Abstract
Purpose
The purpose of this study is to determine the efficacy of adjuvant immunotherapy with autologous cytokine-induced killer (CIK) for postoperative patients with gastric cancer and to investigate the impacts of the predictors on the efficacy of CIK immunotherapy.
Patients and methods
Ninety-two gastric cancer patients who have accepted radical resection were enrolled. The CIK and control groups were established by 1:1 matching on their baseline. As prognosis indicators, preoperative blood cell counts, the neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) were analyzed, respectively. Statistical analyses were done using IBM SPSS Statistics ver.19.0.
Results
CIK treatment significantly prolonged disease-free survival (DFS) (p < 0.05) and there was a tendency of longer overall survival (OS) in the CIK group (p = 0.057). Preoperative NLR was an independent prognostic factor for DFS (p < 0.05). When patients were classified into low and high NLR groups using the cutoff value of 2.995, patients in the low NLR group had a better DFS (p < 0.05). Subset analysis showed that CIK immunotherapy significantly prolonged the DFS in the low NLR group (p = 0.017) but not in the high NLR group (p = 0.695) except that it did well clearly within 17 months. Compared to the low NLR group, lymphocyte decreased significantly, neutrophil increased steeply and white blood cell (WBC) elevated subsequently (p < 0.001in all cases) in the high NLR group.
Conclusion
Adjuvant immunotherapy with the CIK cells prolongs DFS in postoperative patients with gastric cancer and the preoperative NLR is an independent prognostic factor for DFS. Low NLR predicts significant benefits from the CIK immunotherapy while high NLR forebodes the requirement of more cycles of CIK treatment or other stronger immunotherapy to improve the survival rate of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is distinguished from other cancers by its high mortality and morbidity, and is considered a major public health threat worldwide (Miller et al. 2016; Chen et al. 2016). Surgical resection with adjuvant chemotherapy remains the key modality for gastric cancer (Takahashi et al. 2013). Many investigations have been conducted to improve the quality of life and extend the survival rate of patients (Lazăr et al. 2016; Goode and Smyth 2016; Joo et al. 2016). However, unfortunately, there are still limited clinical benefits due to high rate of tumor metastasis.
It is well known that immune system plays an important role in the control of carcinogenesis, tumor progression, and metastasis (Cole et al. 1985; Kiessling et al. 1999; Gabrilovich and Pisarev 2003; Wang and DuBois 2015). Immunosuppression is remarkably common in patients with cancer, which makes tumor incurable, easy to recur or metastasize. Reversing immunosuppression by adjuvant immunotherapy, such as immune response cells, may lead to improved survival rate of gastric cancer patients (Stroncek et al. 2010; Auphan-Anezin et al. 2013; Slaney et al. 2014; Jäkel et al. 2014; Schmeel et al. 2015; Lee et al. 2015). Combination the cytokine-induced killer (CIK)-based immunotherapy is one of the promising strategies that are widely demonstrated in cancers by many studies, including our previous data (Shi et al. 2012; Jäkel et al. 2014; Schmeel et al. 2015; Lee et al. 2015; Li et al. 2015). In short, the CIK-based immunotherapy can strengthen systemic antitumor immunity of patients by abundantly infusing the T cells after activation and/or specialization in vitro (Schmidt Wolf et al. 1991). It has been shown that combination of the CIK-based immunotherapy and postoperative chemotherapy can improve the survival rate of cancer patients, preventing recurrence and extending survival, presumably due to the ability of the processed immune cells to suppress residual tumor cells. In addition, the CIK-based immunotherapy is an available, promising and inexpensive method for cancer patients, and its safety and effectiveness are well verified in clinical practices in the past few decades. However, there are still many uncertainties about how to select the potential patients who may effectively benefit from such a treatment method, and about how to predict their prognosis.
