Abstract
Mesoderm origin in Bryozoa is largely unknown. In this study, embryonic and early larval stages of Membranipora membranacea, a bryozoan exhibiting a planktotrophic cyphonautes larva, are investigated using mainly ultrastructural techniques. Shortly after the onset of gastrulation, an ectodermal cell, which is situated centrally at the prospective anterior pole of the larva, can be recognized by its constricted apical surface and enlarged basal part. It is also distinct from other ectodermal cells by the composition of its cytoplasm. In later stages, it has left the epidermis, lost its epithelial character, and is situated subepithelially, between the basal sides of the ectodermal and endodermal sheets. A blastocoelic cavity is not present at this stage. This cell divides and gives rise to a group of cells forming a muscular and neuronal strand at the anterior side of the larva. The majority of the larval musculature originates from this ingression. Despite this evidence for an ectodermal origin, additional sources of mesoderm can so far not be excluded. The literature on mesoderm origin in Bryozoa is reviewed and the results are compared to known data from other metazoan taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In contrast to the increased interest and growing body of data on the origin of mesoderm in the different groups of Bilateria (Technau and Scholz 2003), we are facing a considerable lack of detailed knowledge about early embryogenesis in Bryozoa. On the one hand, this is surprising considering both the long history of research on this animal group and their yet uncertain phylogenetic position. But on the other hand, bryozoans are not easily accessible, especially for experimental developmental studies. This is, for example, reflected in the lack of established laboratory protocols and “model bryozoans”. In fact, several detailed descriptive studies on bryozoan development are available, mainly from the late 19th and early 20th century (summarized in Nielsen 1971; Ström 1977; 1990; Reed 1991; Zimmer 1997; Ostrovsky et al. 2008), which have contributed tremendously to our present knowledge of bryozoan biology. Most of the earlier embryological studies, however, comprise purely external observations, which provide only limited resolution for tracing internal structures like mesoderm. Therefore, hypotheses on the origin of mesoderm in Bryozoa have remained rather speculative.
Traditionally, three bryozoan subtaxa are distinguished, the Phylactolaemata, Stenolaemata, and Gymnolaemata. Although recent molecular data support the monophyly of each of these groups, their relationships remain ambiguous (Fuchs et al. 2009). Species of Gymnolaemata, the largest bryozoan subtaxon, exhibit different larval types, but the early development is comparatively uniform (Ström 1977; Zimmer 1997). Zooids of most species are proterandrous hermaphrodites. Outbreeding is widespread (Silén 1966). There is indication that the eggs are usually fertilized within the maternal zooid, and zygotes stay arrested in their cell cycle until spawning (Temkin 1996; see Ostrovsky 2008a for review). Within Gymnolaemata, the planktotrophic cyphonautes larva as well as several types of lecithotrophic larvae are known (Zimmer and Woollacott 1977). Embryogenesis in species with cyphonautes larvae takes place in the water column. Lecithotrophic larvae are brooded in various types of brood chambers and develop either from macrolecithal eggs or are nourished via placental structures (reviewed in Ström 1977; Reed 1991; Ostrovsky 2008b).
Many of the embryological studies that address the question of mesoderm origin assume an origin from a quartet of cells, situated at the vegetal pole, which are also likely to produce the endoderm (e.g., Vigelius 1886; Calvet 1900; Pace 1906; d’Hondt 1983). Thus, this lineage is often referred to as the endomesoderm. However, apart from the mentioned low resolution for internal structures, most of these earlier studies were on species whose larvae lack a functional gut. As this likely represents a derived condition within the Bryozoa (Zimmer and Woollacott 1977), data from the possibly more ancestral feeding larvae, the cyphonautes, are suggested to be of greater phylogenetic significance. Membranipora membranacea (Linnaeus, 1767) is a common cosmopolitan bryozoan that forms large colonies preferentially on laminarian kelp blades. Its cyphonautes larvae are widespread in the plankton during spring and summer months. The larvae are up to 800 μm in length and are characterized by their compressed triangular shape with two lateral, presumably chitinous, shells.
In this study, different embryonic and early larval stages of M. membranacea were examined beginning shortly before gastrulation until formation of the early swimming cyphonautes in order to get information about the origin and development of the mesoderm and to provide a structural groundwork for comparisons with other taxa as well as for forthcoming developmental studies on bryozoans. Serial sectioning for transmission electron microscopy as well as fluorescence staining techniques were applied for this approach.
Materials and methods
Colonies of Membranipora membranacea (Linnaeus, 1767) were collected near the docks of Friday Harbor Laboratories, San Juan Island, WA, USA in May and June 2004 and kept in running seawater for several days. Reproductive colony parts were identified by observation using a dissecting microscope. The zygotes present in the zooidal body cavities were obtained by carefully piercing the membranous frontal walls. The opened colonies were subsequently rinsed with seawater and the zygotes were separated from foreign matter using nylon meshes of different sizes. Zygotes were transferred into 200-ml custard dishes with 0.1-μm filtered seawater which were kept on a floating table at approx. 12°C. Concentrations ranged from 2 to 5 cells/ml. The cell cycle is arrested as long as the zygotes are inside the body cavity. Under natural circumstances, they become activated during release through the intertentacular organ. Activation of artificially obtained zygotes can be accomplished in vitro by exposure to seawater containing 0.01 mM EDTA (Reed 1987). When activated, the cells change their shape from disk-like to roundish. Zygotes were transferred back to 0.1-μm filtered seawater within 30 min after activation. Development was followed at constant temperature of 12°C under natural illumination. Late gastrulae and early cyphonautes were fed on an algal suspension containing a mixture of Rhodomonas sp., Isochrysis galbana, and Dunaliella sp. Water in the bowls was exchanged once daily and debris was removed manually by pipetting. Developmental stages were fixed at distinct intervals for fluorescence and electron microscopy.
Embryonic stages were fixed in 2.5% glutaraldehyde buffered either with 0.1 M PBS containing 0.2 M NaCl or 0.1 M sodium cacodylate containing 0.14 M NaCl for 30 min at 4°C. Specimens were stored in buffer until post-fixation with 1% osmium tetroxyde (30 min, 4°C), subsequently washed in buffer, dehydrated in a graded ethanol series, and embedded into Epon with propylene oxide as intermedium. Series of silver-interfering ultrathin sections were produced using a Leica UC6 microtome and Diatome diamond knives. Sections were placed on formvar-coated single-slot copper grids and automatically stained with uranyl acetate and lead citrate using a prototype of an automatic stainer. For examinations, a Philips CM 120 transmission electron microscope was used at 60 kV. Images were taken on Ditabis erasable photoplates, scanned and processed using analySIS, Adobe Photoshop and ImageJ software packages.
