Abstract
Background
Patients suffering from cancer often make use of complementary and alternative medicine (CAM). Only few data exist on the prevalence and clinical significance of interactions of a biological CAM method and conventional drugs.
Methods
From February 2014 to March 2016, consecutive patients from five oncological practices in Germany were asked to fulfill a standardized questionnaire regarding use of CAM. Data on diagnosis, date of first diagnosis, ECOG and the past and current treatment were derived from the patients’ files. Interactions were evaluated by systematically using a database on potential interactions.
Results
From 1000 patients asked to participate, we received a total of 720 questionnaires of which 711 were completed and eligible for evaluation. 29% of the patients reported any CAM usage. Women showed a significantly higher use of CAM with 35.6 versus 23.6% of men. For 54.9% of CAM users (15.9% of all patients), we found a combination of conventional drugs and biological based CAM methods with a risk for interactions. Vitamins A, C and E were the most frequently used CAM substances in these cases (39.3%), followed by herbs with 17.5%.
Conclusion
There was a risk of interactions between a biological CAM method and conventional drugs in 54.9% of the patients using CAM. To raise knowledge on interactions a better training for doctors with respect to CAM is strongly needed. Furthermore, patients’ awareness should also be raised and communication between physician and patient on the topic improved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A majority of cancer patients use complementary and alternative medicine (CAM) (Berretta et al. 2016; Horneber et al. 2012; Huebner et al. 2014a, b). The National Center for Complementary and Integrative Health defines Complementary Medicine as non-mainstream practice used together with conventional medical treatment. In contrast, alternative medicine describes the use of non-mainstream medicine instead of conventional medicine (NCCIH 2016).
Complementary health approaches can be divided into several main categories as, for example, natural products (such as herbs, vitamins, minerals and probiotics), mind and body practices (including yoga, meditation, massage, acupuncture, etc.) and so-called holistic approaches (e.g., Traditional Chinese medicine, homeopathy, Ayurvedic medicine, etc.) (NCCIH 2016).
An average of 40% of cancer patients use CAM at least once during or after treatment (Horneber et al. 2012). A recent multicenter survey from Italy revealed a CAM user rate of 49% in cancer patients (Berretta et al. 2016). In addition, two studies from Germany showed a user rate of CAM by cancer patients between 50 and 60% (Huebner et al. 2014b; Wortmann et al. 2016) at a comprehensive cancer center. Especially breast cancer patients have a high user rate with up to 80% in the USA (Saxe et al. 2008) and 76% in a recent online survey in Germany (Huebner et al. 2014a, b).
Regarding disclosure to the physician, rates vary highly between 23 and 90% (Robinson and McGrail 2004).
Using complementary medicine may help the patients to get active by themselves (Huebner et al. 2014a, b). Besides this very positive effect, there are also risks. These result from side effects and interactions (Huebner et al. 2013). While naturopathy allegedly has only low risks, in cancer care there may be serious risks. Side effects may mimic side effects from cancer therapy (for example nausea, abdominal pain or diarrhea). If the attending physician knows nothing about the additional naturopathic therapy and assumes that the actual tumor therapy has too many side effects, cancer treatment could reduce or even stopped in the worst case. Missing communication skills may be one reason in this case (Hillen et al. 2014). In addition, even life-threatening side effects from various herbs and other traditional drugs have been described (Pourroy et al. 2017). Moreover, herb-anticancer drug interactions may increase side effects of the conventional therapy (Meijerman et al. 2006). Furthermore, interactions may also happen between CAM and supportive therapy or CAM and drugs for comorbidities (Pourroy et al. 2017).
So far, only few data exist on the prevalence and clinical significance of these interactions. In a survey with breast cancer patients, we found at least one-third of all patients on active cancer therapy running the risk of interactions. Of those choosing to use CAM substances in general three quarters were in danger of interactions (Zeller et al. 2013).
