Abstract
Purpose
The N6-methyladenosine (m6A) has been involved in the regulation of cell proliferation and metastasis in multiple cancers. However, the biological significance of m6A reader IGF2BP2 in oral squamous cell carcinoma (OSCC) and the mechanism of IGF2BP2 itself have not been fully investigated.
Methods
The cellular phenotypes of OSCC cells were determined by CCK-8 and transwell migration assays. The energy metabolism was detected using glucose uptake/lactate production assay and extracellular acidification rate analysis. The molecular interaction was tested by RNA immunoprecipitation assay.
Results
Here, results indicated that IGF2BP2 was up-regulated in OSCC and that it acted as a predictor of poor prognosis. IGF2BP2 promoted the proliferation, migration and Warburg effect of OSCC cells in vitro. Mechanistical assays illustrated that IGF2BP2 directly interacted with HK2 mRNA by binding the 3′-UTR m6A site. Moreover, IGF2BP2 positively promoted the stability of HK2 mRNA and thus the protein level of HK2 increased upon IGF2BP2 overexpression.
Conclusions
In conclusion, the IGF2BP2/m6A/HK2 axis accelerated the abnormal energy metabolism of OSCC. Taken together, these findings revealed a novel mechanism by which IGF2BP2 functions in OSCC progression, which may provide new therapy options for OSCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinoma (OSCC) acts as the most common malignant head and neck tumors, accounting for 80–90% of head and neck malignancy (Metsäniitty et al. 2021; van der Kamp et al. 2022). To make matters worse, the incidence rate and mortality have continued to rise in recent years (Bienkowska et al. 2021). Despite the marked improvement made by recent advances, including radiotherapy and chemotherapy, the mortality of OSCC is still high, especially the very low 5-year survival rate (Shahoumi 2021; Haldar and Singh 2022). High metastasis or insensitivity to chemotherapy is still one of the critical factors for the poor prognosis of OSCC. Nevertheless, whether and how OSCC could be effectively treated is largely unclear.
N6-methyladenosine (m6A), one of the most abundant chemical modifications of mRNAs in eukaryotes, has been reported to play a significant role in many human tumors (Chen et al. 2022; Huang et al. 2021). The methyltransferase, also known as “writers”, activates the m6A activity by installing m6A in nitrogen atoms (Relier et al. 2022). The demethylase, also known as “erasers”, wipes off the m6A from the methylated RNA (Mu et al. 2022). Moreover, the recognition protein, also known as “readers”, recognizes the m6A-modified RNAs to stabilize or degrade them (Li et al. 2021a; Tong et al. 2021). numerous papers have reported the functions of m6A methyltransferase in OSCC,. For instance, methyltransferase METTL3 is consistently up-regulated in OSCC samples and the high expression is associated with OSCC poor prognosis. Moreover, METTL3 mediates the m6A modification of BMI1 mRNA in its 3′-UTR to promote BMI1 translation under the cooperation with IGF2BP1 (Liu et al. 2020). Moreover, demethylase also regulates the tumorigenesis of FTO. For example, the fat mass and obesity-associated protein (FTO) is significantly up-regulated in areca nut-chewing OSCC tissue samples. The depletion of FTO attenuates the arecoline-induced chemoresistance and oncogenicity through negatively regulating transcription factor forkhead box protein A2 (Li et al. 2021b).
Regarding m6A reader, the functions and potential mechanism are still unclear. Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) is a critical m6A reader responsible for recognizing the m6A modification site. Here, we performed functional assays to investigate the function and mechanism of IGF2BP2 in OSCC. In the present research, we found that IGF2BP2 was up-regulated in the OSCC tissue and cells. IGF2BP2 combined with the m6A modification site at 3’-UTR of HK2 mRNA.
Materials and methods
Clinical samples
The OSCC tissue of patients who underwent surgery were gathered. None of the OSCC patients received radiotherapy or chemotherapy before surgery. After resection, tissue specimens were immediately stored in liquid nitrogen and subsequently stored at − 80 °C. Diagnosis was pathologically confirmed by two independent pathologists.
