Abstract
The fine structure of the mesodermal organs (water-vascular system, somatocoel derivatives, connective tissue, and mesenchymal cells) in late doliolaria, pentactula, and 1-month-old juveniles of the holothurian Apostichopus japonicus was studied. It has been shown that all the structures of the water-vascular system, the longitudinal muscle band anlagen, extracellular matrix, and different types of mesenchymal cells are present at the late doliolaria stage. However, even in 1-month-old juveniles, many organs do not reach their full development as in adults. During the developmental period under study, several stages of myogenesis can be distinguished, ranging from the appearance of single myoepithelial cells to the beginning of muscle bundle formation. These data are consistent with our hypothesis of the origin, development, and evolution of the muscular system in echinoderms. One-month-old juveniles already have all the main types of coelomocytes characteristic of adults. This means that the immune system of A. japonicus is already formed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesoderm is the most important evolutionary novelty of animals (Rieger and Ladurner 2003; Technau and Scholz 2003; Martindale et al. 2004; Heger et al. 2020). This germ layer gives rise to many tissues and organs in Bilateria such as mesothelium, the muscular, circulatory, excretory, immune, and reproductive systems. Furthermore, connective-tissue formations, including the vertebrate skeleton, are considered a derivative of the mesoderm. In different groups of animals, specific organs can be composed of cells of the third germ layer. In particular, the unique water-vascular system of echinoderms, which is characteristic only of this animal phylum, is developed from the mesoderm. Despite the importance of derivatives of the mesoderm, its formation in ontogeny has not been studied in sufficient detail for Bilateria.
Echinoderms are an ancient group of marine invertebrates. Having a common ancestor with vertebrates and comprising with them the group Deuterostomia, they are of major research interest. In all deuterostome animals, the mesoderm is formed by the enterocoely, i.e., through the separation of the coeloms from the archenteron (Technau and Scholz 2003). Most classes of echinoderms have three pairs of coeloms: axocoel, hydrocoel, and somatocoel (Brusca and Brusca 2003). Axocoels and hydrocoels give rise to the organs of the water-vascular system. Somatocoels, while growing, form the mesothelium of internal organs, with its cavity becoming the animal’s secondary body cavity. Further transformation and specialization of mesodermal cells in echinoderms are almost unstudied. There have been only a few publications considering the development of certain organs and tissues like muscles (Dolmatov and Ivantey 1993; Dolmatov et al. 2007; Dolmatov 2010), coelomocytes (Li et al. 2014; Ho et al. 2016), and the water-vascular system (Udagawa et al. 2022a, b).
Among echinoderms, holothurians attract special attention. These animals are an important target species of fisheries and aquaculture in many countries worldwide (Purcell 2010). One of the most commercially valuable holothurian species in the Far Eastern region is Apostichopus japonicus (Selenka, 1867). A large number of papers consider various aspects of the biology of this species (Yang et al. 2015). Nevertheless, the morphological pattern of development has not been sufficiently studied. Previously, we investigated the features of the formation of ectodermal and endodermal derivatives at late stages of larval development, metamorphosis, and formation of juvenile in A. japonicus (Dolmatov et al. 2016, 2017, 2018). In our present study, we focus on mesoderm transformation in this holothurian species.
Materials and methods
Larvae and settled specimens of the holothurian A. japonicus were reared at the Research Module for Growing of Larvae of Commercial Species of Marine Invertebrates, A.V. Zhirmunsky National Scientific Center of Marine Biology, Far Eastern Branch, Russian Academy of Sciences (NSCMB FEB RAS), Vladivostok, Russia, and kindly provided by S.I. Maslennikov. The material for transmission electron microscopy (TEM), immunocytochemistry, and 3D reconstruction was processed as described previously (Dolmatov et al. 2016, 2017, 2018). Larvae and settled specimens were cut transversely.
For TEM analysis, samples were fixed with 2.5% glutaraldehyde for 1–7 days at 4 °C and then with a 1% solution of OsO4 in cacodylate buffer for 1 h. The material was embedded in Araldite. Sections were cut with glass knives on an Ultracut E ultramicrotome (Reichert, Vienna, Austria). Ultrathin (50–70 nm) sections were contrasted with uranyl acetate and lead citrate and examined under a Carl Zeiss Libra 120 transmission electron microscope. For immunocytochemical analysis, the material was fixed for 8 h in 4% paraformaldehyde. Larvae and settled specimens were incubated in monoclonal mouse antibodies against alpha-tubulin (SIGMA, USA) and then in secondary antibodies against mouse immunoglobulins labeled with Alexa 546 (Invitrogen, Molecular Probes). Subsequently, the material was incubated with FITC-labeled phalloidin (diluted 1/300) for 2 h at room temperature and embedded in anti-fade Vectashield medium (Invitrogen, Molecular Probes), containing DAPI. The material was analyzed using an LSM 780 (Carl Zeiss) confocal laser scanning microscope.
Results
Late doliolaria
Doliolaria larva is the last larval development stage in holothurians with planktotrophic larvae. In A. japonicus, it forms on days 14–15 post-fertilization. Late doliolaria larva has a spindle-like shape (Fig. 1a, b). Its main organs have already formed (Dolmatov et al. 2016, 2017, 2018). Larvae swim vertically in the water column, with their future anterior end oriented upwards. Mesoderm derivatives are represented by the water-vascular system, mesothelium (coelomic epithelium) of internal organs, and also by connective tissue and mesenchymal cells.
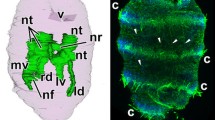
Late doliolaria of A. japonicus. a Three-dimensional reconstruction of the body and digestive, water-vascular, and nervous systems (view from the dorsal side); orange color indicates water-vascular system, green color—nervous system, yellow color—digestive system, violet—body wall. b General view from the ventral side; green color indicates actin; red color—tubulin; blue color—nuclei. c Three-dimensional reconstruction of the water-vascular system (view from the right side). a anus, ci ciliary ring, g gut, hc hydroporic canal, ld left dorsal radial water-vascular canal, ln left dorsal radial nerve cord, mvc mid-ventral radial water-vascular canal, nr nerve ring, rd right dorsal radial water-vascular canal, rn right dorsal radial nerve cord, rv right ventral radial water-vascular canal, t tentacle, tf tube foot, v vestibulum, wr water-vascular ring
Hydrocoel derivatives (water-vascular system)
The water-vascular system of late doliolaria larva consists of a ring canal and five radial canals, five tentacular canals, a polian vesicle, and a hydroporic canal (Fig. 1c). Radial canals of the water-vascular system are developed to varying degrees (Fig. 1c). At this stage, the mid-ventral and dorsal water-vascular canals are developed to the greatest extent. At the end of the mid-ventral water-vascular canal, there is an anlage of the tube foot canal. The small polian vesicle grows from the left ventro-lateral radius of the water-vascular ring and is located between the gut and the coelom wall. The hydroporic canal extends from the water-vascular ring in the dorsal interradius. It crosses the coelom and the primary body cavity and opens through a hydropore on the dorsal side of the larva. The madreporite is located in the extracellular matrix (ECM) outside of the coelom wall.