Inflammation has been recognized to alter the balance between pro-and anti-tumor immunity, thereby preventing the immune system from rejecting malignant cells, and providing a tumor-friendly environment for progressive disease (Karin and Greten 2005; Ostrand-Rosenberg 2008). Reduced inflammation in the tumor microenvironment had suppressed tumor metastasis or progression (Bunt et al. 2007; Said and Theodorescu 2012). Moreover, there are evidences showing that neutrophils can promote metastasis in various tumor models (Kowanetz et al. 2010; Huh et al. 2010; Coffelt and de Visser 2014), while in clinical trials, tumor-infiltrating neutrophils, elevated blood neutrophils and elevated blood neutrophil/lymphocyte ratio (NLR) have been associated with poor clinical outcomes in several human cancers (Donskov 2013). Recently, inflammatory markers have been explored extensively to quantify the worse outcomes of gastric cancers in the clinical trials, such as blood cell counts, differential counts, C-reactive protein (CRP), NLR, and platelet/lymphocyte ratio (PLR) (Kim et al. 2015; Grenader et al. 2016; Wang et al. 2016). Among these markers, the preoperative NLR and PLR have been recognized as the most reliable prognosis indicators for gastric cancer. NLR can reflect immune status of the host, the host’s tumor load, and the advancement status of cancer. Elevated NLR level is associated with recurrence and poorer survival rate in gastric cancer, and it also indicates unfavorable changes of systemic immunity in the host.
Therefore, the purpose of this study was to first confirm that the CIK immunotherapy combining postoperative adjuvant chemotherapy can improve the prognosis of gastric cancer after curative surgery, and next to confirm that some of inflammatory markers are associated with the prognosis of these patients, especially, being able to predict the efficacy of the CIK treatment on patients. Our data showed that the CIK treatment significantly prolonged disease-free survival (DFS) and borderline significantly prolonged the overall survival (OS) duration of the patients. The NLR value is an independent predictor of DFS, and the low NLR value is associated with a better survival rate of patients. Subgroup analysis indicated that the low NLR group markedly benefited from the CIK treatment, but not the high NLR group. Notably, within 17 months (or, equivalently, several months after the patients have completed the CIK treatment), the additional immunotherapy undoubtedly delayed the progression of the patients in the high NLR group.
Materials and methods
Patients and treatments
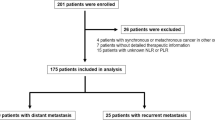
This is a retrospective study including all of the gastric patients who were admitted in Liaoning Cancer Hospital and Institute and accepted radical resection between January 2011 and December 2014. Patients who underwent the CIK immunotherapy combined with the regular adjuvant protocol after operation were defined as trial group. Then control group was established by 1:1 matching on their clinicopathological characteristics and completed chemotherapy among patients without the CIK treatment. All patients with distant metastasis or preoperative chemotherapy/irradiation were excluded. If the first CIK cell transfusion was started after DFS, the case was also excluded. There were no special selection criteria regarding whether the patients who would receive the CIK treatment. In our hospital, the CIK treatment has been approved by the institutional ethics committee, and we have obtained written consent from each patient.
A total of 92 patients were enrolled in this study; none of these patients was enrolled in any clinical trials before. All the data used in this study were from the electronic medical record system of our hospital.
All patients were followed up regularly until February 2016 or until death every 6 months for the first 2 years from surgery and then every year thereafter. The median follow-up period was of 24 months.
Treatments
The treatments conformed to the NCCN Clinical Practice Guidelines in Oncology. Patients received multidrug adjuvant chemotherapy based on 5-Fu within 1 month after surgery, except one patient with stage I. The patients in the immunotherapy group received the CIK treatment subsequently or simultaneously with adjuvant chemotherapy, unless recurrence was ascertained. The patients in the control group did not receive the CIK treatment. More than 5–10 × 109 CIK cells were transfused into patients via superficial vein at the day 15 of each cycle. The next cycle accomplished at the intervals of about 2–4 weeks until they no longer agreed to continue the treatment. The median duration of the CIK treatment was five cycles (range 1–15 cycles). When the patients in either the CIK group or the control group were diagnosed with recurrence, second-line chemotherapy or palliative surgery was performed.