For fluorescence staining, embryos were fixed in 4% paraformaldehyde in 0.05 M PBS containing 0.3 M NaCl for 30 min at room temperature. Specimens were rinsed in the same buffer. For storage, a small amount of NaN3 was added. For f-actin staining, specimens were permeabilized with a solution of 0.1% Triton-X 100 in PBS and stained with AlexaFluor-568-labeled phalloidin for 2 h. For antibody staining, blocking buffer (PBS with 0.1% Triton-X 100, 0.1% bovine serum albumin) was applied for 2 h. Specimens were incubated with primary antibody (anti-ac-α-tubulin, Sigma T-6793) at a dilution of 1:500 in blocking buffer for 10 h at room temperature. After several washing steps, specimen were incubated with secondary antibody (AlexaFlour568 goat-anti-mouse, Molecular Probes A-11004) in similar way. In order to determine numbers of cells in the different stages, some embryos were counterstained with the nuclear dye propidium iodide dissolved in PBS for 20 min. Stained embryos were mounted in Citifluor glycerol solution on glass slides. Confocal image stacks were photographed on a Leica TCS SPE and processed using ImageJ. Numbers of examined specimens are given in Table 1.
Results
Early cleavages
The activated zygotes (Fig. 1a) are of roundish shape and 50–60 μm in diameter. They are surrounded by a transparent fertilization membrane. It fits relatively loosely around the egg and exhibits lots of wrinkles. In TEM sections (Figs. 2, 3c), the membrane appears as a 1–1.5 μm thick, homogeneously electron-dense layer with a slightly grained outer surface. The membrane possibly shrinks during glutaraldehyde fixation, because in TEM sections it is often seen much closer to the larval surface. The zygotes are polarized: the cytoplasm is uniformly opaque except at one pole, where a translucent spot, obviously containing less yolk is found (Fig. 1a). This region of the zygote corresponds to the animal pole of the embryo. Polar bodies were not observed. In some eggs, the sperm axoneme still stuck in the membrane (see Fig. 1b), however, because of the loose fit of the fertilization membrane, the relation of the sperm entry point to the first cleavage planes could not be determined safely.
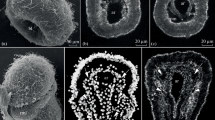
Cleavage and early embryonic development, light micrographs, Nomarsky optics (DIC). a Zygote shortly after activation (see text). Yolk is not equally distributed in the cell. A region at the prospective apical pole (ap) appears considerably brighter. The fertilization membrane (fm) lies in folds around the cell. b 2-cell stage, 1 h post activation (hpa). In some embryos, a sperm axoneme (sa) was observed sticking in the fertilization membrane. c 8-cell stage, 5 hpa, view on animal pole, focal plane is on the animal tier of cells. The first two cleavages are meridional, the third is equatorial. d 16-cell stage, 9 hpa, view on vegetal pole. Fourth cleavage is parallel to first and results in a biradially symmetric embryo with two tiers of eight cells. In the vegetal tier, cleavage is slightly unequal resulting in little larger central blastomeres. e 64-cell stage, 18 hpa, view on animal pole. Yolk-rich macromeres at the vegetal pole have become internalized. f Mid-gastrula, 22 hpa. In this stage, about 160 cells are present in average. A rim is formed around the blastopore (bp) g Late gastrula, 30 hpa. An apical organ (ao) has formed. The atrial invagination (at) has shifted the blastopore toward the inside. The prospective corona (co) develops ciliation, which enables the embryo to swim. h Early cyphonautes, 44 hpa. In this stage, a continuous gut is present. The atrium extends and develops ciliated structures for particle capturing. i Early cyphonautes, 72 hpa. In the atrium (at), lateral ciliated ridges (cr) become visible. st stomach
Mid-gastrula stages, TEM micrographs. a Sagittal section of 22 hpa stage. At the anterior side of the embryo, the prospective mesodermal cell (me), which is distinct from the other ectodermal cells, exhibits an expanded basal side that extends between the two epithelial sheets. b Horizontal section of 23.5 hpa stage. At this stage, the cell has ingressed into a subepithelial position, thus can be regarded as mesodermal. c Detail of apical region of cell ingressing from the ectoderm into blastocoel. Apical adherens junctions to the neighboring cells are still present (arrowheads). The cytoplasm exhibits an exceptional amount of electron-lucent vesicles. d Detail of the center of the archenteron. Apical regions of endodermal cells are connected by adherens junctions (arrowhead). e Detail of first mesodermal cell. an animal, ant anterior, bp blastopore, ec ectoderm, en endoderm, fm fertilization membrane, pos posterior, veg vegetal
All cleavages are total. Two-cell stages (Fig. 1b) occur as early as about 60 min post activation and further cleavages follow at slightly increasing intervals. The first and second cleavages are meridional and orthogonal to each other, resulting in a radially symmetrical embryo. The third cleavage is equatorial (Fig. 1c). No significant differences could be observed between the resulting upper and lower tier, so that up to this point, all cleavages are equal. The fourth cleavage breaks the radial symmetry. Cleavage planes are parallel to the plane of the first cleavage, therefore resulting in an embryo with an animal and vegetal tier, each consisting of 2 × 4 cells (Fig. 1d). The cell divisions in this cleavage are more or less equal in the animal half, but not in the vegetal half, where the resulting central cells are slightly bigger than the peripheral ones. In the 32-cell stage (not shown), two tiers of 8 cells are located in the animal half. The vegetal half consists of four larger cells at the vegetal pole. Above these is a tier of 12 cells, which resemble in size those of the animal half. The cells in the animal half now seem to divide faster than those in the vegetal half, but cleavages become hard to follow by light microscopy from the 32-cell stage on. No formation of a blastocoelic cavity was observed.
Early gastrula
Gastrulation begins at the 64-cell stage (Figs. 1e, 7a) and shows characteristics of both invagination and epiboly. The cells at the vegetal pole bend inwards forming the blastopore and the beginning archenteron (Fig. 2a, b). The ectodermal cells are much smaller and begin to overgrow the vegetal cells laterally. The archenteron is still without a lumen, but the cells of the endoderm are coupled by adherens junctions, thus representing an epithelial layer. Apical junctional complexes are, though delicate, also present in the ectodermal layer. Yolk is present in the form of homogeneously electron-dense granules, ovoid to round in shape and up to 2.5 μm in diameter. The granules are found in all cells, but are most abundant in cells of the endoderm and the blastopore rim. Additionally, electron-lucent vesicles of similar size occur. A blastocoel is still absent. The basal cell membranes of the ectodermal and endodermal cells are directly adjacent without a visible ECM or basal lamina in between. Cell–cell junctions between ecto- and endodermal cells have not been observed. The ectodermal cells are smallest at the embryonic equator.
Mid-gastrula
In 22-h stages (Fig. 1f), which contain approximately 160 cells, the blastopore becomes clearly visible on the vegetal side of the embryo. A specific ectodermal cell is recognized at the anterior side of the embryo (Figs. 3a, 7b). This cell is distinct from the other ectodermal cells by its larger volume. Its cytoplasm contains a large quantity of electron-lucent vesicles (Fig. 3a, c), and its basal region is considerably broadened in comparison with the other ectodermal cells. The apical surface is reduced, but the cell still exhibits apical adherens junctions to the neighboring cells (Fig. 3c). This cell, therefore, most likely shows the beginning of an ingression process, as seen in embryos of slightly later temporal stages (23.5 hpa, Fig. 3b), where a cell with similar cytological characteristics is found in a similar location. In these stages, the cell is completely ingressed and situated between endoderm and ectoderm (Figs. 3e, 7c). It has lost its polarity and apical junctions and therefore resembles a mesenchymal cell.