All parts of the water-vascular system have a similar histological organization and consist of a luminal (water-vascular) epithelium, an external coelomic epithelium, and a connective-tissue layer. The luminal epithelium is represented by low ciliated cells (Fig. 2a, b). On the apical surface, they bear short microvilli. The cells are connected to each other via septate junctions and desmosomes and to the basal lamina via hemidesmosomes. Nuclei contain a moderate amount of heterochromatin and a nucleolus. In the apical part of the cytoplasm, small vesicles and sometimes multivesicular bodies are found. Moreover, the cells have mitochondria, the Golgi apparatus (GA), and oval or elongated cisterns of the rough endoplasmic reticulum (RER). The RER often contains material of medium electron density. Phagosomes are sometimes present in the cytoplasm.
Microanatomical organization of the water-vascular system of the late doliolaria of A. japonicus. a Transverse section through the mid-ventral water-vascular canal. b Myoepithelial cell in the epithelium of mid-ventral water-vascular canal. c The water-vascular epithelium of the tentacular canal. d Basal part of the water-vascular epithelium of the tentacular canal. e The water-vascular epithelium of the tube foot canal. f Longitudinal section of the hydroporic canal. g Semithin longitudinal section of the stone canal. h The water-vascular epithelium of the stone canal. i Distal part of the hydroporic canal. bl basal lamina, c cilia, ce coelomic epithelium, ct connective tissue, d desmosome, e epidermis, hc hydroporic canal, hd hemidesmosome, m mitochondrion, mb multivesicular body, mf myofilaments, mp madreporite, mt mitotic cell, mv microvilli, ph phagosome, rn radial nerve cord, rr rough endoplasmic reticulum, rw water-vascular canal, s sclerocyte, sc stone canal, sj septate junction, sv secretory vacuole, v vesicle
The water-vascular epithelium of the ring canal and the ventro-lateral canals does not contain myoepithelial cells. Dorsal and, especially, mid-ventral radial canals are more developed. In their epithelium, single myoepithelial cells are found in the anterior part of the animal’s body and are located on the side facing the nerve cords (Fig. 2b). These are low ciliated cells. The basal part of the cytoplasm contains a small bundle of myofilaments.
The water-vascular epithelium of the tentacular canals consists of ribbon-shaped myoepithelial cells located along the tentacle, forming its longitudinal musculature (Fig. 2c). Each cell bears a cilia and long microvilli on its apical surface. The basal cytoplasm is filled with bundles of myofilaments. The cells extend numerous processes embedded in the basal lamina (Fig. 2d). The thickness of the basal lamina is 0.3–0.5 µm. The cytoplasm contains numerous expanded RER cisterns filled with a homogeneous content of medium electron density. In addition, the cells have a GA, mitochondria, and, sometimes, phagosomes.
The luminal epithelium of the tube foot canal has the same structure (Fig. 2e). The cells are located on the basal lamina having a thickness of 0.2–0.4 µm or up to 0.9 µm in some areas. Unlike the luminal epithelium of the tentacles, the epithelial cells of the tube foot exhibit secretory activity. In their apical part, vacuoles of 0.2–0.3 µm in diameter with electron-lucent substance are located. The vacuole content is excreted into the foot canal cavity.
In the late doliolaria larva, a polian vesicle begins to form. It is a small protrusion of the ring canal wall in the ventral interradius. Its structure does not differ from that of the ring canal. At this developmental stage, the proximal part of the hydroporic canal is transformed into the stone canal (Fig. 2f–h). Its luminal epithelium is composed of columnar cells located on a relatively thick basal lamina that forms small plicae. The cells are tightly adjacent to each other, connected via septate junctions and desmosomes. They bear cilia and numerous long microvilli on their apical surface. The cells contain RER cisterns, a GA, mitochondria, and numerous free ribosomes. In the apical cytoplasm, there are small vesicles of different diameters. The stone canal ends with the forming madreporite. The madreporite consists of groups of sclerocytes surrounding the stone canal. Inside the madreporite, cavities are formed that are lined by flattened ciliated cells.
Distally of the madreporite, the luminal epithelium of the hydroporic canal is formed by cells whose basal parts are embedded in connective tissue (Fig. 2f, i). The apical surface of the cells bears long cilia and microvilli. Their nuclei are located in the basal part of the epithelial layer, are rounded or irregular in shape, contain large amounts of heterochromatin, and have a nucleolus. The cytoplasm contains a GA, RER cisterns, mitochondria, lipoprotein granules, and free ribosomes. Numerous electron-lucent vesicles of various diameters are found in the apical cytoplasm. In the area of the hydropore, the luminal epithelium of the hydroporic canal passes into the epidermis (Fig. 2i).
Derivatives of somatocoels
The coelomic epithelium of internal organs is formed by low cells of a cubic, prismatic, or elongated shape (Fig. 3a, b). They have an oval or irregularly shaped nucleus with clumps of heterochromatin and one or two nucleoli. On the apical surface, the cell bears a cilium and rare short microvilli. In the cytoplasm, there are small vesicles, mitochondria, RER cisterns, and free ribosomes.
Microanatomical organization of somatocoel derivatives of the late doliolaria of A. japonicus. a Semithin transverse section through the middle part of the late doliolaria. b Coelomic epithelium. c Myoepithelial cell of the coelomic epithelium. d LMB anlage in mid-ventral ambulacrum, asterisks indicate myoepithelial cells. e Myoepithelial and peritoneal cells of LMB anlage. bl basal lamina, bw body wall, c cilia, cc coelomic cavity, ce coelomic epithelium, d desmosome, g gut, hs hyaline sphere, mc myoepithelial cell, mf myofilaments, pc peritoneal cell, pv polian vesicle, rr rough endoplasmic reticulum, rw radial water-vascular canal, sj septate junction, tf tube foot
The muscle system of late doliolaria larvae is represented by visceral musculature of the intestinal tube and the body wall, as well as by longitudinal muscle bands (LMBs) that can be considered as somatic musculature. The formation of the gut contractile system was described earlier (Dolmatov et al. 2017) and, therefore, we will not consider it here.
The visceral musculature of the body wall is formed by myoepithelial cells that are part of the coelomic epithelium (Fig. 3c). At the late doliolaria stage, most of them are located in the anterior half of the larva’s body. The cell shape varies from flattened to prismatic. Myoepithelial cells are usually located below peritoneocytes and are separated from the coelom by peritoneocytes’ lateral processes. Their nuclei contain a nucleolus and a small amount of heterochromatin. The basal parts of the cells are filled with myofilaments. The cytoplasm contains RER cisterns, mitochondria, ribosomes, and electron-lucent vesicles.
In A. japonicus at the late doliolaria stage, the LMB formation begins in the anterior segment of the mid-ventral ambulacrum (Dolmatov et al. 2016). The LMB anlage is a group of myoepithelial cells of the body wall coelomic epithelium at the point of its contact with the radial water-vascular canal (Fig. 3d). The apical processes of the peritoneocytes located on the sides of this group cover the myoepithelial cells and separate them from the coelom (Fig. 3d). The cells of the LMB anlage contain small bundles of myofilaments in their basal part (Fig. 3e). They lose cilia, but centrioles are often retained in the cytoplasm. Myoepithelial cells and peritoneocytes are located on a common basal lamina and are connected to each other via apical desmosomes and septate junctions.