CIK cells preparation
The CIK cells were prepared as described in previous studies (Shi et al. 2012; Lee et al. 2015; Li et al. 2015). To summarize the process briefly, 50 mL of heparinized peripheral blood was obtained from each eligible patient. Then, PBMCs (about 2 × 107 cells) were isolated by Ficoll-Hypaque density gradient centrifugation (LTS107705, Tianjin Haoyang Biological Manufacture CO. LDT, China). PBMCs were firstly incubated in 50 mL X-VIVO 15 serum-free medium (Lonza, Walkersville, MD, USA), containing 1000U/mL recombinant human interferon (IFN)-γ (PeproTech, Rocky Hill, NJ) and 5% inactivated autogeneic serum, at 37 °C with 5% CO2. After 24 h, 100 ng/mL anti-CD3 antibody to stimulate the CIK cell growth (e-Bioscience, Scan Diego, CA), 1000 U/mL recombinant human interleukin 2 (IL-2) (Shandong Quangang Pharmaceutical Co., Quangang, China) and 1 ng/mL recombinant human interleukin (IL)-1 alpha (PeproTech, Rocky Hill, NJ) were added. Then the fresh X-VIVO 15 serum-free media with 1000 U/mL IL-2 were continually supplemented to maintain a suitable density of cells in the culture system. The CIK cells were cultured for 14 days before being analyzed for phenotype and cytotoxicity and being used to treat patients. Safety testing was performed during the course of cell culture. All products were free of bacterial and fungal contamination, negative for mycoplasma, and contained <5 Euendotoxin. The viability of the CIK cells was above 90%.
Data collection of complete blood cell count
We collected the data obtained from blood routine examination within 1 week before the radical resection in our hospital, including white blood cell (WBC) counts, lymphocyte counts, lymphocyte percentage, neutrophil counts, neutrophil percentage, red blood cells (RBC) counts, hemoglobin contents, and platelet counts. The NLR was defined as the neutrophil count divided by the lymphocyte count. The PLR was defined as the platelet count divided by the lymphocyte count.
Statistical analysis
Differences in demographic and clinical characteristics between the two groups were evaluated using the t test, χ2 test, or the Fisher exact test as appropriate with an alpha less than 0.05. The cumulative survival rates were compared using the Kaplan–Meier method and the log-rank test. Furthermore, the Cox proportional hazards model for multivariate analysis was applied to establish the independent prognostic factors. Data were analyzed using IBM SPSS Statistics ver.19.0 (IBM Co., Armonk, NY, USA). Results with the p values less than 0.050 were considered as statistically significant.
Results
Baseline characteristics of the patients
The demographic and clinical characteristics are shown in Table 1. No significant differences were found between the CIK group and control group in terms of age, gender, differentiation, stage or unfavorable prognostic factors (p > 0.05). We further analyzed blood cell counts and differential counts as well as the neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR). All data are showed in Table 1 in terms of median, range, mean and standard deviation. There were no statistical significance between the CIK group and control group (p > 0.05).
Effects of the CIK immunotherapy on DFS and OS
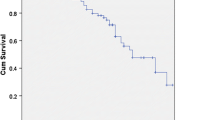
The DFS and OS curves between the CIK group and the control group are presented in Fig. 1, respectively. The patients in CIK group had a significantly better DFS and a tendency of better OS than the patients in the control group (log-rank, p = 0.044 for DFS and p = 0.057 for OS), respectively. The median DFS duration was 35.0 months in the control group and it has not been reached in the CIK group. The median OS duration has not been reached in both groups. The average DFS duration and the average OS duration were 34.0 months (95% CI, 25.2–43.6) and 48.3 months (95% CI, 41.3–55.3) in the control group versus 46.1 months (95% CI, 40.4–51.8) and 53.2 months (95% CI, 49.6–56.8) in the CIK group. Furthermore, compared with the patients in the control group, the patients in the CIK group had significantly reduced risk of recurrence (HR, 0.779; 95% CI, 0.211–0.998).
Multiple factor analysis of DFS
As shown in Table 2, the influence of each significant predictor was first identified by univariate analysis using Cox’s proportional hazards mode. Neither demographic nor clinicopathologic characteristics of the patients showed distinction between the CIK and control groups (p > 0.05). No significant differences have been observed between two groups in terms of the preoperative blood cell counts and differential counts (p > 0.05). It was the value of NLR not of PLR that markedly associated with poor prognosis in gastric cancer (p < 0.05). Furthermore, except neutrophil and lymphocyte, the items were assessed by multivariate analysis using Cox’s proportional hazards mode. Male patients with poorly differentiated cancer cells, with more unfavorable prognosis factors or with higher NLR had a worse DFS (p < 0.05). Noticeably, NLR was the only promising predictor for DFS of gastric cancer confirmed by both univariate analysis and multivariate analysis using Cox’s proportional hazards mode (p < 0.05).