Late gastrula
During the next cell division cycles, the shape of the embryo changes considerably (Fig. 1g). The first externally visible new feature is the apical organ. This knob-like organ is formed from a group of epidermal cells at the animal pole that develop cilia and become elongate and columnar in shape. The cilia break through the fertilization membrane. On the oral side of the embryo, the blastopore broadens. Ectodermal cells around the blastopore invaginate, forming the beginning atrium. During this process, the blastopore gets shifted deeper toward the inside of the embryo. The cells that surround the atrial invagination form a conspicuous ciliated rim, the prospective corona, and the fertilization membrane ruptures in this region. The cilia already beat regularly, thus embryos at these stages start swimming. Apart from the apical organ, the ectoderm differentiates into two distinct regions (Fig. 4a). At the anterior and posterior side of the embryo, the epithelium becomes squamous, with the cells sometimes only 1 μm in height. In contrast, the epidermal cells situated laterally acquire a columnar shape, measuring up to 10 μm in height. These cells begin to secrete the lateral shells (Fig. 4a, e), which are characteristic of the cyphonautes larva. The cells exhibit a well-developed endoplasmic reticulum and golgi cisternae. Numerous electron-dense vesicles are found in the apical region of the cells, directly underneath the apical membrane. The vesicles are almost 150 nm in diameter and consist of a dense core surrounded by a slightly brighter halo. The developing shell is a longitudinally textured layer that contains numerous dark spots. These might originate from the earlier mentioned vesicles when they expel their contents via exocytosis. Outside of the developing shell, the fertilization membrane begins to rupture. The apical surfaces of the aboral ectodermal cells never bear microvilli.
Late gastrula stages, TEM micrographs. a Horizontal section from central region as indicated in inset. Mesodermal cells are situated at the anterior side of the larva. An archenteron (ar) has formed. b Horizontal section from oral region as indicated in inset. Nerve bundles (ne) encircle the oral opening of the atrium (at), underneath the prospective corona c, d Details of anterior region showing mesodermal cells (me) located between ectoderm (ec) and endoderm (en). A large central cell is flanked by two thinner lateral ones that exhibit strands of myofilaments (my). Adjacent to the mesoderm are two nerve bundles coming from the apical organ and running toward the blastopore rim. e Detail of shell (sh) secretion. Lateral epidermal cells bear specific electron-dense vesicles. ant anterior, fm fertilization membrane pos posterior
The cells of the endoderm still contain the majority of yolk vesicles. The endoderm has developed a clearly visible lumen, the archenteron (Fig. 4a). The apical sides of the endodermal cells bear short micovilli and a thin layer of glycocalyx. Apical adherens junctions are now well developed, however, septate junctions have not been observed until this stage. The distance between the basal sides of the ectodermal and endodermal cells has become wider, indicating the beginning formation of a basal lamina. On the anterior side, mesodermal cells are found (Figs. 4a–d, 7d). These are mesenchymal, apolar, and arranged in a strand reaching from the prospective corona toward the basal side of the apical organ. About four cells are present in this stage, overlapping each other in the strand. They are flanked laterofrontally by two nerve bundles (Fig. 4c, d) running from the apical organ to the prospective corona, where they proceed posteriorly (Fig. 4b). Laterally, the mesodermal cells exhibit bundles of myofilaments, characterizing them as muscle cell precursors.
Early cyphonautes larvae
The characteristics of the cyphonautes larva can be recognized in stages of about 44 h (Figs. 1h, 7e). These stages exhibit a roughly triangular shape with externally visible lateral shells. The remainder of the fertilization membrane still covers the anterior and posterior side (Fig. 5a–d). The gut has curved to the posterior end of the oral side and opens with an anus there. The pharyngeal region can be distinguished from the stomach by its wide lumen and its ciliation (Fig. 5b). The pharynx forms the ascending part of the gut while the stomach and intestine form the descending part. The pharynx epithelium is corrugated with its cells being rather thin (0.5–3 μm). They bear short apical microvilli and a delicate glycocalyx. The stomach is characterized by its large voluminous gastrodermic cells which exhibit long apical microvilli and lack cilia. The intestine is a short, narrow unciliated duct connecting stomach and anus. The larvae start foraging immediately after the anus has formed, so that algal cells can be found in the gut. The atrium has extended considerably (Fig. 5c, d). Its epithelium is densely ciliated, especially along two lateral ridges (Figs. 5c, f, 6f). The cells of the oral surface bear microvilli and a glycocalyx, resembling those of the pharyngeal cells.
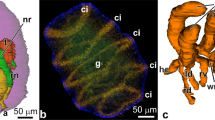
Early cyphonautes larva. a–d TEM micrographs. a–c Selected horizontal sections, planes as indicated in d. a Apical region. Section of apical organ (ao) and tangential section of gut epithelium (ge) ensheathed with mesodermal muscle cells (me). b Middle region, cross section of anterior muscle and nerve strand, pharynx (ph), and stomach (st). c Oral region showing atrium (at) and ciliated ridges (cr). d Sagittal section. e Phalloidin staining of musculature, lateral view, projection of full confocal stack. f α-tubulin staining (cilia and nerve processes), lateral view, projection of full confocal stack. am anterior median muscle, an anterior nerve, ant anterior, bc primary body cavity, cm coronal muscle, co corona, ec ectoderm, fm fertilization membrane, lm lateral muscles, pm posterior muscle, pos posterior, sh shell, st stomach
Early cyphonautes larva, details of mesoderm and musculature from regions indicated by squares in Fig. 5a–c, TEM micrographs. a Tangential section of curved intestinal tract. The gut epithelium (ge) is surrounded by myofilamentous processes (my) of mesodermal cells (me). b A cell located centrally in the apical organ (ao) bears vertically arranged bundles of myofilaments. c The anterior muscle strand is formed by several mesodermal cells that reside in the blastocoel (bc) and are adjacent to the anterior pharyngeal (ph) wall. Nerve bundles (ne) run parallel to the muscle cells. d The pharynx epithelium is surrounded by circular muscle fibers. Longitudinal muscle cells lie at the posterolateral side of the pharynx e Longitudinal muscle cells at the posterior end between gut and epidermis f Muscles in the lateral body cavity and ciliated ridge (cr). at atrium, en endoderm, sh shell
The apical organ (Fig. 5a, d) has increased in size and exhibits a centrally located cell that bears vertically arranged bundles of myofilaments (Figs. 5e, 6b). Characteristic in this stage is the occurrence of an extensive fluid-filled primary body cavity between epidermis and gut epithelium. Coelomic spaces are absent. The mesoderm mainly comprises the already well-differentiated musculature. Different muscular compartments can be recognized. On the anterior side, a strand composed of a few muscle cells connects the basal side of the apical organ to the site of the developing pyriform organ (Figs. 5b, e, 6c). Parallel to the muscles run 2–3 nerve bundles (Figs. 5b, f, 6c). The pharynx is encompassed by myofilamentous processes of mesodermal cells (Figs. 5a, b, e, 6a, d). Lateral muscles are present in the body cavity and underneath the ciliated ridges (Figs. 5b, c, e, 6d, f). Further muscles are found on the posterior side of the larva and parallel to the corona (Figs. 5b, e, 6e).