Connective-tissue structures
Connective tissue of late doliolaria larva is represented by ECM, hyaline spheres, skeletal elements, and also by mesenchymal cells. ECM fills entirely the primary body cavity (Fig. 2g). It is composed of an amorphous component and small bundles of collagen fibers. Various mesenchymal cells, probably fibroblasts and coelomocytes, as well as sclerocytes, are found in connective tissue. At this stage, larvae retain five pairs of hyaline spheres (Dolmatov et al. 2016). All of them are located in the primary body cavity and are surrounded by bundles of collagen fibers (Fig. 4a, b). Hyaline spheres consist of a granular substance of medium electron density. Collagen fibers are also found in them (Fig. 4c).
Microanatomical organization of connective tissue of the late doliolaria of A. japonicus. a Hyaline sphere. b Bundles of collagen fibers surrounding the hyaline sphere. c Granular substance and collagen fibers in the hyaline sphere. d Semithin transverse section of the posterior part of the late doliolaria. e Sclerocyte of the calcareous body. f Sclerocytes forming the madreporite, arrows indicate centrioles. g Pinocytotic vesicle of the sclerocyte. h Amoebocyte. i Amoebocyte-like cells. j Cisterns of the rough endoplasmic reticulum of amoebocyte-like cell. k Vacuoles of amoebocyte-like cell. l Phagosomes in the cytoplasm of amoebocyte-like cells, asterisks indicate fragments of cells of ciliary rings. m Morula cell with electron-lucent granules. n Morula cell with heterogeneous granules. ac amoebocyte-like cell, bl basal lamina, cb calcareous body, cf collagen fibers, ci ciliary ring, eg electron-lucent granule, ga Golgi apparatus, hg heterogeneous granule, hs hyaline sphere, ls lysosome, m mitochondrion, ph phagosome, rr rough endoplasmic reticulum, va vacuole
The largest skeletal elements of late doliolaria are two calcareous bodies retained from the auricularia stage (Dolmatov et al. 2016). These are located in the posterior part of the larval body under the last pair of hyaline spheres (Fig. 4d). The calcareous bodies are surrounded by sclerocyte processes (Fig. 4e). Furthermore, the formation of skeletal structures characteristic of adult holothurian is observed at this stage. Calcification of ECM occurs between the tentacular canals and the anterior segments of the radial water-vascular canals. Subsequently, this will result in the formation of a calcareous ring. A madreporite is observed to form (Fig. 2f, g). Under the body epidermis, as well as under the epidermis of the tips of the tentacles and foot, there is a large number of sclerocytes forming spicules.
Sclerocytes, regardless of their localization, are similar in structure. These are separated from ECM by the basal lamina (Fig. 4e, f). The cell nuclei are rounded in shape and contain a nucleolus. The cytoplasm is characterized by a large number of free ribosomes. Also, it contains a small GA, short rounded RER cisterns, mitochondria, and electron-lucent vacuoles with heterogeneous contents. Centrioles are often located near the nucleus. Pinocytotic vesicles are found on the cell surface (Fig. 4g). Many sclerocytes contain electron-lucent vacuoles (Fig. 4f). Apparently, these are vacuoles with calcite crystals that were removed during the decalcification of samples. Larger calcite crystals are surrounded by groups of sclerocytes.
Coelomocytes
Several types of coelomocytes are present in the cavities of the water-vascular system and in the ECM of late doliolaria larvae. Quite many of them are found near degrading structures such as hyaline spheres and ciliary rings (Fig. 4a). Amoebocytes are found in the cavities of the water-vascular system. These cells have a rounded nucleus containing small clumps of heterochromatin (Fig. 4h). The cytoplasm of amoebocytes has RER cisterns, microvesicles, secretory vacuoles, and also lysosomes with electron-dense contents.
Amoebocyte-like cells are the cells found most frequently in connective tissue (Fig. 4a, i). They have a rounded or elongated nucleus with small clumps of heterochromatin and a large nucleolus. Their cytoplasm contains a well-developed GA apparatus, a large number of RER cisterns filled with a substance of medium electron density, and also large vacuoles with electron-lucent contents (Fig. 4i, j). A layer of heterogeneous material of medium electron density is frequently observed along the periphery of vacuoles (Fig. 4k). Furthermore, electron-dense lysosomes and phagosomes with heterogeneous contents are found in the cytoplasm of amoebocyte-like cells. The phagosomes may contain fragments of resorbing larval structures: the cells of ciliary rings and the substance of hyaline spheres (Fig. 4k, l). In addition, bacterial cells are often present within phagosomes, which indicates the protective functions of amoebocytes. Amoebocyte-like cells containing phagosomes form aggregates, thus, building structures similar to the brown bodies of adults (Fig. 4l).
Two types of morula cells are found in the connective tissue of late doliolaria larva. The first type contains large electron-lucent granules in the cytoplasm (Fig. 4m). A characteristic feature of the second type is the presence of rounded heterogeneous granules of up to 1–2 µm in diameter (Fig. 4n). The granules consist of a fibrillar substance with an electron-dense “core”. The cytoplasm contains a GA, short RER cisterns, and mitochondria.
Pentactula
Hydrocoel derivatives (water-vascular system)
During the metamorphosis, animals lose their ability to swim and settle on the substrate. All organs of the water-vascular system increase in size compared to the late doliolaria stage. Changes in the radial canals become particularly noticeable. The dorsal and mid-ventral canals reach the posterior end of the animal’s body (Fig. 5a). The ventro-lateral water-vascular canals have a shorter length and end approximately in the middle part of the pentactula. The polian vesicle and the tube foot increase in size. The hydroporic canal and the hydropore are retained (Fig. 5). The luminal epithelium cells in most organs of the water-vascular system are flattened. Nevertheless, their structure shows almost no changes compared to the previous stage.
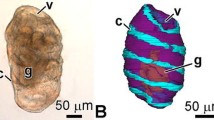
Pentactula of A. japonicus. a Three-dimensional reconstruction of the body with digestive and water-vascular systems (view from the right-dorsal side); orange color indicates water-vascular system, blue color—calcareous ring, yellow color—digestive system, violet—body wall. b General view from the right-dorsal side; green color indicates actin; red color—tubulin; blue color—nuclei. c Three-dimensional reconstruction of the water-vascular system (view from the right-dorsal side). cr calcareous ring, g gut, hc hydroporic canal, ld left dorsal radial water-vascular canal, lm longitudinal muscle band, lv left ventral radial water-vascular canal, lvn left ventral radial nerve cord, mp madreporite, mvc mid-ventral radial water-vascular canal, pv polian vesicle, rd right dorsal radial water-vascular canal, rv right ventral radial water-vascular canal, t tentacle, tf tube foot, wr water-vascular ring
In all radial water-vascular canals of pentactula, numerous myoepithelial cells are found on the side facing the radial nerve (Fig. 6a, b). In the ventro-lateral canals, myoepithelial cells are present only in their anterior parts. In the dorsal and mid-ventral canals, these are arranged all along their lengths. With the development of myoepithelial cells, their lateral sides form outgrowths. Outgrowths of adjacent cells intertwine with each other, forming a complex network of interdigitations (Fig. 6a). The nuclei of myoepithelial cells are rounded or irregular in shape. The basal cytoplasm contains myofilaments, with their number greater than that at the late doliolaria stage. Furthermore, there are RER cisterns, ribosomes, mitochondria, and electron-lucent vesicles in the cytoplasm; lipoprotein granules are also frequently found.