Cell culture
OSCC cells (SCC-9, CAL-27) and normal oral epithelium keratinocytes (HOK) were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM with 10% fetal bovine serum (FBS) and streptomycin and penicillin (1%, GE Healthcare, Chicago, IL, USA).
Cell transfection
To up-regulate or silence the expression of IGF2BP2, full length and shRNA sequence against IGF2BP2, as well as empty vectors, were ligated into pIRES2-EGFP vector or pGPU6/GFP/Neo vector, respectively (GenePharma). Cells were plated in a 24-well culture dish at 70–80% confluence, and transfection was performed using Lipofectamine LTX Reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. The transfection efficiency was identified using qRT-PCR.
Western blotting
For the OSCC cells, total proteins were extracted and subjected to 10% SDS-PAGE for separation and then transferred to PVDF membranes. Primary antibodies (anti-IGF2BP2, 1:1000, Abcam, ab128175) were used for incubation on nonfat milk-blocked PVDF membranes at 4 °C overnight. After incubation, the PVDF membranes were rinsed with Tris-buffered saline containing Tween 20 and further incubated with secondary antibody for 1 h at room temperature. Lastly, a chemiluminescence detection kit was used to detect the signals from different proteins. The relative integrated density values were analyzed using ImageJ software.
Real-time quantitative PCR (RT-qPCR)
Firstly, the total RNA was extracted from OSCC cells using TRI Reagent and the extracted RNA was quantified. Then, the cDNA was reversely transcribed using PrimeScript RT reagent kit (Takara, Dalian, China). Subsequently, the relative expressions of IGF2BP2 and HK2mRNA were examined using SYBR green dye (Toyobo, Osaka, Japan) by a PCR reaction system, including a cDNA template (3 µL), SYBR green dye (10 µL), ddH2O (6.5 µL) and paired primers (0.5 µL, final concentration: 0.3 µM). The quantitative gene expression of each group was detected (2−ΔΔCT). The premiers are listed in Table S1.
CCK-8 assay
A cell proliferation assay was carried out by CCK-8 (Dojindo Laboratories) according to the protocol of the manufacturer. Approximately. 2000 per/well OSCC cells were seeded in six-well plates. After culturing for 48 h, the cells were added with 10 mL CCK-8 solutions. After that, the OD value was measured using Thermomax microplate reader (bio-tekEL) at 450 nm wavelengths.
Transwell migration assay
Transwell migration assay was performed using Transwell inserts (8 μm pore size; Costar). About 2 × 104 OSCC cells in serum‐free medium were seeded into the upper chamber uncoated with Matrigel (BD Biosciences, San Jose, CA). In the lower chamber, completed DMEM medium was filled. After 24 h of culturing, the cells through the upper chamber were removed and then fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The migrated cell numbers were counted under a light microscope.
Glucose uptake and lactate production assay
After transfection, OSCC cells were cultured and then replaced by high-glucose culture medium. After 24 h, the supernatants of OSCC culture medium were collected separately. The glucose uptake was detected using a glucose assay kit (BioVision, Milpitas, California, USA). The lactate production was detected using a lactate assay kit (BioVision) according to the manufacturer’s instructions.
Measurement of extracellular acidification rate (ECAR)
The extracellular acidification rate (ECAR) was estimated as previously described using Seahorse XF Glycolysis Stress Test Kit according to the manufacturer’s instructions (Chen et al. 2018). In brief, the OSCC cells (1 × 104 cells/well) were seeded into Seahorse XF 96-cell culture microplates for adhere culture overnight. Then, cells were washed with DMEM medium supplemented with l-glutamine (2 mM, pH = 7.4) and incubated at 37 °C for 1 h in CO2-free incubator. The glycolysis rate and glycolytic capacity were detected using Seahorse XF 96 Extracellular Flux Analyzer (Agilent Technologies, Santa Clara, CA, USA).
Half-life measurement
To analyze the stability of HK2 mRNA, half-life assay was performed with the treatment of transcriptional inhibitor actinomycin D (Act D). OSCC cells were treated with actinomycin D (2 mg/mL, Sigma-Aldrich, Louis, MO, USA) to halt the transcription and then harvested at 0, 3 and 6 h after the treatment. The cellular RNAs were isolated and analyzed using qRT-PCR. Beta-actin acted as an internal reference.