The aim of our study was to evaluate the risk of interactions in a more diverse sample of cancer patients to better inform physicians, nurses and pharmacists of the entailing risks and to sensitize professionals as well as patients to the topic.
Patients and methods
Questionnaire
We used a standardized questionnaire developed by experts of the working group Prevention and Integrative Oncology of the German Cancer Society which has been published before (Huebner et al. 2014a, b).
The questionnaire consisted of three main sections:
-
Demographic data and data on the tumor
-
Patient’s lifestyle and their opinion on the cause of their disease
-
Use of CAM, reasons for usage and the usage of different, most common CAM methods with the resulting satisfaction
This questionnaire was coupled with a part to be filled in by the oncologist concerning the diagnosis, date of first diagnosis, ECOG and the past and current treatment.
The questionnaire was pseudonymized and the patients were informed that the oncologist would not get any information concerning their answers. The patients returned the questionnaire in a closed envelope which was only opened by the study staff.
Patients
From February 2014 to March 2016, overall 1000 consecutive patients from five oncological practices in Germany were asked by the staff of the practice to participate. Inclusion criteria were age 18 years and older, being able to fill in the questionnaire, active cancer therapy (all types of cancer and all types of therapy).
Evaluation of interactions
We formerly have developed and published a method to evaluate risks for interactions (Zeller et al. 2013). This analysis is based on data derived from the website of the Memorial Sloan Kettering Cancer Center “about herbs” (“Integrative Medicine: Search About Herbs|Memorial Sloan Kettering Cancer Center” 2018), the compendium of Cassileth on herb-drug interactions (Cassileth and Lucarelli 2003) and the database DrugDigest (“Drug Information—Express Scripts®” 2018). The following risk categories were used:
-
1.
Interaction unlikely (no evidence in the literature regarding interaction)
-
2.
Interaction possible or likely
-
3.
No data
In the case of ambiguous data we decided to grade the interaction as possible.
Classified as potentially leading to interactions with the conventional treatment were medicinal herbs, healing plants, antioxidants unless when only treated with antibodies, mistletoe and medicinal mushrooms in combination with any drugs that may entail immunological reactions (antibodies, taxanes, oxaliplatin, etc.) and enzymes during treatment with bevacizumab (for potentially increased risk for bleeding).
Statistics
IBM SPSS Statistics 24 was used for data evaluation, analysis of frequencies and correlations using Chi-square tests; p < 0.05 was considered significant.
Ethical vote
The survey got a positive ethical vote from the Ethical Committee of the J.W. Goethe University (FF 55/2008). The patients signed a consent form. This includes information on the process of pseudonymisation and on the fact that the oncologist would not get to know their answers.
Results
Demographic data
From 1000 patients asked to participate, we received 720 questionnaires of which nine questionnaires were not eligible due to non-completion or the patient not suffering from cancer. In total 711 questionnaires were completed and eligible for evaluation. The demographic data and types of cancer are shown in Table 1. Of the participating patients, 47.7% were men and 47.0% were women. The mean age was 65.0 years, with the oldest participant being 88 and the youngest 23 years. Concerning participants highest level of education most patients went to a lower secondary school (66.9%), a fifth attended university (20.4%), 4.4% graduated at university entry level, and 2.8% did not get any education. Most of the patients were married (68.2%), 12.2% widowed and 4.4% had children under the age of 15 years.
Regarding the type of cancer, most common types were leukemia and lymphoma with 24.2% and colon cancer with 21.5%, followed by lung cancer with 17.2% and breast cancer with 14.91%. 38.4% of participants were diagnosed less, 30.9% more than a year and 21.1% less than a month ago. Nearly half of the patients (45.7%) had metastases. More than half (51.2%) had ECOG stage 0 and 36.6% stage 1. The vast majority of patients (94.0%) stated currently receiving cancer treatment. Chemotherapy was the most frequent treatment with 52.0%. Regarding the treatment situation, 32.2% were not previously treated, while 24.2% were operated on and 16.7% received an operation and chemotherapy. Information provided by the oncologists on treatment showed that 77.1% of the patients received a cytokine, 33.7% a platinum-based chemotherapy and 29.1% antibodies.