Microanatomical organization of the water-vascular system of the pentactula of A. japonicus. a Transverse section through the mid-ventral water-vascular canal. b Transverse section through the ventro-lateral water-vascular canal. c Interdigitations of myoepithelial cells of the tentacle. d Luminal epithelium of tentacle. e Luminal epithelium of the polian vesicle. bl basal lamina, c cilia, d desmosome, hd hemidesmosome, ij interdigitations, lf lipofuscin, m mitochondrion, mf myofilaments, mv microvilli, pc peritoneal cell, rn radial nerve cord, v vesicle, va vacuole
Upon metamorphosing and settling, the animals have tentacles that increased in size. In their luminal epithelium, adjacent myoepithelial cells form numerous interdigitations (Fig. 6c). The number of myofilaments increases (Fig. 6d). In addition, the tube foot shows significant growth after the metamorphosis. Its luminal epithelium is similar in structure to that of the tentacles.
After the metamorphosis, myoepithelial cells appear in the luminal epithelium of the polian vesicle (Fig. 6e). Peritoneal and myoepithelial cells are arranged on a 0.2 µm thick basal lamina, attached to it via hemidesmosomes. The cells are interconnected via apical desmosomes and septate junctions. Peritoneocytes are flattened in shape. On the apical surface, they bear cilia and microvilli. The nuclei of the cells are irregular in shape. The cytoplasm contains numerous microvesicles, electron-lucent vacuoles of various diameters, multivesicular bodies, RER cisterns, and mitochondria. Myofilaments are located in the basal part of myoepithelial cells. Peritoneocytes’ processes separate the myofilament-containing parts of myoepithelial cells from the polian vesicle cavity. The rest of the cytoplasm contains the same organelles and inclusions as those in peritoneocytes. The hydroporic canal epithelium does not change compared to that at the previous stage.
Derivatives of somatocoels
After the metamorphosis, the structure of the coelomic epithelium of the body wall and internal organs shows only little changes. As in the previous stage, in pentactula it is represented by flattened peritoneal and myoepithelial cells (Fig. 7a). At this stage, the formation of the LMB is observed in the anterior parts of all five ambulacra. In the examined individuals, the myogenesis stages differ between radii. The initial stage of LMB formation occurs in the ventro-lateral ambulacra. Single myoepithelial cells are found in the coelomic epithelium bordering the radial water-vascular canal (Fig. 7b). These are separated from the coelom by apical processes of neighboring peritoneocytes. In the dorsal radii, the number of myoepithelial cells in the LMB increases, and they form a continuous layer (Fig. 7c). Most of their cytoplasm is filled with myofilaments. This layer of myoepithelial cells is also separated from the coelom by bodies and processes of the peritoneocytes located on the sides relative to the LMB. Thus, both types of cells are attached to a single basal lamina.
Microanatomical organization of somatocoel derivatives of the pentactula of A. japonicus. a Coelomic epithelium of the body wall. b LMB anlage in ventro-lateral ambulacrum. c LMB anlage in dorsal ambulacrum. d Mid-ventral LMB. e Separate groups of myoepithelial cells in mid-ventral LMB. bl basal lamina, c cilia, ct connective tissue, dc dorsal water-vascular canal, hd hemidesmosome, lf lipofuscin, mc myoepithelial cell, mf myofilaments, mvc mid-ventral radial water-vascular canal, pc peritoneal cell, vc ventro-lateral water-vascular canal
The content of ECM in mid-ventral LMB increases (Fig. 7d). Furthermore, the connective-tissue layer separating the LMB from the radial water-vascular canal develops. Myoepithelial cells, primarily their basal parts with myofilaments, are surrounded by ECM. Their parts containing nuclei remain located apically, under the bodies and processes of peritoneocytes. The single layer of myoepithelial cells breaks down into separate groups. However, myoepithelial cells and peritoneocytes are located on a common basal lamina (Fig. 7e).
Connective-tissue structures
As in late doliolaria, the entire primary body cavity in pentactula between the epidermis and the coelom is filled with ECM (Fig. 8a). The outer, denser layer is 8–20 µm thick. Besides a large number of collagen fibers, it contains spicules (Fig. 8b). The inner, 20–30 µm thick layer is looser. Numerous mesenchymal cells are found in it.
Microanatomical organization of connective tissue of the pentactula of A. japonicus. a Semithin transverse section of the anterior part of the pentactula. b Semithin transverse section of the body wall, asterisks indicate amoebocyte-like cells. c Remnants of hyaline sphere. d Semithin transverse section of the calcareous ring. e Sclerocytes of the calcareous ring. f Sclerocyte in the wall of tube foot. g Sclerocyte in the body wall. h Centriole (arrow) in sclerocyte cytoplasm. i Multivesicular bodies and myelin-like bodies in the cytoplasm of sclerocytes. bl basal lamina, co coelom, cr calcareous ring, ct connective tissue, ex external layer of connective tissue, ga Golgi apparatus, hc hydroporic canal, hs hyaline sphere, in internal layer of connective tissue, m mitochondrion, mb multivesicular body, mi myelin-like body, og outgrowth, s sclerocyte, t tentacle, v vesicle, va vacuole
Remnants of hyaline spheres persist in pentactula. They have an irregular, flattened shape and are devoid of the surrounding in the form of collagen fibers (Fig. 8b, c). Amoebocyte-like cells with phagosomes can be found inside and on the surface of hyaline spheres. Some pentactula have brown bodies instead of hyaline spheres.
After the metamorphosis, the small (right) calcareous body disintegrates, while the large (left) calcareous body is partially retained in the posterior part of the pentactula body. The madreporite embraces the dorsal and lateral sides of the stone canal with a narrow ring (Fig. 5a, b). Between the base of the tentacular canals and the anterior segments of the radial water-vascular canals, the amount of ECM and collagen fibers increases (Fig. 8d). Sclerocytes are found in the forming connective tissue (Fig. 8e). These cells have extensive vacuoles, which indicates the formation of calcite crystals. The increase in the amount of ECM and its calcification causes a calcareous ring to form (Fig. 5a).
Numerous rounded spicules are located in the surface layer of the connective tissue of the animal’s body wall. Spicules are also present in ECM of the tips of the tentacles and tube foot, as well as in the connective tissue of the cloaca. These are formed both by single sclerocytes and their groups (Fig. 8f, g). In the latter case, a common vacuole is formed.
Sclerocytes of different skeletal structures have similar structure. As in the previous stage of holothurian development, they are surrounded by the basal lamina and have euchromic nuclei with a large nucleolus (Fig. 8f–i). The cytoplasm contains RER cisterns, ribosomes, and numerous mitochondria. Centrioles are often found near the nucleus (Fig. 8h). In sclerocytes of pentactula, the GA becomes more pronounced, and the number of dictyosomes increases. Multivesicular bodies and myelin-like bodies appear in the cytoplasm of sclerocytes (Fig. 8i).
Coelomocytes
As in the late doliolaria, the cavities of the water-vascular system and ECM in pentactula contain various amoebocyte-like and morula cells. Their composition and structure have not changed compared to the previous developmental stage.
One-month-old juveniles
Hydrocoel derivatives (water-vascular system)
Animals at this developmental stage reach a body length of 700–800 µm. On the outside, they are covered with numerous spicules. The arrangement of the internal organs changes little compared to the previous stage (Fig. 9). Anteriorly of the tube foot, there is a second foot. In addition, another two tube feet are laid down in the anterior half of the juvenile’s body. On each of the dorsal water-vascular canals, two canals of dorsal papillae are located. The hydroporic canal in juveniles retains a connection with the environment and opens on the dorsal side through a narrow hydropore.