RNA immunoprecipitation (RIP) assay
RIP assays were performed using EZ-Magna RIP Kit (Millipore) according to the manufacturer’s protocol. SCC-9 and CAL-27 cells were lysed in complete RIP lysis buffer (Magna RIP Kit, Millipore, MA, USA) at 4 °C via disruptive sonication. Protein A/G agarose beads were conjugated with specific antibodies (anti-IGF2BP2, 11601-1-AP, Proteintech, China) or control IgG and then incubated with cell extract for 2 h at 4 °C. HK2 mRNA was analyzed through quantitative reverse-transcription polymerase chain reaction (qRT-PCR).
Xenograft mouse model
Male BALB/c nude mice (5–6 weeks of age) were provided by Type Culture Collection of the Chinese Academy of Medical Sciences (Beijing, China) and acclimatized for a week prior to this assay. Nude mice were subcutaneously injected with 5 × 106 CAL27 cells. Every 5 days, the tumor weight and length week were monitored with a caliper for the tumor volume following the formula (0.52 × a × b2, a for the long and b for the short diameter, respectively). After 30 days, mice were killed and tumors were dissected out for weight. The assay was approved by the Ethics Committee of Cangzhou Central Hospital.
Statistical analysis
Data are expressed as means ± SD (standard deviation). Comparison was performed using variance analysis. Least significant difference procedure analysis was performed when there were more than two groups. P value < 0.05 was considered statistically significant.
Results
IGF2BP2 was highly expressed in OSCC tumor tissue cohort and cells
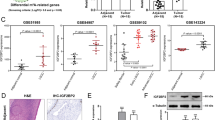
Given the critical roles of m6A methylation key enzymes in human cancer, we focused on the novel m6A reader IGF2BP2 in OSCC. Firstly, the large cohort data survey based on TCGA (https://www.tcga.org/) found that the expression of IGF2BP2 was significantly up-regulated in the HNSC cohort (Fig. 1A). Because OSCC was a subclass of HNSC, the data from the database indicated that IGF2BP2 was over-expressed in OSCC, which might act as an oncogene in OSCC. Moreover, the level of IGF2BP2 mRNA was found to be up-regulated in OSCC cells (SCC-9, CAL-27) (Fig. 1B). Moreover, the IGF2BP2 protein was detected using western blot analysis and it was found that the IGF2BP2 protein was up-regulated in OSCC cells (SCC-9, CAL-27) (Fig. 1C). With regard to the survival rate of OSCC with high/low IGF2BP2 expression, public database GEPIA (http://gepia.cancer-pku.cn/index.html) (Fig. 1D) and Kaplan–Meier plotter (http://kmplot.com/analysis/) (Fig. 1E) revealed that patients with high IGF2BP2 expression had close correlation with low survival rate. Overall, the above data indicated that IGF2BP2 was highly expressed in OSCC and might function as a risk factor for OSCC.