Psychological support was utilized by 8.2% of participants and only 0.8% participated in self-help group.
Complementary and alternative medicine (CAM)
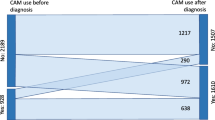
Only 13.9% of participants stated using CAM. Yet, 4.6% not having marked using CAM reported reasons for CAM usage and marked a CAM method when asked to mark methods in a standardized list of CAM methods in the questionnaire and another 10.5% marked only methods. Accordingly, a total of 29% of the patients reported any CAM usage in at least one of the three questions. The user rate when looking at the five different oncological institutions varied between 24.8 and 48.8%.
Women showed a higher use of CAM with 35.6 versus 23.6% of men. Patients with higher education used CAM significantly more often than those with lower (university level 44.6%, university entry level 35.5% versus lower secondary school level 24%; p = < 0.05). Age had an influence on CAM usage. Based on the type of cancer highest user rates were found in gynecological cancers (ovarian cancer 44.4% and other gynecological tumors 38.5%) and breast cancer (39.6%). Next highest rates were in patients with pancreatic (35.1%), esophageal and bile duct system cancer (33.3%).
Of those using CAM 35.9% informed their doctor. This was in about two-thirds the oncologist (68.9%) and/or the general practitioner (66.2%). About a quarter (25.7%) of all patients marked having a doctor or alternative practitioner competent in questions regarding CAM. In 14.8% this was the oncologist and in 13.1% the general practitioner. Patients having a physician with experience in CAM reported using CAM methods more often (51.4%) than those who did not have a CAM competent physician (21.8%) (p < 0.05).
As leading reasons for using CAM, patients listed wanting to support their immune system (12.8% of all patients), wanting to actively do something against their disease (9.6%), not wanting to miss a chance (9.4%), wanting to reduce the side effects of their treatment (8.2%) and wanting to minimize the risk of a relapse (7.0%) (Fig. 1).
CAM methods
From the list of CAM methods, the most often used non-biological based one were prayers (6.5% of all patients, 22.3% of those using CAM). For biological based methods, 11.8% of all participants stated using or having used Vitamin A, C or E (40.8% of those using CAM), 6.2% selenium other trace elements a (20.9%) and 5.1% herbs (17.5%) (Fig. 2).
Interactions
Of those declaring CAM usage most (82%) currently received a therapy with a cytostatic and 35.4% with antibodies. From all of the patients treated with cytostatics, 10.5% took vitamins and 6.0% used selenium and other trace elements. For those getting antibodies, the numbers were 14.1% for vitamins and 7.9% for trace elements. Considering type of prior treatment, the highest CAM user rate (50%) was found in patients having received a multimodal therapy with operation, radiation and systemic therapy. In about one-third of the patients after operation and systemic therapy (33.6%) or sole systemic therapy (31.4%) the use of CAM was indicated.
For 54.9% of CAM users (15.9% of all patients), we found a combination of conventional drugs and biological based CAM methods with a risk for interactions. Most of those used one CAM method (44.7%), 9.2% reported using two and 1% three. Vitamins A, C and E were the most frequently used CAM substances in these cases (39.3%), followed by healing plants with 17.5%. Patients reporting to have told their physician on their CAM usage were not less often at risk for interactions. Additionally, those patients having a physician competent in CAM had a higher rate of potential interactions (p = < 0.05).