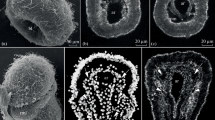
One-month-old juvenile of A. japonicus. a Three-dimensional reconstruction of the body with digestive and water-vascular systems (view from the right side); orange color indicates water-vascular system, blue color—calcareous ring, yellow color—digestive system, red color—longitudinal muscle band; gray—body wall. b General view from the right-dorsal side; green color indicates actin; red color—tubulin; blue color—nuclei. cr calcareous ring, dn dorsal radial nerve cord, g gut, hc hydroporic canal, lm longitudinal muscle band, lvn left ventral radial nerve cord, mp madreporite, mvc mid-ventral radial water-vascular canal, pa papilla, pv polian vesicle, rd right dorsal radial water-vascular canal, rv right ventral radial water-vascular canal, t tentacle, tf tube foot
The luminal epithelium of the water-vascular system organs has not undergone significant changes compared to the previous stage. It is formed by flattened ciliated cells (Fig. 10a, b). Myelin bodies and lipoprotein granules vanish in the cell cytoplasm. In the basal part of the luminal epithelium of the stone canal, myoepithelial cells appear (Fig. 10c, d). These are distinguished by bundles of myofilaments present in the cytoplasm. A layer of denser connective tissue containing sclerocytes and calcite crystals appears around the stone canal (Fig. 10c).
Microanatomical organization of water-vascular system of the 1-month-old juvenile of A. japonicus. a Luminal epithelium of water-vascular ring. b Luminal epithelium of polian vesicle. c Transverse section of the stone canal. d Myoepithelial cells in the luminal epithelium of the stone canal. e Transverse section of the posterior segment of ventral water-vascular canal. f Myoepithelial cells in the luminal epithelium of water-vascular canal. g Luminal epithelium of water-vascular canal of tube foot. h Nerve cell processes with dense core vesicles (arrows). i Longitudinal section of the luminal epithelium of tentacle. j Transverse section of the luminal epithelium of tentacle. bl basal lamina, c cilia, ct connective tissue, hd hemidesmosome, ij interdigitations, m mitochondrion, mc myoepithelial cell, mf myofilaments, mv microvilli, ns processes of the neurosecretory cell, nsc neurosecretory cell, pc peritoneal cell, rr rough endoplasmic reticulum, s sclerocyte
In all radial water-vascular canals, the luminal epithelium contains myoepithelial cells. They are absent only from the posterior segments of the ventral canals (Fig. 10e). The bodies of myoepithelial cells and their processes with myofilaments are located in the basal part of the epithelium and are covered with peritoneocytes’ processes (Fig. 10f).
One-month-old individuals have two tube feet (Fig. 9a). In their luminal epithelium, the number of processes of neurosecretory cells with large electron-dense granules increases (Fig. 10g). Bodies of these cells are sometimes found. Within the epithelium, nerve cell processes containing dense core vesicles appear for the first time (Fig. 10h).
The structure of the luminal epithelium of the tentacles has changed compared to that in the previous stage (Fig. 10i, j). Most of the cytoplasm of myoepithelial cells is filled with myofilaments. The number of interdigitations between cells decreases. Unlike the foot canal, neurosecretory cells and their processes are not found in the luminal epithelium of the tentacles.
Derivatives of somatocoels
In 1-month-old juveniles, the structure of the coelomic epithelium of the body wall and internal organs has changed little compared to that of the previous stage. Unlike those in pentactula, myoepithelial cells are found in all interradii. They form the circular musculature of the animal. In the anterior half of the body, the body wall coelomic epithelium creates numerous deep plicae formed by tall peritoneal and myoepithelial cells (Fig. 11a–c). In the posterior part of the body, it consists of flattened myoepithelial and peritoneal cells (Fig. 11d).
Microanatomical organization of coelomic epithelium of the 1-month-old juvenile of A. japonicus. a Semithin transverse section of the dorsal ambulacrum in the anterior part of the body. b Peritoneocytes of the coelomic epithelium of the anterior part of the body. c Myoepithelial cells of the coelomic epithelium of the anterior part of the body. d Coelomic epithelium of the posterior part of the body. e Apical part of peritoneal cells. f Nerve processes in the coelomic epithelium. bl basal lamina, c cilia, ce coelomic epithelium, d desmosome, ga Golgi apparatus, lm longitudinal muscle band, m mitochondrion, mc myoepithelial cell, mf myofilaments, mv microvilli, np nerve processes, rn radial nerve cord, rr rough endoplasmic reticulum, v vesicle
The apical surface of the peritoneal cells bears cilia and a moderate number of long microvilli. The nuclei of cells are oval or irregular in shape, with a nucleolus and a moderate content of heterochromatin. The cytoplasm is almost entirely filled with numerous RER cisterns (Fig. 11e). In addition, the cells have a GA, electron-lucent vesicles, and mitochondria, there are also heterophagosomes, multivesicular bodies, and myelin-like bodies.
Myoepithelial cells are separated from the coelom by processes and bodies of peritoneocytes. The apical surface of myoepithelial cells is devoid of microvilli and cilia; the basal bodies of the latter are sometimes found in the cytoplasm. The nucleus situates in the apical part of the cell. The basal part of the cell is filled with myofilaments. In the basal part, numerous thin cytoplasmic processes without myofilaments are observed. The cytoplasm contains RER cisterns, ribosomes, mitochondria, electron-lucent vesicles, and sometimes multivesicular bodies. Within the body wall coelomic epithelium, processes of nerve cells are found (Fig. 11d, f).
In 1-month-old juveniles, the LMB development continues. The ventro-lateral and dorsal LMBs have similar structures and consist of a single layer of myoepithelial cells separated from the coelom by peritoneocytes (Fig. 12a, b). Nevertheless, in the ventro-lateral LMBs, the width of the myoepithelial cell layer is smaller. Furthermore, the accumulation of ECM between peritoneal and myoepithelial cells begins in the dorsal LMBs. The connective-tissue layer separating the LMBs from the radial water-vascular canal increases.
Microanatomical organization of longitudinal muscle bands of the 1-month-old juvenile of A. japonicus. a Transverse section of dorsal LMB. b Transverse section of ventral LMB. c Transverse section of mid-ventral LMB. d Neurosecretory cells in the LMB. e Coelomic epithelial cell embedding into LMB. bl basal lamina, bm muscle bundle, c cilia, co coelom, ct connective tissue, dc dorsal radial water-vascular canal, hd hemidesmosome, m mitochondrion, mc myoepithelial cell, mf myofilaments, ns processes of the neurosecretory cell, pc peritoneal cell, rr rough endoplasmic reticulum, v vesicle
In the mid-ventral LMB, peritoneocytes are completely separated from myoepithelial cells and form the flattened epithelium covering the LMB (Fig. 12c). Below, the volume of ECM, in which rare collagen fibers are visible, increases. At this myogenesis stage, myoepithelial cells of the mid-ventral LMB are more correctly referred to as myocytes. These are separated from the surrounding connective tissue by their own basal lamina, thus, forming muscle bundles (Fig. 12c). The basal lamina is absent only from the apical part of the cells. Myocytes form hemidesmosomes not only in the basal part but also on the lateral and apical sides. Processes of neurosecretory cells are found in the muscle bundles and in the LMB connective tissue (Fig. 12d).