IGF2BP2 was highly expressed in OSCC tumor tissue cohort and cells. A The large cohort data survey based on TCGA (https://www.tcga.org/) revealed the up-regulated expression of IGF2BP2 in an HNSC cohort. B RT-PCR was performed to detect the expression of IGF2BP2 in OSCC cells (SCC-9, CAL-27). C Western blot analysis was performed to analyze the IGF2BP2 protein level in OSCC cells (SCC-9, CAL-27). D Public database GEPIA (http://gepia.cancer-pku.cn/index.html) revealed the survival rate of OSCC with high/low IGF2BP2 expression. E Kaplan–Meier plotter (http://kmplot.com/analysis/) showed the survival rates of OSCC patients with high/low IGF2BP2 expression. *p < 0.05; **p < 0.01
IGF2BP2 promoted the migration, proliferation and Warburg effect (aerobic glycolysis) of OSCC
To investigate the function of IGF2BP2 in OSCC, IGF2BP2 overexpression or knockdown transfection was performed in the OSCC cells (SCC-9, CAL-27). The transfection efficiency was detected using RT-PCR and western blot (Fig. 2A, B). Transwell migration assay indicated that IGF2BP2 overexpression accelerated the migration of SCC-9 cells, and IGF2BP2 knockdown reduced the migration of CAL-27 cells (Fig. 2C). CCK-8 assays illustrated that IGF2BP2 overexpression promoted the proliferation of SCC-9 cells, and IGF2BP2 knockdown decreased the proliferation of CAL-27 cells (Fig. 2D). Glucose uptake analysis (Fig. 2E) and lactate production analysis (Fig. 2F) found that IGF2BP2 overexpression accelerated the glucose uptake and lactate production of SCC-9 cells, while IGF2BP2 knockdown reduced the glucose uptake and lactate production of CAL-27 cells. Extracellular acidification rate (ECAR) analysis displayed that IGF2BP2 overexpression accelerated the acidification rate of SCC-9 cells and IGF2BP2 knockdown weakened the acidification rate of CAL-27 cells (Fig. 2G). Overall, the above data indicated that IGF2BP2 promoted the migration, proliferation and aerobic glycolysis of OSCC.
IGF2BP2 promoted the migration, proliferation and Warburg effect (aerobic glycolysis) of OSCC. A IGF2BP2 overexpression or B knockdown transfection was performed in the OSCC cells (SCC-9, CAL-27). The transfection efficiency was detected using RT-PCR and western blot, respectively. C Transwell migration assay indicated the migration of SCC-9 cells of IGF2BP2 overexpression and IGF2BP2 knockdown of CAL-27 cells. D CCK-8 assays illustrated the proliferation of SCC-9 cells of IGF2BP2 overexpression and IGF2BP2 knockdown of CAL-27 cells. E Glucose uptake analysis and F lactate production analysis revealed the glucose uptake and lactate production of SCC-9 cells of IGF2BP2 overexpression and IGF2BP2 knockdown of CAL-27 cells. G Extracellular acidification rate (ECAR) analysis displayed the acidification rate of SCC-9 cells and CAL-27 cells. *p < 0.05; **p < 0.01
IGF2BP2 targeted HK2 mRNA via the m6A modification site
To discover the potential targets for IGF2BP2, we detected the expression levels of aerobic glycolysis markers (GLUT1, HK2, LDHA). Results indicated that HK2 showed significant overexpression upon IGF2BP2 up-regulation (Fig. 3A), and decreased levels upon IGF2BP2 silencing (Fig. 3B). In public database, HK2 expression was positively correlated to IGF2BP2 expression (Fig. 3C). Using SRAMP online tools (http://www.cuilab.cn/sramp), we found that there were several potential m6A sites in the HK2 genomic sequences (Fig. 3D). Therefore, we noticed that IGF2BP2 targeted HK2 mRNA via the m6A modification site.
IGF2BP2 targeted HK2 mRNA via the m6A modification site. A, B RT-PCR analysis indicates the expression levels of aerobic glycolysis markers (GLUT1, HK2, LDHA) in SCC-9 cells with IGF2BP2 up-regulation, as well as in CAL-27 cells with n IGF2BP2 silencing. C The interaction within HK2 expression and IGF2BP2 expression in an HNSC cohort in a public database (http://gepia.cancer-pku.cn/index.html). D SRAMP online tools (http://www.cuilab.cn/sramp) revealed that there were several potential m6A sites in HK2 genomic sequences. *p < 0.05; **p < 0.01
IGF2BP2 enhanced the stability of HK2 mRNA via an m6A-dependent manner
We found that HK2 acted as a target of IGF2BP2, and IGF2BP2 might target HK2 via m6A modification. Subsequently, the IGF2BP2 motif was identified (https://rna.sysu.edu.cn/rmbase/) (Fig. 4A). The potential m6A modification site on the HK2 genome was identified (GGACU). RIP-qPCR assay revealed that, compared with the control IgG, IGF2BP2 antibody-precipitated complexes enriched the expression of HK2 mRNA in OSCC (Fig. 4B). RNA stability assay indicated that IGF2BP2 overexpression up-regulated the enrichment of HK2 mRNA, while IGF2BP2 silencing decreased the HK2 mRNA enrichment (Fig. 4C, D). Western blot analysis found that IGF2BP2 overexpression up-regulated the enrichment of HK2 protein, while IGF2BP2 silencing decreased the HK2 protein enrichment (Fig. 4E, F). Overall, these data suggested that IGF2BP2 enhanced the stability of HK2 mRNA via an m6A-dependent manner.