Discussion
Only 29% of the patients participating in our study reported using any kind of CAM method and an even lower rate of 13.9% stated using CAM. This is much lower than those found in other studies, which show a CAM usage between 40 and 60% (Berretta et al. 2016; Huebner et al. 2014b; Wortmann et al. 2016). One explanation might be a limitation of our study, namely that patients had to return the closed envelope to the oncologist’s practice. It is conceivable that despite assurance of anonymity patients might have been afraid of disclosure to the physician. In a review of papers on disclosure, the rate varied between 23 and 90%. Leading reasons for non-disclosure were the expectation of a negative response from the practitioner, thinking that the physician did not need to know of the use as it was not relevant and not being asked about it (Robinson and McGrail 2004).
Accordingly, establishing a relationship built on trust and open communication between the patient and the physician is essential to achieve higher rates of disclosure and limit the risks of interactions (Hillen et al. 2014).
The discrepancy between participants declaring the use of CAM (13.9%) and those marking any kind of CAM (29%) shows that many do not know what is considered CAM and what effects and risks it may have. One reason for this might be that participants, who believed not to use any CAM, did not carefully read the explanation in the questionnaire since it only was given after the general question on CAM usage.
More than half of the patients using CAM (54.9%) stated using a combination with conventional cancer therapy potentially leading to interactions. This rate lies between the rate we found in studies with melanoma patients (Loquai et al. 2017) and with breast cancer patients (Zeller et al. 2013).
While 62.1% of patients disclosing their usage to a physician were at risk for interactions, only 45.1% of those doing not so were. This raises the question if this even higher risk rate points to the doctors not reacting accordingly, possibly not being aware of an interaction potential, or the patient’s being non-compliant to a warning. The fact that having a physician competent in CAM did not lower this risk gives a strong hint to the physicians underestimating the risk. In fact, competence from the point of the patient may go along with the physician being in favor of CAM, which might also be a sign for non-evidence-based advocating.
Limitations to our analysis derive from the lack of data on the patients’ comorbidities and supportive therapy not related to the oncologist’s therapy regimen since these could lead to more interactions (Loquai et al. 2016). Moreover, the results do not represent interactions which have been found clinically and we do not have any data on how many clinically relevant interactions have been observed as this was not explicitly asked in the questionnaire for physicians. Yet, the most important limitation is the non-existence of a reliable database on relevant clinical interactions with CAM. Most data are derived from preclinical studies and we had to revert to assumptions and hypotheses (Zeller et al. 2013). With increasing comorbidities due to the cancer treatment of elderly patients and the western lifestyle leading to growing numbers of obesity and diabetes the problem of interactions between conventional therapy and CAM will become more important. In addition a longer duration of cancer therapy in the future is to be expected because of cancer turning into a chronic disease (Loquai et al. 2016). In fact, there is no extensive evaluation of interactions between drugs for comorbidities and CAM.
The central conclusion of our study and other data is that there is an urgent need for a better training for health professionals regarding complementary and alternative medicine. The practitioners need to be properly educated on the potential interactions between CAM and the conventional therapy. Awareness of this topic should be the goal. This also includes a detailed anamnesis and education of the patients, because most patients underestimate the influence of vitamins or other over the counter drugs. To raise the knowledge on and awareness for CAM introducing evidence-based statements in CAM and especially pointing to interactions within national guidelines would be helpful. Furthermore, quality assured information on CAM should be offered for general practitioners and other physicians not specialized in oncology. Pharmacists could assist physicians who plan to recommend CAM by checking for interactions. Beyond that, institutions should only get certification for their courses in CAM when offering evidence-based teaching. Moreover, information booklets with evidence-based health information could help to inform patients.
To minimize potential risks, if there is of lacking evidence or doubt in a biological substance, a non-biological method should be recommended instead. Through which patients will continue to have the opportunity to become active themselves.