In 1-month-old individuals, the onset of a myogenic transformation of the coelomic epithelium cells covering the LMB is observed. This process occurs in the mid-ventral LMB. Epithelial cells form long processes extending into the LMB connective tissue. Bundles of myofilaments are visible in these processes (Fig. 12e).
Connective tissue structures
At this ontogeny stage, the ECM formation continues. The animal’s body wall consists of three layers (Fig. 13a). The outer, densest layer contains spicules and a large number of fibers. In all internal organs, the connective-tissue layer between the luminal and coelomic epithelia grows. The number of collagen fibers and mesenchymal cells in it also increases. At this developmental stage, another mesenchymal cell type, granulocytes, is found in the body wall ECM (Fig. 13b). These cells have an amoeboid shape. Their cytoplasm contains numerous granules filled with a substance having a medium electron density. The diameter of the granules is ca. 0.2 µm. The cytoplasm also contains RER cisterns and mitochondria.
Microanatomical organization of connective tissue of the 1-month-old juvenile of A. japonicus. a Semithin transverse section of body wall. b Granulocyte. c Semithin transverse section of the calcareous ring. d General view of sclerocyte. e Long narrow RER cisterns in the cytoplasm of sclerocyte. f Small juvenile cell. g Juvenile cell with increased volume of the cytoplasm. h Crystal cell. bl basal lamina, cr calcareous ring, ex external layer of connective tissue, g granules, in internal layer of connective tissue, m mitochondrion, mb multivesicular body, n nucleus, og outgrowth, rr rough endoplasmic reticulum, s sclerocyte, sr smooth endoplasmic reticulum, tw water-vascular canal of the tentacle, v vesicle, va vacuole
The left calcareous body, previously located in the posterior part of the animal, disappears. Simultaneously, 1-month-old juveniles show an increase in the size and thickness of the calcareous ring (Figs. 9a, 13c). Not only the lateral but also the posterior walls of the tentacular canals undergo calcification. The juvenile’s body is covered with numerous needle-like spicules (Fig. 13a). Unlike spicules in pentactula, these are distributed all over the animal’s surface. Spicules are also present under the epidermis of the tips of the tentacles and tube feet, as well as in the cloaca connective tissue. Sclerocytes of juveniles are distinguished by a large number of mitochondria and long narrow RER cisterns (Fig. 13d, e). Centrioles and lipoprotein granules are sometimes found in their cytoplasm.
Coelomocytes
The diversity of coelomocyte types increases in juveniles. In addition to amoebocytes and morula cells, juvenile cells are also found. These are cells of 3–5 µm in diameter with a high nuclear-cytoplasmic ratio (Fig. 13f, g). They differ in the cytoplasm volume and the degree of development of the synthetic apparatus. Small cells of ca. 3 µm in diameter have an oval nucleus with large clumps of heterochromatin. A small volume of cytoplasm contains single microvesicles. In cells of 4–5 µm in diameter, the volume of cytoplasm increases, it contains short RER cisterns, microvesicles of smooth endoplasmic reticulum, and a GA. Single mitochondria are present.
Crystal cells are found in the cavities of the water-vascular system. They have a large electron-lucent vacuole occupying the major part of the cytoplasm (Fig. 13h). Vesicles of various diameters are present in the vacuole. The cell nucleus is elongated, hyperchromic, and displaced to the cell periphery. The cytoplasm contains mitochondria, RER cisterns, small granules, and multivesicular bodies.
Discussion
Our previous studies showed that A. japonicus larvae at the late doliolaria stage have all the main organs typical of the adult organism (Dolmatov et al. 2016, 2017, 2018). In addition to the digestive and nervous systems, the coeloms transform and various mesodermal organs develop. Thus, by the time of metamorphosis and settling, juvenile individual becomes almost completely formed. It has external appendages (a tube foot and tentacles) and also the digestive, muscular, and nervous systems. However, these organs are developed insufficiently and are, apparently, not fully functional. The metamorphosis in A. japonicus and, probably, in other holothurians with planktotrophic larva consists in changing the body shape and destroying the ciliary rings (Smiley 1986; Nakano et al. 2006; Dolmatov et al. 2016).
The third germ layer emerged in the evolution of Bilateria due to the need to develop and complicate the muscular system (Rieger and Ladurner 2003; Technau and Scholz 2003; Martindale et al. 2004). However, the origin and stages of the formation of complexly organized contractile systems remain poorly understood. In this regard, echinoderms are convenient model objects, since they show the recapitulation of the stages of the muscular system evolution during muscle development (Dolmatov and Ivantey 1993; Dolmatov et al. 2007; Dolmatov 2010). Furthermore, certain stages of differentiation of coelomic epithelial cells during their transformation into myocytes can be distinguished in A. japonicus. However, different coeloms had, apparently, somewhat different evolutionary trajectories, which is related to their eventual destination.
Hydrocoel derivatives
Hydrocoels in echinoderms give rise to a unique system of organs, the water-vascular system. It is represented by tubular or sac-like formations whose luminal epithelium is formed from the epithelium of hydrocoels. Late doliolaria of A. japonicus already possesses all the components of this system. Changes in metamorphosis and further development are mainly associated with the myogenic differentiation of the water-vascular epithelium cells. Two stages can be distinguished in this process. The initial stage is associated with the myogenic differentiation of epithelial cells and myoepithelium formation. It is characterized by the emergence and increase in the number of myofilaments in the cytoplasm of myoepithelial cells. Different phases of this stage can be identified in the organs of late doliolaria, from the epithelium containing no myoepithelial cells (water-vascular ring canal) to the myoepithelium (tube foot). The second stage of complication of the water-vascular epithelium is associated with the onset of organs’ functioning and consists in strengthening the mechanical connection between adjacent myoepithelial cells, as well as between these cells and ECM. This is, apparently, required for more efficient transmission of contractions from epithelium to connective tissue of the organ. The formation of cell processes embedded in the basal lamina of the myoepithelium can be considered the first phase of this process, which can be observed in the tentacles of late doliolaria. The processes allow better attachment of myoepithelial cells to the connective tissue. The following phase is the development of interdigitation between adjacent myoepithelial cells (the tentacles and tube foot of pentactula). The interdigitation formation leads to even greater mechanical adhesion of contractile cells and allows the epithelium to contract as an integral whole. Such interdigitations between contractile cells are also characteristic of vertebrates (Cullinan et al. 1986; Yoshida et al. 2010).
Interestingly, the myogenic differentiation of epithelial cells of the water-vascular radial canals begins with the side of the nerve cords. In the juveniles that we examined, myoepithelial cells occupy the entire part of the epithelium facing the nerve cord. It is not clear whether the myogenesis expands to the remaining part of the epithelium during further development. There is a lack of data on the morphology of radial water-vascular canals in adult holothurians. Sea urchins and sea stars have these canals lined by nonmuscular epithelium (Cavey and Märkel 1994; Chia and Koss 1994).
In 1-month-old individuals of A. japonicus, myoepithelial cells were identified in the stone canal epithelium. No similar cells have been described from stone canals of other holothurian species. These have not been found in ophiurans, sea stars, and sea urchins as well. However, myoepithelial cells are present in the water-vascular lining of stone canals of crinoids (Heinzeller and Welsch 1994). According to Heinzeller and Welsch (1994), these form a circular musculature, whose contraction interferes with ciliary activity and prevents the fluid from flowing into the water-vascular system. The absence of myoepithelial cells from the stone canal epithelium in most echinoderms is probably explained by the insufficient knowledge of this organ. More detailed studies are expected to identify these cells.