Discussion
General embryology
The embryology of species with cyphonautes larvae has been examined by Prouho (1892) in Alcyonidium albidum Alder, 1857, Hypophorella expansa Ehlers, 1876, and Electra pilosa (Linnaeus, 1768), by Marcus (1926) in E. Pilosa and Farrella repens (Farre, 1837), by Cook (1962) in Conopeum seurati (Canu, 1928) and Electra crustulenta (Pallas, 1776), as well as in Conopeum tenuissimum (Canu, 1928) (Dudley 1973) and Membranipora serrilamella Osburn, 1950 (Mawatari and Mawatari 1975). The early stages of Triticella koreni Sars, 1874, investigated by Ström (1969), resemble those in species with cyphonautes larvae, but this larva is probably not planktotrophic. More recently, Wood (2008) described the development of the cyphonautes larva in the freshwater ctenostome Hislopia malayensis Annandale, 1916. With respect to internal structures, the most detailed investigation is that of Prouho (1892), who applied sectioning and histology on the embryos.
The cleavage pattern and the overall early embryogenesis reported in these works are mostly in accordance with the results of the present study and will, therefore, not be discussed further. However, some discrepancies have been identified: One point is the occurrence of a blastocoelic cavity in blastula and gastrula stages. This was reported by Prouho (1892) and Wood (2008), and is also found in many lecithotrophic embryos (see in the following paragraphs), but could not be substantiated in the present study. A fluid-filled primary body cavity is not formed until the early shelled stages. This partly influences the description of the gastrulation, as an invagination is per definition linked to a coeloblastula. In Membranipora membranacea, the prospective endoderm is partly overgrown by the ectoderm. This mode thus resembles an epibolic gastrulation. Another point is the occurrence of a blastopore and its closure. Prouho (1892) assumed that the blastopore is closed after the internalization of the prospective endodermal cells and that both the larval mouth and anus originate secondarily. This is also described from lecithotrophic larvae. The present results argue for the persistence of the blastopore as larval mouth (protostomy) in M. membranacea. However, because of the atrial invagination, it is not unproblematic to localize the position of the larval mouth exactly.
Mesoderm formation in Gymnolaemata
The results presented here (summarized in Fig. 7) provide evidence for an ectodermal origin of the first mesodermal cell. This cell is situated in the anterior epidermis and ingresses to a position between the basal sides of ectoderm and endoderm. No blastocoel is present at this stage. The cell looses its polarity and acquires mesenchymal characters. Such a process is generally referred to as epithelial–mesenchymal transformation (EMT) and is involved in developmental processes (e.g., gastrulation, mesoderm formation) in many metazoan taxa (see Shook and Keller 2003 for review). The described ingression is so far the only observed event of mesoderm formation in the development of M. membranacea. However, due to the low temporal resolution of the applied method, it can so far not be excluded that, in the course of embryogenesis, additional mesoderm can originate from either ectoderm or endoderm. Prouho (1892) observed in embryonic stages of Alcyonidium albidum two mesodermal cells to appear shortly after gastrulation and subsequent blastopore closure. These cells are distinct from the other internal cells and reside laterally in the anterior end of the embryo. However, Prouho refrained from making definitive statements about the origin of these cells. During later development, he observed mesodermal cells to arrange in a row at the apical end of the larva and suspected these cells to give rise to musculature. The situation in the other species examined by him (Hypophorella expansa Ehlers, 1876, Electra pilosa) is said to be essentially similar. Marcus (1926) described gastrulation in both E. pilosa and Farrella repens to occur by polar ingression of the vegetal cells. He did not observe the first mesodermal cells mentioned by Prouho, but in later stages of F. repens, he was able to differentiate between larval mesenchyme situated in the anterior end of the larva and the forming intestinal tract in the posterior side. Mawatari and Mawatari (1975) stated the gastrula in M. serrilamella to be filled with ectodermal cells, but it remains unclear why the authors regard them as ectodermal and if they actually have observed their formation.
Schematic representation summarizing results from present study. a Early gastrula b Mid-gastrula with beginning ingression of prospective mesodermal cell. c Mid-gastrula with ingressed mesodermal cell. d Late gastrula e Early cyphonautes larva. am anterior muscle, an anterior nerve, ao apical organ, at atrium, bp, blastopore, co corona, st stomach, ph pharynx, pm posterior muscle
A larger number of observations on the origin of mesodermal cells have been made in species with lecithotrophic larvae. Most of these suggest an endodermal origin, but the presumed mechanisms differ considerably. Barrois (1880) described in Lepralia sp. the temporary occurrence of two strips of cells lateral to a mass of endodermal cells. In later stages, these strips reunite with the remaining endoderm to a homogeneous mass. The author interpreted the mesodermal strips as correlates to germ bands, which occur e.g., in polychaetes. However, it is unclear which species he actually investigated, since Lepralia is not a valid genus name and has become a synonym of many other genera. Calvet (1900) observed in Bugula simplex Hincks, 1886 (as B. sabatieri), Cellaria fistulosa (Linnaeus, 1758), and Bowerbankia pustulosa (Ellis and Solander, 1786) a proliferation of cells from the vegetal pole into the blastocoel. He designated them as endoderm though they never give rise to an intestinal tract in these species but instead to mesenchyme and musculature. Pace (1906) described a mesenchymal mass of mesendodermal cells, derived from the vegetal quartet, to fill the blastocoel of early embryos in Flustrellidra hispida (Fabricius, 1780) which has a shelled lecithotrophic larva. Endodermal and mesodermal cells are not distinguishable in this mass. The blastopore is stated to be closed subsequently, and a nonfunctional pharynx is formed during a later invagination of the oral epithelium. In Bugula flabellata (Thompson in Gray, 1848), a species exhibiting a coronate larva, localized delamination of the vegetal cells leads to the formation of an archenteron (Corrêa 1948). The archenteron was described as later disintegrating, giving rise to mesenchyme which soon fills the entire blastocoel. This tissue also forms swellings around the apical organ and the neuromuscular strand. Corrêa (1948) interpreted the neuromuscular strand as consisting of ectomesenchyme, but without further explanation or documentation. Vigelius (1886) described the internalization of the four vegetal macromeres in Bugula calathus Norman, 1868 to occur by epiboly. Neither an archenteron nor a persistent blastopore was found in later embryonic stages. He observed no distinct mesodermal anlage, thus regarding the internal mesenchyme as mesendodermal. Based mainly on observations on Alcyonidium gelatinosum (Linnaeus, 1767) (as A. polyoum (Hassall, 1841)) and in comparison with previous observations in other species, d’Hondt (1983) assumed that the vegetal quartet generally gives rise to mesoderm in bryozoan species lacking a gut and to mesoderm and endoderm in species with a gut.