References
Berretta M, Della Pepa C, Tralongo P, Fulvi A, Martellotta F, Lleshi A et al (2016) Use of complementary and alternative medicine (CAM) in cancer patients: an Italian multicenter survey. Oncotarget 8(15):24401–24414
Cassileth BR, Lucarelli CD (2003) Herb–drug interactions in oncology. BC Decker, Inc, Hamilton
Complementary, Alternative, or Integrative Health: What’s In a Name? | NCCIH (2016) National center for complementary and integrative health. https://nccih.nih.gov/health/integrative-health. Accessed 30 Nov 2017
Drug Information—Express Scripts® (2018) https://www.express-scripts.com/medco/consumer/ehealth/druginfo/dlmain.jsp?WC=N. Accessed 5 Jan 2018
Hillen MA, de Haes HCJM., Stalpers LJA, Klinkenbijl JHG, Eddes EH, Butow PN et al (2014) How can communication by oncologists enhance patients’ trust? An experimental study. Ann Oncol 25(4):896–901
Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M (2012) How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther 11(3):187–203
Huebner J, Muenstedt K, Muecke R, Micke O, Stoll C, Kleeberg UR et al (2013) Counseling cancer patients on complementary and alternative medicine: background, theory, and implementation of nationwide counseling facilities. Strahlenther Onkol 189(8):613–617
Huebner J, Muenstedt K, Prott FJ, Stoll C, Micke O, Buentzel J et al (2014a) Online survey of patients with breast cancer on complementary and alternative medicine. Breast Care (Basel Switzerland) 9(1):60–63
Huebner J, Micke O, Muecke R, Buentzel J, Prott FJ, Kleeberg U et al (2014b) User rate of complementary and alternative medicine (CAM) of patients visiting a counseling facility for CAM of a German comprehensive cancer center. Anticancer Res 34(2):943–948
Integrative Medicine: Search About Herbs | Memorial Sloan Kettering Cancer Center (2018) https://www.mskcc.org/cancer-care/diagnosis-treatment/symptom-management/integrative-medicine/herbs/search. Accessed 5 Jan 2018
Loquai C, Dechent D, Garzarolli M, Kaatz M, Kaehler KC, Kurschat P et al (2016) Risk of interactions between complementary and alternative medicine and medication for comorbidities in patients with melanoma. Med Oncol 33(5):52
Loquai C, Schmidtmann I, Garzarolli M, Kaatz M, Kahler KC, Kurschat P et al (2017) Interactions from complementary and alternative medicine in patients with melanoma. Melanoma Res 27(3):238–242
Meijerman I, Beijnen JH, Schellens JHM (2006) Herb-drug interactions in oncology: focus on mechanisms of induction. Oncologist 11(7):742–752
Pourroy B, Letellier C, Helvig A, Chanet B, De Crozals F, Alessandra C (2017). Development of a rapid risk evaluation tool for herbs/drugs interactions in cancer patients: a multicentric experience in south of France. Eur J Cancer Care 26(6):e12752
Robinson A, McGrail MR (2004) Disclosure of CAM use to medical practitioners: a review of qualitative and quantitative studies. Complement Ther Med. https://doi.org/10.1016/j.ctim.2004.09.006
Saxe GA, Madlensky L, Kealey S, Wu DPH, Freeman KL, Pierce JP (2008) Disclosure to physicians of CAM use by breast cancer patients: findings from the Women’s Healthy Eating and Living Study. Integr Cancer Ther 7(3):122–129
Wortmann JK, Bremer A, Eich H, Wortmann HK, Schuster A, Fühner J et al (2016) Use of complementary and alternative medicine by patients with cancer: a cross-sectional study at different points of cancer care. Med Oncol 33(7):78
Zeller T, Muenstedt K, Stoll C, Schweder J, Senf B, Ruckhaeberle E et al (2013) Potential interactions of complementary and alternative medicine with cancer therapy in outpatients with gynecological cancer in a comprehensive cancer center. J Cancer Res Clin Oncol 139(3):357–365
Funding
There was no funding for this review and study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Firkins, R., Eisfeld, H., Keinki, C. et al. The use of complementary and alternative medicine by patients in routine care and the risk of interactions. J Cancer Res Clin Oncol 144, 551–557 (2018). https://doi.org/10.1007/s00432-018-2587-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2587-7