doi.org/10.1007/s11756-023-01421-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01421-7