In fully grown gymnolaemate larvae, several different mesodermal tissues are recognized (Reed 1991). The musculature of the cyphonautes larva shows distinct functional groups (Gruhl 2008), which already differentiate early in development, as shown in the present study. Apart from muscle cells, the most abundant cell type is mesenchyme. These cells fill most of the space between ectoderm and endoderm resulting in a compact organization of the larval body without blastocoelic spaces. Three different types of mesenchymal cells have been distinguished in Bowerbankia gracilis Leidy (Reed and Cloney 1982). Some of the mesenchymal cells become phagozytotic during metamorphosis and are possibly involved in histolytic processes. In Bugula neritina (Linnaeus, 1758), pigment cells are situated underneath the larval epidermis (Reed and Woollacott 1982). Some, often well-organized, mesodermal components have been identified as contributing to the anlage of the ancestrular polypide, thereby constituting precursors of the adult mesoderm, more specifically the coelomic epithelium: In larvae of B. neritina and other cellularioid cheilostomes, so-called mesodermal blastemata are situated underneath the apical disk (Reed 1991). In vesiculariid ctenostome larvae, the polypide rudiment is represented by a median mesodermal band connected to the roof of the internal sac (Reed and Cloney 1982; Zimmer and Woollacott 1993). Both these elements are found in larvae of Watersipora arcuata Banta, 1969 (Zimmer and Woollacott 1989). It is not clear whether these different mesodermal cells arise independently or originate from a single mesodermal anlage. Woollacott and Zimmer (1971) hypothesized an ectodermal origin of the mesodermal blastema in Bugula neritina based on ultrastructural similarities with the associated ectodermal cells of the apical disk. In larvae of M. membranacea, however, the mesoderm is relatively uniform (Stricker et al. 1988). An accumulation of mesodermal cells, which might contribute to the polypid anlage, is described underneath the apical organ, but these cells are undifferentiated, resembling mesenchymal cells.
Cyclostomata and Phylactolaemata
In terms of mesoderm formation, both cyclostomes and phylactolaemates most likely exhibit extremely derived patterns. In cyclostomes, the origin of mesoderm is obscured by the process of polyembryony, which is commonly found in these species (Harmer 1893, 1898; Calvet 1900; Robertson 1903; Borg 1927; Nielsen 1970, 1971). The zygotes cleave irregularly forming a solid mass from which secondary embryos are divided off. The latter appear bilayered, but it is not clear how the central layer originates. Only Robertson (1903) described newly budded secondary embryos to be solid and later to consist transitionally of only one layer surrounding a blastocoel-like cavity in Crisia eburnea (Linnaeus, 1758). Even a determination of the site of proliferation would not contribute to the understanding of mesoderm origin as polarity of these embryos is uncertain. The larvae are lecithotrophic and remain gutless. Metamorphosis in cyclostomes is not fully understood. A bilayered polypide bud occurs. However, it is uncertain if the central cells contribute to this. Nielsen (1970) suggested that the prospective mesodermal layer of the bud originates from the ectodermal layer by delamination.
The larvae of phylactolaemate bryozoans are hardly comparable to those of gymnolaemates. They lack most of the organs usually found in gymnolaemate larvae, but exhibit fully developed polypides. Because of this precocious character and the, in comparison with gymnolaemate larvae, low proportion of transitory tissues, they are sometimes regarded as representing swimming young colonies rather than larvae (Nielsen 1971). Embryology has been observed in Cristatella mucedo Cuvier, 1798, Plumatella polymorpha (Kraepelin, 1887) (Davenport 1891), Plumatella sp. (Kraepelin, 1892), Plumatella fungosa (Pallas 1768) (Braem 1890, 1897; Brien 1953), Fredericella sultana (Blumenbach, 1779) (Braem 1908), and Lophopus crystallinus (Pallas, 1768) (Marcus 1934). The fertilized eggs are brooded in internal embryo sacs which are attached to the body wall. The embryos are oval and polarized according to their orientation in the embryo sac. The pole facing the maternal zooids body wall is usually referred to as distal, the opposite one as proximal. All workers agree that at some point during development, an elongated embryo appears, which exhibits two epithelial layers surrounding a central lumen. Observations on the formation of this stage, however, remain contradictory: Davenport (1891) reported the ingression of four cells from the distal pole at an early stage. He postulates these cells to give rise to the internal cell layer, said to represent mesoderm and endoderm. Kraepelin (1892) confirmed Davenports results, whereas Braem (1897) in Plumatella fungosa also observed this ingression, but found these cells as isolated internal cells in a later monolayered stage. The internal layer is said to originate from a second phase of proliferation, also from the distal pole. These proliferated cells form an internal cavity, a coelom, which confines the blastocoel toward the proximal pole in the course of development. The author, therefore, interpreted the first proliferation as gastrulation, the second as mesoderm formation. Brien (1953) also found a stage like the gastrula observed by Davenport and Braem, but did not confirm a second ingression. In contrast, Braem (1908) reported the occurrence of a blastula stage in F. sultana that is hollow only in the distal part, but solid in the proximal part. Mesodermal cells proliferate from the border region between both parts into the upper blastocoel. At a later stage, a coelomic cavity is formed in the proximal part, whereas the blastocoel persists in the distal part. Marcus (1934) also observed a central constriction in the embryos of L. crystallinus, but with both resulting parts being hollow. Internal cells occur in both parts, whereas those in the upper part are hypothesized as endoderm, those in the lower part as mesoderm. However, the exact origins of these tissues were not observed. d’Hondt (2005) interpreted the formation of thickened cells at the distal part as an aberrant form of gastrulation. Thus, according to the author, mesoderm proliferating from these regions should be regarded as endomesoderm.
Comparison with other taxa
Mesoderm in most spiralian taxa is of dual origin (see Nielsen 2001, 2004, 2005; Lambert 2008). Mesodermal elements that go back to the 4d micromere are usually referred to as endomesoderm. These typically comprise the lateral germ bands, which give rise to longitudinal musculature as well as, in annelids, to coelomic sacs and segmental musculature. The second type, ectomesoderm, is in most cases derived from the second and/or third quartet of micromeres, members of otherwise ectodermal lineages. Ectomesoderm is sometimes also referred to as larval mesoderm, because in many cases it forms musculature of the pretrochal region, which vanishes during metamorphosis or later development. However, this split may not apply in all species and the exact lineage of ectomesoderm is variable within the Spiralia (Boyer and Henry 1998; Ackermann et al. 2005; Hejnol et al. 2007). Nevertheless, both types of mesoderm might have already been present in the ancestral spiralian, and modifications in the extent and exact origin of ectomesoderm are probably due to different adaptations in different larval types (Boyer et al. 1996, 1998; Henry and Martindale 1998, 1999).