Prognostic value of NLR on patients in the CIK and control groups
When exploratory subset analysis was performed, we used a receiver-operating characteristic (ROC) curve to determine an appropriate cutoff value of the NLR. The area under curve was 0.580. We selected the 2.995 as cutoff value. Then patients were classified into low NLR (NLR < 2.995) and high NLR (NLR > 2.995) groups. The numbers of patients in the low and high NLR groups were 74 and 18, respectively (Table 3). Age, gender, differentiation, stage, the numbers of unfavorable factors and whether or not added CIK immunotherapy were all balanced between the low and high NLR groups (p > 0.05). The DFS rates of the patients in the low NLR group were significantly higher than those in the high NLR group (p = 0.022) (Fig. 2a). Addition of immunotherapy to the regular postoperative adjuvant protocol significantly prolonged the DFS duration in the subset of patients with low NLR (p = 0.017) (Fig. 2b). Another subset of high NLR did not show any distinctions between the CIK and control groups (p = 0.695) (Fig. 2c). However, the KM curve of the CIK group was obviously higher than that of the control group within about 17 months. To confirm the value of NLR, we used the mean (NLR = 2.250) and the median (NLR = 1.960) as the cutoff value to classify the patients into low and high NLR groups, respectively. When groups were classified by the mean of NLR (Fig. 2b), we found that the CIK treatment also significantly increased DFS in the low NLR group (p = 0.036) and observed a similar form of the KM curves separating within 20 months in the high NLR group. When groups were classified by the median of NLR (Fig. 2c), the CIK treatment showed a similar prognostic trend but not statistical significant in the low and high NLR groups (p = 0.193 and p = 0.144, respectively).
Drifts in blood cell counts between the low and high NLR groups
To explain the findings, we determined the disparity between the blood cell counts in the low and high NLR groups. As shown in Table 4, compared to the data of the low NLR group, lymphocyte counts/percentage significantly decreased in the high NLR group (p < 0.001), while neutrophil counts/percentage more steeply increased (p < 0.001). Consequently, an elevated level of the WBC counts appeared in the high NLR group instead of maintaining equilibrium (p < 0.001). Inversely, the RBC counts, hemoglobin contents, and platelet counts did not show any drifts (p > 0.05).
Discussion
In this study, we assessed the efficacy of autologous CIK treatment adding to regular postoperative protocol in patients with gastric cancer after radical resection. In line with the previous reports (Shi et al. 2012; Jäkel et al. 2014; Schmeel et al. 2015; Lee et al. 2015; Li et al. 2015), the patients with the CIK immunotherapy significantly and borderline significantly benefited on DFS and OS, respectively. Our median follow-up period was of 24 months, which may be suitable for observation of DFS, but longer follow-up time is needed for OS. On the other hand, most of patients accomplished their CIK treatment plan within 12 months, and the median courses of the CIK treatment were five cycles (range: 1 ~ 15 cycles). Given the potential of the CIK immunotherapy against residual tumor cells (Schmidt Wolf et al. 1991; Shi et al. 2012; Jäkel et al. 2014; Schmeel et al. 2015; Lee et al. 2015; Li et al. 2015), it is conceivable that combination of the CIK and chemotherapy may prevent cancers from recurring or metastasizing. To date; however, there has been no way to tell which patients need more cycles or longer persistent period of immunotherapy that is enough to prevent tumor relapse or metastasis.