Somatocoel derivatives
The analysis of the LMB development in A. japonicus is consistent with our hypothesis of the origin, development, and evolution of the muscular system in echinoderms (Dolmatov and Ivantey 1993; Dolmatov et al. 2007; Dolmatov 2010). Since the development of ambulacra in holothurians occurs unevenly (Dolmatov and Yushin 1993; Mashanov and Dolmatov 2000; Dolmatov et al. 2016), this provides the opportunity to observe all the major myogenesis stages. In late doliolaria, one of the early myogenesis stages occurs in the mid-ventral LMB: the development of single myoepithelial cells above the water-vascular canal. Then the number of these cells increases, which results in the formation of a typical echinoderm pseudostratified myoepithelium (Rieger and Lombardi 1987). The following stage of the muscular system complication is the surrounding of contractile cells by connective tissue. Initially, the amount of ECM under the myoepithelium increases. This stage is observed in dorsal LMBs of 1-month-old individuals. With the further development of holothurians, this process will continue and, as a result, the connective-tissue basis of LMB will be formed. Then the ECM begins to accumulate also between the peritoneocytes located on the LMB surface and the myoepithelial cells. This stage, in our opinion, recapitulates the evolutionary stage of embedding contractile cells into connective tissue. The ECM accumulation leads to the origin of submerged myoepithelia that consist of externally located peritoneocytes and myoepithelial cells embedded in connective tissue. In this case, all cells are arranged on a common basal lamina.
The following stage of LMB formation is the separation of myoepithelium into epithelial and muscle cells. In 1-month-old juveniles of A. japonicus, this stage is not yet completed, since the muscle bundles have not yet formed. They are not completely separated from the ECM by the basal lamina. However, the process of new muscle bundle formation is already beginning in 1-month-old juveniles of A. japonicus through the myogenic differentiation and embedding of coelomic epithelial cells into the formed ECM of LMB.
Connective tissue
A significant change in the connective tissue of A. japonicus occurs in the process of metamorphosis and definitive organogenesis. The spaces of the primary body cavity between developing organs and somatocoels are gradually filled with ECM. This leads to the formation of various structures and organs in holothurians: the body wall, the digestive tube, and the water-vascular system. The ECM contains a large number of various mesenchymal cells. During the ontogeny of holothurians, these give rise to several cell types (Smiley 1994). However, despite the advanced development of connective tissue, no typical fibroblasts are found. It is likely that amoebocyte-like cells perform the function of synthesis of collagen fibers and ECM.
Sclerocytes of A. japonicus have a structure typical of holothurians (Stricker 1986). Their feature, which distinguishes this cell type from other mesenchymal cells, is the presence of basal lamina. Its function is still unclear. According to Stricker (1986), the presence of the basal lamina indicates the ectodermal origin of sclerocytes. However, these are most likely of mesodermal origin. The basal lamina is probably necessary for the fixation of spicules in ECM.
The structure of sclerocytes gradually alters during the transition from doliolaria to a 1-month-old juvenile. In their cytoplasm, the number of mitochondria and dictyosomes of GA increases, and numerous RER cisterns are formed. These changes apparently indicate an intensification of the synthetic activity of cells and the calcification process. After settling, animals have a large number of spicules formed on their body surface and in the tentacles and tube foot, as well as the development of the calcareous ring and madreporite.
One of the noteworthy and intriguing structures of holothurian larvae is hyaline spheres. According to Peters-Didier and Sewell (2019), these function as nutrient storage structures for accumulating neutral lipids. Besides lipoproteins, they include collagen fibers (Dautov 1997; this article). In A. japonicus, the hyaline spheres degrade after metamorphosis, which is confirmed by previously obtained data (Dautov and Kashenko 1995; Dautov 1997).
After the metamorphosis, A. japonicus show also changes in the composition of mesenchymal cells (coelomocytes). Echinoderm coelomocytes represent a heterogeneous population of immune cells (Chia and Xing 1996; Ballarin et al. 2021). At the late doliolaria stage, there are already two populations of coelomocytes: amoebocyte-like cells and morula cells. Such a division into two populations occurs early in the ontogeny, at the gastrula stage (Ho et al. 2016). Amoebocyte-like cells are involved in the removal of structures degraded during the metamorphosis, primarily hyaline spheres and ciliary rings. In A. japonicus, the first morula (spherule) cells are found at the blastula stage (Li et al. 2014). At the late doliolaria stage, two types of these cells are identified: with electron-lucent and heterogeneous granules. These types of morula cells persist during further development and are found in adults (Eliseikina and Magarlamov 2002; Xing et al. 2008).
In 1-month-old juveniles of A. japonicus, the diversity of coelomocytes increases. Crystal and juvenile cells appear. Crystal cells are probably involved in the regulation of salt metabolism in echinoderms (Chia and Xing 1996). Juvenile cells (lymphocytes) are considered progenitor cells that give rise to other coelomocyte types in echinoderms (Smiley 1994; Eliseikina et al. 2010; Zavalnaya et al. 2020). Cells that differ in the volume of cytoplasm and the degree of organelles’ development probably represent various stages of differentiation of juvenile cells.
Thus, our study has shown that the main mesodermal organs are already formed at the late doliolaria stage. All the structures of the water-vascular system, the LMB anlagen, extracellular matrix, and different types of mesenchymal cells are present. However, even in 1-month-old juveniles, many organs do not reach their full development as in adults. This particularly applies to the contractile system. Nevertheless, 1-month-old juveniles already have all the main types of coelomocytes characteristic of adults. This means that the immune system of A. japonicus is already formed.
Data availability
All data used in this study are available upon personal request to the authors.