In most descriptive studies on phoronid development, the mesoderm is assumed to originate from single cells ingressing from the archenteron (e.g., Herrmann 1980; Zimmer 1980). On the contrary, fate-mapping studies conducted by Freeman and Martindale (2002) have shown that mesoderm arises from both endoderm and ectoderm and that its formation is likely to be induced at the endoderm–ectoderm boundary. Mesodermal cells migrate freely in the blastocoel and differentiate into muscle, nerve, and mesenchymal cells. It is not known whether ectodermally and endodermally derived cells give rise to different larval tissues. In support of a dual origin of mesoderm in Phoronida, Bartolomaeus (2001) found mesenchymal cells in the preoral lobe of the actinotrocha larva of Phoronis muelleri Selys-Longchamps, 1903, while differentiated coelom is already present in the larval trunk. Ingression of mesenchymal cells before gastrulation as reported by some authors (e.g., de Selys-Longchamps 1907; Zimmer 1964; Herrmann 1986) has not been corroborated by studies on Phoronis pallida Silén, 1952 (Santagata 2004). Grobe (2008) found mesodermal cells, possibly delaminated from vegetal cells, in blastula stages of Phoronis ovalis Wright, 1856, but not before gastrula stages in P. muelleri.
Though several earlier studies assume an archenteric origin of mesoderm and coelomic cavities, Brachiopoda do not appear to show a uniform pattern of mesoderm formation. This coincides with a considerable diversity in cell fates and specification mechanisms found among brachiopod subtaxa (Freeman 2003). A comprehensive review of brachiopod development is found in Nielsen (2005). Mesoderm develops from the boundary between endoderm and ectoderm in the inarticulates Glottidia pyramidata (Stimpson, 1860) (Freeman 1995) and Discinisca strigata (Broderip, 1833) (Freeman 1999) during gastrulation, but from the archenteric walls in later gastrulae in Novocrania anomala (O. F. Müller, 1776) (Nielsen 1991; Freeman 2000). In the latter species, the mesoderm is thought to originate either from a single anlage growing from near the blastopore toward the anterior pole (“modified enterocoely”; Nielsen 1991) or by multipolar ingression over the whole length of the archenteron (Freeman 2000). In the articulates, mesoderm is reported to arise from the archenteron either by lateral outpouching (Long and Stricker 1991), delamination (Freeman 1993) in Terebratalia septentrionalis (Couthouy, 1838) or by outgrowth of an initially compact cell mass in Calloria inconspicua (Sowerby, 1846) and Notosaria nigricans (Sowerby, 1846) (Lüter 2000). There is no evidence for an ectodermal origin of mesodermal elements in Brachiopoda. Larval musculature is mainly formed from coelomic myoepithelial cells (Lüter 2000).
Basal deuterostomes are generally considered to completely lack ectomesoderm. In enteropneusts, the protocoel unequivocally arises by enterocoely (Nielsen 2001), and larval muscles are part of this coelomic epithelium (Ruppert and Balser 1986). Meso- and metacoels arise from the wall of the stomach either by outpouching or from cells delaminating from the gut. Only the hydropore and the distal part of the hydrocanal are likely to originate from ectoderm (Ruppert and Balser 1986). Additionally, scattered mesenchyme cells are found in the blastocoel (Tagawa et al. 1998). These have been suggested to be of ectodermal origin in earlier works (Morgan 1891). However, fate-mapping studies by Henry et al. (2001) showed that vegetal micromeres give rise to both the archenteron and the mesoderm (protocoel, wandering mesenchyme, muscle strand, hydrocanal). In the pterobranch Rhabdopleura compacta Hincks, 1880, primary mesenchyme is formed in the blastula stage, whereas mesoderm is formed by ingression from the archenteron (Dilly 1973).
In many echinoderms also two mesenchyme cell ingressions occur. Ingression of primary, skeletogenic, mesenchyme takes place before gastrulation and goes back to a group of vegetal micromeres. Secondary mesenchyme ingresses from the archenteron during gastrulation. Later, the anterior part of the archenteron gives rise to coelomic sacs and muscle cells (Wray 1997). Primary mesenchyme ingression is possibly derived by heterochrony within echinoderms. Anyway, due to its origin before gastrulation, no ectodermal relationship can be established (Davidson et al. 1998). As in the tornaria, the hydropore and distal part of the pore canal might be ectodermal (Ruppert and Balser 1986).
Conclusion
The phylogenetic significance of mesoderm origin and formation has been subject to much recent discussion (see Rieger and Ladurner 2003; Technau and Scholz 2003; Nielsen 2005; Burton 2008; Lambert 2008) and this continues. Of course, the mechanisms of mesoderm formation are still not fully understood, thus phylogenetic inferences have to be drawn carefully. The present results show that there is reason to regard at least part of the mesoderm in Bryozoa to originate from the ectoderm. This parallels the situation found in spiralians and eventually in phoronids. Considering the currently hypothesized position of Bryozoa within the Lophotrochozoa (Passamaneck and Halanych 2004, 2006; Halanych 2004; Waeschenbach et al. 2006; Helmkampf et al. 2008; Dunn et al. 2008; Jang and Hwang 2009; Sun et al. 2009), the question arises whether the character of ectodermally derived larval mesoderm represents an ancestral lophotrochozoan trait, and, if this is the case, whether it is plesiomorphic, thus lost in deuterostomes, or apomorphic. These questions are tempting, however, the evidence summarized here still appears insufficient to address such fundamental questions. Further studies, most suitably combining morphological, cell lineage, and gene expression approaches on origin and early differentiation of mesoderm have to be awaited.