The preoperative blood routine examination has been acknowledged as a simple, applicable, and cost-effective approach to obtain some reliable prognostic indices for gastric cancer (Hwang et al. 2012; Kim et al. 2015; Grenader et al. 2016; Wang et al. 2016). In the previous research works, it was shown that leukocytosis, lymphocytopenia, neutrophilia, thrombocytosis, and anemia were all associated with worse prognosis of cancers (Pattison et al. 1987; Satomi et al. 1995; Ikeda et al. 2002; De Giorgi et al. 2012; Hattar et al. 2014; Takahashi et al. 2015). Recently, preoperative PLR and NLR values have been recognized as better predictors of recurrence, metastasis and survival in gastric cancer (Hwang et al. 2012; Kim et al. 2015; Grenader et al. 2016; Wang et al. 2016). In this study, we found that only NLR can be used as an independent risk factor for DFS in Cox regression analysis. Consistent with the results from other studies (Hwang et al. 2012; Kim et al. 2015; Grenader et al. 2016; Wang et al. 2016), patients with high NLR had a worse DFS than those with low NLR. In most of previous studies, Youden’s index calculated using the ROC curve was often used as the cutoff value of NLR. Kim et al. (2015) investigated a big sample including 1986 patients who underwent curative surgery for gastric cancer. The cutoff value of NLR for recurrence was set at 3. Likewise, in our study, Youden’s index of NLR values calculated using the ROC curve was 2.995. Keizman et al. (2012) evaluated the association of pre-treatment NLR with response rate, PFS and OS in 109 patients who were treated with sunitinib for mRCC. A low base-line blood NLR (≤3) was independently correlated with the response to sunitinib, and independently correlated with favorable PFS and OS. The optimized cutoff value may be different in various investigations (Hwang et al. 2012; Keizman et al. 2012; Donskov 2013; Kim et al. 2015; Grenader et al. 2016; Wang et al. 2016), but the conclusion was highly consistent; that is, the higher NLR was independent risk factor for recurrence, metastasis or survival in gastric cancer as well as in other cancers.
Furthermore, the analysis in the subgroup revealed the association of preoperative NLR and the CIK immunotherapy. In the low NLR group, the adjuvant CIK immunotherapy significantly conduced benefits on DFS. It is well known that a weakened immune system leads to cancers including gastric cancer (Cole and Humphrey 1985; Kiessling et al. 1999; Gabrilovich and Pisarev 2003; Karin and Greten 2005; Ostrand-Rosenberg 2008; Auphan-Anezin et al. 2013; Wang and DuBois 2015) and the lymphocyte response is known to be a major factor in the suppression of cancer progression (Cole and Humphrey 1985; Kiessling et al. 1999; Gabrilovich and Pisarev 2003; Whiteside 2006; Stroncek et al. 2010; Auphan-Anezin et al. 2013; Slaney et al. 2014; Wang and DuBois 2015). However, the level of lymphocyte in the patients with cancer frequently gets much lower than in healthy adults (Takahashi et al. 2015; Sun et al. 2016). And more serious impairment of immunity may be coming after surgery and adjuvant chemotherapy (Cole and Humphrey 1985; Kiessling et al. 1999; Shi et al. 2012). Therefore, the presumable explanation was that, at the right moment, the CIK immunotherapy properly replenished the activated and/or specialized T cells that the body needs. While, in the high NLR group, the CIK treatment seemed powerless for reversing immunosuppression of the host. Interestingly, the KM curves showed a meaningful shape, which indicated that the CIK treatment worked indubitably but transiently or inadequately. The results from three independent analyses were much similar, using the cutoff value of NLR set at Youden’s index, mean or median of NLR. The phenomenon in the high NLR group may be explained with the fact that there were unfavorable factors in immune system of the host. The high NLR reflects an enhanced neutrophil response to tumors (Ostrand-Rosenberg 2008; Donskov 2013; Coffelt and de Visser 2014; Kim et al. 2015; Grenader et al. 2016; Wang et al. 2016) except dysfunction of lymphocytes (Cole and Humphrey 1985; Kiessling et al. 1999; Gabrilovich and Pisarev 2003; Auphan-Anezin et al. 2013; Wang and DuBois 2015). Circulating neutrophils, a major source of circulating angiogenetic and growth factors (Kusumanto et al. 2003), are crucially involved in the process of tumor angiogenesis, and therefore, elevated blood neutrophils stimulate tumor and accelerate tumor growth and metastasis (Stockmann et al. 2014). Depending on the inflammatory mediators in various microenvironments of body, neutrophils can promote metastasis in situ or distant specific organs (Kowanetz et al. 2010; Huh et al. 2010; Coffelt and de Visser 2014). On the other hand, circulating neutrophils also inhibit cytotoxic activity of lymphocytes, natural killer cells and activated T cells (Petrie et al. 1985). In addition, the abnormal phenotype of the tumor may stimulate an influx of inflammatory cells into tissues around it, and the tissue destruction and disruption caused by the physical effects of the tumor may trigger a more generalized and nonspecific inflammatory response (Nagtegaal et al. 2001). In turn, the inflammation results in thrombocytosis, lymphocytopenia, neutrophilia, and leukocytosis (Pattison et al. 1987; Satomi et al. 1995; Ikeda et al. 2002; Hwang et al. 2012; De Giorgi et al. 2012; Hattar et al. 2014; Takahashi et al. 2015). Consistently, in this study, the WBC count increased along with the reduction of lymphocytes and the sharp augmentation of neutrophils in the high NLR group. In other words, the patients with high NLR got incurable, easy to recur and metastasize due to the multiple impairment of antitumor immunity in the body. Therefore, it may be feasible that we clinically select such patients according to their NLR value that need more cycles of the CIK treatment or more powerful immunotherapy.