IGF2BP2 enhanced the stability of HK2 mRNA via an m6A-dependent manner. A The IGF2BP2 motif was identified (https://rna.sysu.edu.cn/rmbase/). GGACU w as the potential m6A modification site on the HK2 genome. B RIP-qPCR assay revealed the IGF2BP2 antibody-precipitated HK2 mRNA expression in OSCC (SCC-9, CAL-27). C, D Shortened RNA lifetime of HK2 mRNA after IGF2BP2 overexpression (SCC-9) or knockdown (CAL-27). E, F Western blot analysis revealed the HK2 protein upon IGF2BP2 overexpression (SCC-9) or knockdown (CAL-27). *p < 0.05; **p < 0.01
IGF2BP2/HK2 promoted the aerobic glycolysis of OSCC
Given that HK2 acts as a pivotal element in aerobic glycolysis, we investigate the potential role of IGF2BP2/HK2 in OSCC aerobic glycolysis. Glucose uptake analysis (Fig. 5A) and lactate production analysis (Fig. 5B) found that HK2 overexpression promoted the glucose uptake and lactate production of OSCC cells (CAL-27 cells), then IGF2BP2 knockdown (sh-IGF2BP2-1#) reduced the glucose uptake and lactate production, respectively. Then, extracellular acidification rate (ECAR) analysis displayed that HK2 overexpression accelerated the acidification rate, while IGF2BP2 knockdown co-transfection weakened the acidification rate of CAL-27 cells (Fig. 5C). In vivo animal xenograft assay indicated that IGF2BP2 knockdown repressed the tumor growth (Fig. 5D, E). Overall, these data suggested that IGF2BP2/HK2 promoted the aerobic glycolysis of OSCC.
IGF2BP2/HK2 promoted the phenotype of OSCC. A Glucose uptake analysis and B lactate production analysis was detected in CAL27 cells transfected with HK2 overexpression plasmids or co-transfected with IGF2BP2 silencing (sh-IGF2BP2-1#). C Extracellular acidification rate (ECAR) analysis displayed the acidification rate in CAL27 cells transfected with HK2 overexpression and/or IGF2BP2 knockdown (sh-IGF2BP2-1#). D In vivo tumor weight and E volume was calculated in mice
Discussion
Accumulating evidence has continued to implicate m6A in the pathological characteristics of a wide array of human tumors, including OSCC (Huang et al. 2020; Xu et al. 2021). Besides, m6A highlights its potential as a therapeutic target. However, the molecular mechanism by which m6A reader IGF2BP2 influences OSCC remains largely unknown (Zhu et al. 2021; Ai et al. 2021). To explore the role of m6A regulatory reader enzymes IGF2BP2 in OSCC pathogenesis and uncover its regulatory mechanism, we performed a cluster of functional and mechanistic assays related to m6A (Fig. 6).
Increasing evidence suggests that various m6A key enzymes, such as IGF2BP1/2/3, METTL3 and ALKBH5, are implicated in a myriad of biological processes supporting cancer progression. For instance, METTL3 mediates the m6A modification of BMI1 mRNA in its 3′-UTR to promote BMI1 translation under the cooperation with IGF2BP1 (Liu et al. 2020). Moreover, depletion of demethylase fat mass and obesity-associated protein (FTO) attenuates the arecoline-induced chemoresistance of OSCC through negatively regulating transcription factor FOXA2 (Li et al. 2021b). Methyltransferase-like 14 (METTL14) is up-regulated in OSCC and promotes proliferation, migration, and invasiveness of OSCC cells, which directly combines with eukaryotic translation initiation factor gamma 1 (eIF4G1) mRNA and decreases its mRNA stability (Wang et al. 2021). Taken together, these data suggest that m6A is wildly involved in the tumorigenesis and progression of OSCC.