References
Ackermann C, Dorresteijn A, Fischer A (2005) Clonal domains in postlarval Platynereis dumerilii (Annelida : Polychaeta). J Morphol 266:258–280
Barrois J (1880) Mémoire sur la metamorphose des Bryozoaires. Ann Sci Nat Zool Ser 13(9):1–67 + 4pls
Bartolomaeus T (2001) Ultrastructure and formation of the body cavity lining in Phoronis muelleri (Phoronida, Lophophorata). Zoomorphology 120:135–148
Borg F (1927) Studies on recent cyclostomatous Bryozoa. Zool Bidr Uppsala 10:181–507
Boyer BC, Henry JQ (1998) Evolutionary modifications of the spiralian developmental program. Amer Zool 38:621–633
Boyer BC, Henry JJ, Martindale MQ (1996) Dual origins of mesoderm in a basal spiralian: cell lineage analyses in the polyclad turbellarian Hoploplana inquilina. Dev Biol 197:329–338
Boyer BC, Henry JJ, Martindale MQ (1998) The cell lineage of a polyclad turbellarian embryo reveals close similarity to coelomate spiralians. Dev Biol 204:111–123
Braem F (1890) Untersuchungen über die Bryozoen des süssen Wassers. Zoologica 2:1–134, pls. 1–15
Braem F (1897) Die geschlechtliche Entwicklung von Plumatella fungosa. Zoologica 10:1–96
Braem F (1908) Die geschlechtliche Entwicklung von Fredericella sultana. Zoologica 52:1–37
Brien P (1953) Études sur les Phylactolémates Evolution de la zoecie—Bourgeonnement d’accroissement Bourgeonnement statoblastique—Embryogenese—L’Ontogenese multiple. Ann Soc R Zool Belg 84:301–444
Burton PM (2008) Insights from diploblasts: the evolution of mesoderm and muscle. J Exp Zool 310B:5–14
Calvet L (1900) Contribution à l’histoire naturelle des bryozoaires ectoproctes marins. Trav Inst Zool Univ Montpellier 8:1–488
Cook PL (1962) The early larval development of Membranipora seurati (Canu) and Electra crustulenta (Pallas), Polyzoa. Cah Biol Mar 3:57–60
Corrêa DD (1948) A embryologia de Bugula flabellata (J. V. Thompson) (Bryozoa Ectoprocta). Zoologia, Sao Paulo 13:7–71
d’Hondt JL (1983) Sur l’évolution des quatre macroméres du pôle végétatif chez les embryons de Bryozoaires Eurystomes. Cah Biol Mar 24:177–185
d’Hondt JL (2005) État des connaissances sur le développement embryonaire des Bryozoaires Phylactolaemates. Denisia 16:59–68
Davenport CB (1891) Observations on budding in Paludicella and some other Bryozoa. Bull Mus Comp Zool 22:1–114
Davidson EH, Cameron RA, Ransick A (1998) Specification of cell fate in the sea urchin embryo: summary and some proposed mechanisms. Development 125:3269–3290
de Selys-Longchamps M (1907) Phoronis. Fauna Flora Golf Neapel 30:1–280
Dilly PN (1973) The larva of Rhabdopleura compacta (Hemichordata). Mar Biol 18:69–86
Dudley JE (1973) Observations on the reproduction, early larval development, and colony astogeny of Conopeum tenuissimum (Canu). Chesap Sci 14:270–278
Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD, Sorensen MV, Haddock SHD, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G (2008) Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452:745–749
Freeman G (1993) Regional specification during embryogenesis in the articulate brachiopod Terebratalia. Dev Biol 160:196–218
Freeman G (1995) Regional specification during embryogenesis in the inarticulate brachiopod Glottidia. Dev Biol 172:15–36
Freeman G (1999) Regional specification during embryogenesis in the inarticulate brachiopod Discinisca. Dev Biol 209:321–339
Freeman G (2000) Regional specification during embryogenesis in the inarticulate brachiopod Crania anomala. Dev Biol 227:219–238
Freeman G (2003) Regional specification during embryogenesis in rhynchonelliform brachiopods. Dev Biol 261:268–287
Freeman G, Martindale MQ (2002) The origin of mesoderm in phoronids. Dev Biol 252:301–311
Fuchs J, Obst M, Sundberg P (2009) The first comprehensive molecular phylogeny of Bryozoa (Ectoprocta) based on combined analyses of nuclear and mitochondrial genes. Mol Phylogenet Evol 52:225–233
Grobe P (2008) Larval development, the origin of the coelom and the phylogenetic relationships of the Phoronida. Dissertation, Freie Universität Berlin, Germany
Gruhl A (2008) Muscular systems in gymnolaemate bryozoan larvae (Bryozoa: Gymnolaemata). Zoomorphology 127:143–159
Halanych KM (2004) The new view of animal phylogeny. Ann Rev Ecol Syst 35:229–256
Harmer SF (1893) On the occurrence of embryonic fission in cyclostomatous Polyzoa. Q J Microsc Sci N S 34:199–241
Harmer SF (1898) On the development of Tubulipora, and some British and northern species of this genus. Q J Microsc Sci N S 41:73–157
Hejnol A, Martindale MQ, Henry JQ (2007) High-resolution fate map of the snail Crepidula fornicata: the origins of ciliary bands, nervous system, and muscular elements. Dev Biol 305:63–76
Helmkampf M, Bruchhaus I, Hausdorf B (2008) Multigene analysis of lophophorate and chaetognath phylogenetic relationships. Mol Phylogenet Evol 46:206–214
Henry JJ, Martindale MQ (1998) Conservation of the spiralian developmental program: cell lineage of the nemertean, Cerebratulus lacteus. Dev Biol 201:253–269
Henry JJ, Martindale MQ (1999) Conservation and innovation in spiralian development. Hydrobiologia 402:255–265
Henry JQ, Tagawa K, Martindale MQ (2001) Deuterostome evolution: early development in the enteropneust hemichordate, Ptychodera flava. Evol Dev 3:375–390
Herrmann K (1980) Die archimere Gliederung bei Phoronis muelleri (Tentaculata). Zool Jb Anat Ontog Tiere 103:234–249
Herrmann K (1986) Die Ontogenese von Phoronis mülleri (Tentaculata) unter besonderer Berücksichtigung der Mesodermdifferenzierung und Phylogenese des Coeloms. Zool Jb Anat Ontog Tiere 114:441–463
Jang K, Hwang U (2009) Complete mitochondrial genome of Bugula neritina (Bryozoa, Gymnolaemata, Cheilostomata): phylogenetic position of Bryozoa and phylogeny of lophophorates within the Lophotrochozoa. BMC Genomics 10:167
Kraepelin K (1892) Die Deutschen Süsswasser-Bryozoen. Eine Monographie. II. Entwickelungsgeschichtlicher Teil. Abh Naturwiss Verein Hamburg 12:1–67, pls. 1–5
Lambert JD (2008) Mesoderm in spiralians: the organizer and the 4d cell. J Exp Zoolog B Mol Dev Evol 310:15–23
Long JA, Stricker SA (1991) Brachiopoda. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates VI. Echinoderms and lophophorates. Boxwood Press, Pacific Groove, pp 47–84
Lüter C (2000) The origin of the coelom in Brachiopoda and its phylogenetic significance. Zoomorphology 120:15–28
Marcus E (1926) Beobachtungen und Versuche an lebenden Meeresbryozoen. Zool Jb 52:1–102
Marcus E (1934) Über Lophopus cristallinus (Pall.). Zool Jb Anat Ontog Tiere 58:501–606
Mawatari S, Mawatari SF (1975) Development and metamorphosis of the cyphonautes of Membranipora serrilamella Osburn. In: Pouyet S (ed) Bryozoa 1974. Université Claude Bernard, Lyon, pp 13–18, 2pl
Morgan TH (1891) The growth and metamorphosis of tornaria. J Morphol 5:407–458
Nielsen C (1970) On metamorphosis and ancestrula formation in cylostomatous bryzoans. Ophelia 7:217–256
Nielsen C (1971) Entoproct life-cycles and the entoproct/ectoproct relationship. Ophelia 9:209–341