References
Ballarin L et al (2021) Stem cells and innate immunity in aquatic invertebrates: bridging two seemingly disparate disciplines for new discoveries in biology. Front Immunol 12:688106
Brusca RC, Brusca GJ (2003) Invertebrates, 2nd edn. Sinauer Associates, Sunderland
Cavey MJ, Märkel K (1994) Echinoidea. In: Harrison FW, Chia FS (eds) Microscopic anatomy of invertebrates. Echinodermata, vol 14. Wiley-Liss Inc, New York, pp 345–400
Chia FS, Koss R (1994) Asteroidea. In: Harrison FW, Chia FS (eds) Microscopic anatomy of invertebrates. Echinodermata, vol 14. Wiley-Liss Inc, New York, pp 169–245
Chia F-S, Xing J (1996) Echinoderm coelomocytes. Zool Stud 35:231–254
Cullinan V, Campbell JH, Mosse PR, Campbell GR (1986) The morphology and cell culture of the striated musculature of the rat azygos vein. Cell Tissue Res 243:185–191. https://doi.org/10.1007/bf00221867
Dautov SS (1997) Structure and properties of hyaline spheres in holothuroid larvae. Invertebr Reprod Dev 32:155–161. https://doi.org/10.1080/07924259.1997.9672617
Dautov SS, Kashenko SD (1995) Hyaline spheres in auricularia of Stichopus japonicus. Invertebr Reprod Dev 27:61–64
Dolmatov IY (2010) Development and evolution of the muscle system in the Echinodermata. In: Harris LG, Bottger SA, Walker CW, Lesser MP (eds) Echinoderms: Durham. CRC Press, Boca Raton, pp 163–166
Dolmatov IY, Ivantey VA (1993) Histogenesis of longitudinal muscle bands in holothurians. Russ J Dev Biol 24:67–72
Dolmatov IY, Yushin VV (1993) Larval development of Eupentacta fraudatrix (Holothuroidea, Dendrochirota). Asian Mar Biol 10:125–134
Dolmatov IY, Mashanov VS, Zueva OR (2007) Derivation of muscles of the Aristotle’s lantern from coelomic epithelia. Cell Tissue Res 327:371–384
Dolmatov IY, Ginanova TT, Frolova LT (2016) Metamorphosis and definitive organogenesis in the holothurian Apostichopus japonicus. Zoomorphology 135:173–188. https://doi.org/10.1007/s00435-015-0299-y
Dolmatov IY, Ginanova TT, Frolova LT (2017) Digestive system formation during the metamorphosis and definitive organogenesis in Apostichopus japonicus. Zoomorphology 136:191–204. https://doi.org/10.1007/s00435-016-0340-9
Dolmatov IY, Ginanova TT, Eliseikina MG, Frolova LT (2018) Formation of the ectodermal organs during the metamorphosis and definitive organogenesis in the holothurian Apostichopus japonicus. Zoomorphology 137:545–564. https://doi.org/10.1007/s00435-018-0412-0
Eliseikina MG, Magarlamov TY (2002) Coelomocyte morphology in the holothurians Apostichopus japonicus (Aspidochirota: Stichopodidae) and Cucumaria japonica (Dendrochirota: Cucumariidae). Russ J Mar Biol 28:197–202
Eliseikina MG, Magarlamov TY, Dolmatov IY (2010) Stem cells of holothuroid coelomocytes. In: Harris LG, Bottger SA, Walker CW, Lesser MP (eds) Echinoderms: Durham. CRC Press, Boca Raton, pp 163–166
Heger P, Zheng W, Rottmann A, Panfilio KA, Wiehe T (2020) The genetic factors of bilaterian evolution. Elife 9:e45530. https://doi.org/10.7554/eLife.45530
Heinzeller T, Welsch U (1994) Crinoidea. In: Harrison FW, Chia FS (eds) Microscopic anatomy of invertebrates. Echinodermata, vol 14. Wiley-Liss Inc, New York, pp 9–148
Ho EC et al (2016) Perturbation of gut bacteria induces a coordinated cellular immune response in the purple sea urchin larva. Immunol Cell Biol 94:861–874. https://doi.org/10.1038/icb.2016.51
Li Q, Qi R-R, Wang Y-N, Qiao G, Ye S-G, Li H (2014) Ontogenesis of coelomocytes in sea cucumber (Apostichopus japonicus) studied with probes of monoclonal antibody. Fish Shellfish Immunol 41:260–263. https://doi.org/10.1016/j.fsi.2014.09.005
Martindale MQ, Pang K, Finnerty JR (2004) Investigating the origins of triploblasty: “mesodermal” gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa). Development 131:2463–2474. https://doi.org/10.1242/dev.01119
Mashanov VS, Dolmatov IY (2000) Developmental morphology of a holothurian Cucumaria japonica (Dendrochirota, Holothuroidea), a species with accelerated metamorphosis. Invertebr Reprod Dev 37:137–146. https://doi.org/10.1080/07924259.2000.9652412
Nakano H, Murabe N, Amemiya S, Nakajima Y (2006) Nervous system development of the sea cucumber Stichopus japonicus. Dev Biol 292:205–212
Peters-Didier J, Sewell MA (2019) The role of the hyaline spheres in sea cucumber metamorphosis: lipid storage via transport cells in the blastocoel. EvoDevo 10:8. https://doi.org/10.1186/s13227-019-0119-4
Purcell SW (2010) Managing sea cucumber fisheries with an ecosystem approach. FAO Fisheries and Aquaculture Technical Paper. No. 520. FAO, Rome
Rieger RM, Ladurner P (2003) The significance of muscle cells for the origin of mesoderm in bilateria. Integr Comp Biol 43:47–54. https://doi.org/10.1093/icb/43.1.47
Rieger RM, Lombardi J (1987) Ultrastructure of coelomic lining in echinoderm podia: significance for concepts in the evolution of muscle and peritoneal cells. Zoomorphology 107:191–208
Smiley S (1986) Metamorphosis of Stichopus californicus (Echinodermata: Holothuroidea) and its phylogenetic implications. Biol Bull 171:611–631
Smiley S (1994) Holothuroidea. In: Harrison FW, Chia FS (eds) Microscopic anatomy of invertebrates. Echinodermata, vol 14. Wiley-Liss Inc, New York, pp 401–471
Stricker SA (1986) The fine structure and development of calcified skeletal elements in the body wall of holothurian echinoderms. J Morphol 188:273–288. https://doi.org/10.1002/jmor.1051880303
Technau U, Scholz CB (2003) Origin and evolution of endoderm and mesoderm. Int J Dev Biol 47:531–539
Udagawa S, Ikeda T, Oguchi K, Kohtsuka H, Miura T (2022a) Hydrocoel morphogenesis forming the pentaradial body plan in a sea cucumber, Apostichopus japonicus. Sci Rep 12:6025. https://doi.org/10.1038/s41598-022-09691-y
Udagawa S et al (2022b) The pentameric hydrocoel lobes organize adult pentameral structures in a sea cucumber, Apostichopus japonicus. Dev Biol 492:71–78. https://doi.org/10.1016/j.ydbio.2022.09.002
Xing K, Yang H, Chen M (2008) Morphological and ultrastructural characterization of the coelomocytes in Apostichopus japonicus. Aquat Biol 2:85–92
Yang H, Hamel J-F, Mercier A (2015) The sea cucumber Apostichopus japonicus. History, biology and aquaculture. Elsevier, Amsterdam
Yoshida M et al (2010) Weaving hypothesis of cardiomyocyte sarcomeres: discovery of periodic broadening and narrowing of intercalated disk during volume-load change. Am J Pathol 176:660–678. https://doi.org/10.2353/ajpath.2010.090348
Zavalnaya EG, Shamshurina EV, Eliseikina MG (2020) The immunocytochemical identification of PIWI-positive cells during the recovery of a coelomocyte population after evisceration in the holothurian Eupentacta fraudatrix (Djakonov et Baranova, 1958) (Holothuroidea: Dendrochirota). Russ J Mar Biol 46:97–104. https://doi.org/10.1134/S106307402002011X
Acknowledgements
The material was processed and analyzed at the Far Eastern Center of Electron Microscopy and the CKP «Primorsky aquarium» (National Scientific Center of Marine Biology, Far Eastern Branch, Russian Academy of Sciences, Vladivostok, Russia). This study was supported by the Russian Science Foundation (Grant no. 21-74-30004).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by IYD, TTG, MGE and LTF. The first draft of the manuscript was written by IYD, TTG and MGE and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dolmatov, I.Y., Ginanova, T.T., Eliseikina, M.G. et al. Formation of the mesodermal organs during the metamorphosis and definitive organogenesis in the holothurian Apostichopus japonicus. Zoomorphology 142, 357–376 (2023). https://doi.org/10.1007/s00435-023-00605-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00605-8