Given that OSCC is one of the most common malignancies worldwide and aerobic glycolysis acts as an essential factor in the tumorigenesis of OSCC, we designed and performed the current study to analyze the potential role of m6A on OSCC aerobic glycolysis. The present study analyzed the expression of IGF2BP2 in OSCC tissue samples and cells, indicating that IGF2BP2 level increased both in tissue samples and cells and correlated with the poor prognosis. Moreover, bio-functional assays suggested that IGF2BP2 promoted the migration, proliferation, and aerobic glycolysis of OSCC. These data illustrated that IGF2BP2 may act as an oncogene in the OSCC.
Although many risk factors have been reported to be involved in the tumorigenesis and progression of OSCC, the critical pathogenic factors for malignant tumor are closely correlated with abnormal energy metabolism. Aerobic glycolysis is an important energy-providing method for tumor physiological activities, such as differentiation and proliferation. Moreover, glycolysis provides abundant energy supply for OSCC and participates in malignancy progression. In the progression of aerobic glycolysis, key enzyme HK2 plays a critical role and numerous pathological processes are correlated with HK2.
Here, our research found that m6A reader IGF2BP2 directly combined with the m6A modification site on HK2 mRNA, thereby enhancing the RNA stability of HK2 mRNA. Then, the protein level of HK2 was also up-regulated upon IGF2BP2 overexpression and decreased upon IGF2BP2 silencing. In OSCC, HK2 has been identified as an oncogene. For example, HK2 is highly expressed in OSCC patient-derived tissues and cells, and the depletion of HK2 repressed the OSCC cell growth in vivo and in vitro (Li et al. 2020). Overall, our finding demonstrated that HK2 may serve as a potential target of OSCC tumorigenesis.
In conclusion, elucidating the molecular mechanism related to aerobic glycolysis of OSCC cells is essential for the development of accurate diagnostic and individualized therapeutic strategies, which may significantly improve the prevention of OSCC.
Data availability
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
References
Ai Y, Liu S, Luo H, Wu S, Wei H, Tang Z et al (2021) METTL3 intensifies the progress of oral squamous cell carcinoma via modulating the m6A amount of PRMT5 and PD-L1. J Immunol Res 2021:6149558. https://doi.org/10.1155/2021/6149558
Bienkowska KJ, Hanley CJ, Thomas GJ (2021) Cancer-associated fibroblasts in oral cancer: a current perspective on function and potential for therapeutic targeting. Front Oral Health 2:686337. https://doi.org/10.3389/froh.2021.686337
Chen Z, Zuo X, Zhang Y, Han G, Zhang L, Wu J, Wang X (2018) MiR-3662 suppresses hepatocellular carcinoma growth through inhibition of HIF-1α-mediated Warburg effect. Cell Death Dis 9:549. https://doi.org/10.1038/s41419-018-0616-8
Chen P, Hu J, Han X, Chen Y (2022) Advances in the functional roles of N6-methyladenosine modification in cancer progression: mechanisms and clinical implications. Mol Biol Rep. https://doi.org/10.1007/s11033-022-07126-5
Haldar A, Singh AK (2022) The association of long non-coding RNA in the prognosis of oral squamous cell carcinoma. Genes Genomics. https://doi.org/10.1007/s13258-021-01194-w
Huang GZ, Wu QQ, Zheng ZN, Shao TR, Chen YC, Zeng WS, Lv XZ (2020) M6A-related bioinformatics analysis reveals that HNRNPC facilitates progression of OSCC via EMT. Aging 12:11667–11684. https://doi.org/10.18632/aging.103333
Huang M, Xu S, Liu L, Zhang M, Guo J, Yuan Y et al (2021) m6A methylation regulates osteoblastic differentiation and bone remodeling. Front Cell Dev Biol 9:783322. https://doi.org/10.3389/fcell.2021.783322