Nielsen C (1990) Bryozoa Ectoprocta. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates vol IV, Part B, Fertilization, development and parental care. Wiley, London, pp 185–200
Nielsen C (1991) The development of the brachiopod Crania (Neocrania) anomala. Acta Zool 27:7–28
Nielsen C (2001) Animal evolution. Interrelationships of the living phyla. Oxford University Press, Oxford
Nielsen C (2004) Trochophora larvae: Cell-lineages, ciliary bands, and body regions. 1. Annelida and Mollusca. J Exp Zool B 302:35–68
Nielsen C (2005) Trochophora larvae: Cell-lineages, ciliary bands, and body regions. 2. Other groups and general discussion. J Exp Zool B 304:401–447
Ostrovsky AN (2008a) External versus internal and self- versus cross-fertilization in Bryozoa: transformation of the view and evolutionary considerations. In: Wyse Jackson PN, Spencer Jones ME (eds) Annals of bryozoology 2. International Bryozoology Association/Trinity College, Dublin, pp 103–115
Ostrovsky AN (2008b) The parental care in cheilostome bryozoans: a historical review. In: Wyse Jackson PN, Spencer Jones ME (eds) Annals of bryozoology 2. International Bryozoology Association/Trinity College, Dublin, pp 211–245
Ostrovsky AN, Vavra N, Porter JS (2008) Sexual reproduction in gymnolaemate Bryozoans: history and perspectives of the research. In: Wyse Jackson PN, Spencer Jones ME (eds) Annals of bryozoology 2. International Bryozoology Association/Trinity College, Dublin, pp 117–210
Pace RM (1906) On the early stages in the development of Flustrella hispida (Fabricius), and on the existence of a “yolk nucleus” in the egg of this form. Q J Microsc Sci 50:435–478 + 4 pls
Passamaneck YJ, Halanych KM (2004) Evidence from Hox genes that bryozoans are lophotrochozoans. Evol Dev 6:275–281
Passamaneck YJ, Halanych KM (2006) Lophotrochozoan phylogeny assessed with LSU and SSU data: evidence of lophophorate polyphyly. Mol Phylogenet Evol 40:20–28
Prouho H (1892) Contribution a l’histoire des bryozoaires. Arch Zool Exp Gén 10(2.Ser.):557–656
Reed CG (1987) Phylum Bryozoa. In: Strathmann MF (ed) Reproduction and development of marine invertebrates of the Northern Pacific coast. University of Washington Press, Seattle, pp 494–511
Reed CG (1991) Bryozoa. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates VI. Echinoderms and lophophorates. Boxwood Press, Pacific Groove, pp 85–245
Reed CG, Cloney RA (1982) The larval morphology of the marine bryozoan Bowerbankia gracilis (Ctenostomata: Vesicularioidea). Zoomorphology 100:23–54
Reed CG, Woollacott RM (1982) Mechanisms of rapid morphogenetic movements in the metamorphosis of the bryozoan Bugula neritina (Cheilostomata, Cellularioidea) I. Attachment to the substratum. J Morphol 172:335–348
Rieger MR, Ladurner P (2003) The significance of muscle cells for the origin of mesoderm in Bilateria. Integ Comp Biol 43:47–54
Robertson A (1903) Embryology and embryonic fission in the genus Crisia. Univ Calif Publ Zool 1:115–156
Ruppert EE, Balser EJ (1986) Nephridia in the larvae of hemichordates and echinoderms. Biol Bull 171:188–196
Santagata S (2004) Larval development of Phoronis pallida (Phoronida): implications for morphological convergence and divergence among larval body plans. J Morphol 259:347–358
Shook D, Keller R (2003) Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev 120:1351–1383
Silén L (1966) On the fertilization problem in the gymnolaematous Bryozoa. Ophelia 3:113–140
Stricker SA, Reed CG, Zimmer RL (1988) The cyphonautes larva of the marine bryozoan Membranipora membranacea. I. General morphology, body wall, and gut. Can J Zool 66:368–383
Ström R (1969) Sexual reproduction in a stoloniferous bryozoan, Triticella koreni (G.O. Sars). Zool Bidr Uppsala 38:113–127
Ström R (1977) Brooding patterns of bryozoans. In: Woollacott RM, Zimmer RL (eds) Biology of bryozoans. Academic Press, New York, pp 23–55
Sun M, Wu Z, Shen X, Ren J, Liu X, Liu H, Liu B (2009) The complete mitochondrial genome of Watersipora subtorquata (Bryozoa, Gymnolaemata, Ctenostomata) with phylogenetic consideration of Bryozoa. Gene 439:17–24
Tagawa K, Nishino A, Humphreys T, Satoh N (1998) The spawning and early development of the hawaiian acorn worm (hemichordate), Ptychodera flava. Zool Sci 15:85–91
Technau U, Scholz CB (2003) Origin and evolution of endoderm and mesoderm. Int J Dev Biol 47:531–539
Temkin MH (1996) Comparative fertilization biology of gymnolaemate bryozoans. Mar Biol 127:329–339
Vigelius WJ (1886) Zur Ontogenie der marinen Bryozoen. Mitt Zool Stn Neapel 6:499–541
Waeschenbach A, Telford MJ, Porter JS, Littlewood DTJ (2006) The complete mitochondrial genome of Flustrellidra hispida and the phylogenetic position of Bryozoa among the Metazoa. Mol Phylogenet Evol 40:195–207
Wood TS (2008) Development and metamorphosis of cyphonautes larvae in the freshwater ctenostome bryozoan, Hislopia malayensis Annandale, 1916. In: Hageman GS, Key MM Jr, Winston JE (eds) Bryozoan studies 2007. Virginia Museum of Natural History, Martinsville
Woollacott RM, Zimmer RL (1971) Attachment and metamorphosis of the cheilo-ctenostome bryozoan Bugula neritina (Linné). J Morphol 134:351–382
Wray GA (1997) Echinoderms. In: Gilbert SF, Raunio AM (eds) Embryology: constructing the organism. Sinauer Associates, Sunderland, pp 309–329
Zimmer RL (1980) Mesoderm proliferation and formation of the protocoel and metacoel in early embryos of Phoronis vancouverensis. Zool Jb Anat Ontog Tiere 103:219–233
Zimmer RL (1997) Phoronids, brachiopods, and bryozoans, the lophophorates. In: Gilbert SF, Raunio AM (eds) Embryology: constructing the organism. Sinauer Associates, Sunderland, pp 279–305
Zimmer RL, Woollacott RM (1977) Structure and classification of gymnolaemate larvae. In: Woollacott RM, Zimmer RL (eds) Biology of bryozoans. Academic Press, New York, pp 57–89
Zimmer RL, Woollacott RM (1989) Larval morphology of the bryozoan Watersipora arcuata (Cheilostomata: Ascophora). J Morphol 199:125–150
Zimmer RL, Woollacott RM (1993) Anatomy of the larva of Amathia vidovici (Bryozoa: Ctenostomata) and phylogenetic significance of vesiculariform larva. J Morphol 215:1–29
Zimmer RL (1964) Reproductive biology and development of Phoronida. Dissertation, University of Washington
Acknowledgments
I thank Thomas Bartolomaeus (Bonn) for helpful comments and discussions on the manuscript. Many thanks go to Dennis Willows for providing facilities at Friday Harbour Laboratories (Washington, USA) and especially to Richard Strathmann for advice on rearing of Membranipora embryos. I also thank three anonymous reviewers for their valuable remarks.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gruhl, A. Ultrastructure of mesoderm formation and development in Membranipora membranacea (Bryozoa: Gymnolaemata). Zoomorphology 129, 45–60 (2010). https://doi.org/10.1007/s00435-009-0099-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-009-0099-3