There could be some limitations in this study. First, this is a retrospective study conducted at a single institution. In fact, most patients actually made medical decisions of the CIK immunotherapy according to their own wishes and financial situations, which could be a second limitation of this study without a standard regimen of immunotherapy. Therefore, larger and well-designed studies are needed to confirm our findings.
In short, the CIK immunotherapy is a safe, effective, inexpensive and available method for average patients to prevent advancement of gastric cancer and extend survival duration by reversing immunosuppression and strengthening antitumor immunity of the body. Therefore, this strategy is worthy of more investigation to identify valid predictors, by which we can select potential susceptive patients and provide better treatment plan.
References
Auphan-Anezin N, Verdeil G, Grange M, Soudja SM, Wehbe M, Buferne M, Mas A, Schmitt-Verhulst AM (2013) Immunosuppression in inflammatory melanoma: can it be resisted by adoptively transferred T cells? Pigment Cell Melanoma Res 26(2):167–75
Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S (2007) Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res 67(20):10019–26
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015.CA Cancer J Clin 66(2):115–32
Coffelt SB, de Visser KE (2014) Cancer: inflammation lights the way to metastasis. Nature 507(7490):48–9
Cole WH, Humphrey L (1985) Need for immunologic stimulators during immunosuppression produced by major cancer surgery. Ann Surg 202(1):9–20
De Giorgi U, Mego M, Scarpi E, Giuliano M, Giordano A, Reuben JM, Valero V, Ueno NT, Hortobagyi GN, Cristofanilli M (2012) Relationship between lymphocytopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer. Clin Breast Cancer 12(4):264–269
Donskov F (2013) Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol 23(3):200–207
Gabrilovich D, Pisarev V (2003) Tumor escape from immune response mechanisms and targets of activity. Current Drug Targets 4(7):525–36
Goode EF, Smyth EC (2016) Immunotherapy for gastroesophageal cancer. J Clin Med 5(10):pii: E84
Grenader T, Waddell T, Peckitt C, Oates J, Starling N, Cunningham D, Bridgewater J (2016) Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol 27(4):687–692
Hattar K, Franz K, Ludwig M et al (2014) Interactions between neutrophils and non-small cell lung cancer cells: enhancement of tumor proliferation and inflammatory mediator synthesis. Cancer Immunol Immunother 63(12):1297–1306
Huh SJ, Liang S, Sharma A, Dong C, Robertson GP (2010) Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res 70(14):6071–82
Hwang SG, Kim KM, Cheong JH, Kim HI, An JY, Hyung WJ, Noh SH (2012) Impact of pretreatment thrombocytosis on blood-borne metastasis and prognosis of gastric cancer. Eur J Surg Oncol 38(7):562–567
Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, Tatsuta M, Satomi T (2002) Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol 9(3):287–291
Jäkel CE, Vogt A, Gonzalez-Carmona MA, Schmidt-Wolf IG (2014) Clinical studies applying cytokine-induced killer cells for the treatment of gastrointestinal tumors. J Immunol Res 2014:897214
Joo MK, Park JJ, Chun HJ (2016) Recent updates of precision therapy for gastric cancer: towards optimal tailored management. World J Gastroenterol 22(19):4638–50
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5(10):749–59
Keizman D, Ish-Shalom M, Huang P, Eisenberger MA, Pili R, Hammers H, Carducci MA (2012) The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer 48(2):202–8
Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjöberg J, Pisa P, Petersson M (1999) Tumor-induced immune dysfunction. Cancer Immunol Immunother 48(7):353–62
Kim EY, Lee JW, Yoo HM, Park CH, Song KY (2015) The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric cancer? Ann Surg Oncol 22(13):4363–4370
Kowanetz M, Wu X, Lee J et al (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+granulocytes. Proc Natl Acad Sci USA 107(50):21248–55
Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH (2003) Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 6(4):283–287
Lazăr DC, Tăban S, Cornianu M, Faur A, Goldiş A (2016) New advances in targeted gastric cancer treatment. World J Gastroenterol 22(30):6776–99
Lee JH, Lee JH, Lim YS et al (2015) Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 148(7):1383–91.e6
Li Y, Jin A, Chen S, Song C, Zhang G (2015) Efficacy of adjuvant chemotherapy combined with CIK cell immunotherapy in 130 patients with postoperative colorectal cancer [in Chinese]. J Chin Oncol 21(10):843–847
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A (2016) Cancer treatment and survivorship statistics. CA Cancer J Clin 66(4):271–89
Nagtegaal ID, Marijnen CA, Kranenbarg EK et al (2001) Local and distant recurrences in rectal cancer patients are predicted by the nonspecificimmune response; specific immune response has only a systemic effect–a histopathological and immunohistochemical study. BMC Cancer 1:7
Ostrand-Rosenberg S (2008) Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev 18(1):11–8
Pattison CW, Woods KL, Morrison JM (1987) Lymphocytopenia as an independent predictor of early recurrence in breast cancer. Br J Cancer 55(1):75–6
Petrie HT, Klassen LW, Kay HD (1985) Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol 134(1):230–4
Said N, Theodorescu D (2012) RhoGDI2 suppresses bladder cancer metastasis via reduction of inflammation in the tumor microenvironment. Oncoimmunology 1(7):1175–1177
Satomi A, Murakami S, Ishida K, Mastuki M, Hashimoto T, Sonoda M (1995) Significance of increased neutrophils in patients with advanced colorectal cancer. Acta Oncol 34(1):69–73
Schmeel LC, Schmeel FC, Coch C, Schmidt-Wolf IG (2015) Cytokine-induced killer (CIK) cells in cancer immunotherapy: report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol 141(5):839–849
SchmidtWolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL (1991) Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med 174(1):139–49
Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J, Wu C (2012) Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother 61(12):2251–2259
Slaney CY, Kershaw MH, Darcy PK (2014) Trafficking of T cells into tumors. Cancer Res 74(24):7168–74
Stockmann C, Schadendorf D, Klose R, Helfrich I (2014) The impact of the immune system on tumor: angiogenesis and vascular remodeling. Front Oncol 4:69
Stroncek D, Berlyne D, Fox B et al (2010) Developments in clinical cell therapy. Cytotherapy 12(3):425–8
Sun S, Li X, Yu Z, Xu M, Liao Y, Zhang G (2016) The differences of the blood indicators between the healthy people and patients with lung cancer. J Mod Oncol ISSN 1672–4992
Takahashi T, Saikawa Y, Kitagawa Y (2013) Gastric cancer: current status of diagnosis and treatment. Cancers (Basel) 5(1):48–63
Takahashi R, Mabuchi S, Kawano M et al (2015) Prognostic significance of systemic neutrophil and leukocyte alterations in surgically treated endometrial cancer patients: a monoinstitutional study. Gynecol Oncol 137(1):112–118
Wang D, DuBois RN (2015) Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 36(10):1085–93
Wang SC, Chou JF, Strong VE, Brennan MF, Capanu M, Coit DG (2016) Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specific survival in resectable gastroesophageal junction and gastric adenocarcinoma. Ann Surg 263(2):292–297
Whiteside TL (2006) The role of immune cells in the tumor microenvironment. Cancer Treat Res 130:103–124
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
This is a retrospective study. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Li, Y., Wang, C., Xu, M. et al. Preoperative NLR for predicting survival rate after radical resection combined with adjuvant immunotherapy with CIK and postoperative chemotherapy in gastric cancer. J Cancer Res Clin Oncol 143, 861–871 (2017). https://doi.org/10.1007/s00432-016-2330-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2330-1