Li M, Gao F, Zhao Q, Zuo H, Liu W, Li W (2020) Tanshinone IIA inhibits oral squamous cell carcinoma via reducing Akt-c-Myc signaling-mediated aerobic glycolysis. Cell Death Dis 11:381. https://doi.org/10.1038/s41419-020-2579-9
Li H, Xiao W, He Y, Wen Z, Cheng S, Zhang Y, Li Y (2021a) Novel insights into the multifaceted functions of RNA n(6)-methyladenosine modification in degenerative musculoskeletal diseases. Front Cell Dev Biol 9:766020. https://doi.org/10.3389/fcell.2021.766020
Li X, Xie X, Gu Y, Zhang J, Song J, Cheng X, Gao Y, Ai Y (2021b) Fat mass and obesity-associated protein regulates tumorigenesis of arecoline-promoted human oral carcinoma. Cancer Med 10:6402–6415. https://doi.org/10.1002/cam4.4188
Liu L, Wu Y, Li Q, Liang J, He Q, Zhao L et al (2020) METTL3 promotes tumorigenesis and metastasis through BMI1 m(6)A methylation in oral squamous cell carcinoma. Mol Therapy 28:2177–2190. https://doi.org/10.1016/j.ymthe.2020.06.024
Metsäniitty M, Hasnat S, Salo T, Salem A (2021) Oral microbiota—a new frontier in the pathogenesis and management of head and neck cancers. Cancers. https://doi.org/10.3390/cancers14010046
Mu H, Li H, Liu Y, Wang X, Mei Q, Xiang W (2022) N6-methyladenosine modifications in the female reproductive system: roles in gonad development and diseases. Int J Biol Sci 18:771–782. https://doi.org/10.7150/ijbs.66218
Relier S, Rivals E, David A (2022) The multifaceted functions of the fat mass and obesity-associated protein (FTO) in normal and cancer cells. RNA Biol 19:132–142. https://doi.org/10.1080/15476286.2021.2016203
Shahoumi LA (2021) Oral cancer stem cells: therapeutic implications and challenges. Front Oral Health 2:685236. https://doi.org/10.3389/froh.2021.685236
Tong H, Wei H, Smith AO, Huang J (2021) The role of m6A epigenetic modification in the treatment of colorectal cancer immune checkpoint inhibitors. Front Immunol 12:802049. https://doi.org/10.3389/fimmu.2021.802049
van der Kamp MF, Halmos GB, Guryev V, Horvatovich PL, Schuuring E, van der Laan B et al (2022) Age-specific oncogenic pathways in head and neck squamous cell carcinoma - are elderly a different subcategory? Cell Oncol (dordr). https://doi.org/10.1007/s13402-021-00655-4
Wang F, Zhu Y, Cai H, Liang J, Wang W, Liao Y et al (2021) N6-methyladenosine methyltransferase METTL14-mediated autophagy in malignant development of oral squamous cell carcinoma. Front Oncol 11:738406. https://doi.org/10.3389/fonc.2021.738406
Xu L, Li Q, Wang Y, Wang L, Guo Y, Yang R et al (2021) m(6)A methyltransferase METTL3 promotes oral squamous cell carcinoma progression through enhancement of IGF2BP2-mediated SLC7A11 mRNA stability. Am J Cancer Res 11:5282–5298
Zhu F, Yang T, Yao M, Shen T, Fang C (2021) HNRNPA2B1, as a m(6)A reader, promotes tumorigenesis and metastasis of oral squamous cell carcinoma. Front Oncol 11:716921. https://doi.org/10.3389/fonc.2021.716921
Funding
This study was supported by Cangzhou City Key Research and Development Project (Number: 204106118).
Author information
Authors and Affiliations
Contributions
KX is responsible for assays and funding. XD, JW and KW acted as assistants.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The present study was approved by the ethical review committee of the Cangzhou Central Hospital.
Patient consent for publications
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, K., Dai, X., Wu, J. et al. N6-methyladenosine (m6A) reader IGF2BP2 stabilizes HK2 stability to accelerate the Warburg effect of oral squamous cell carcinoma progression. J Cancer Res Clin Oncol 148, 3375–3384 (2022). https://doi.org/10.1007/s00432-022-04093-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04093-z