Abstract
Accurate predictors of survival for patients with advanced gastric cancer treated with neoadjuvant chemotherapy are currently lacking. In this study, we aimed to evaluate the prognostic significance of the neutrophil–lymphocyte ratio (NLR) in patients with stage III–IV gastric cancer who received neoadjuvant chemotherapy FOLFOX 4 as neoadjuvant chemotherapy. We enrolled 70 patients with stage III–IV cancer stomach in this study. Patients received FOLFOX 4 as neoadjuvant chemotherapy. Blood sample was collected before chemotherapy. The NLR was divided into two groups: high (>3) and low (≤3). Univariate analysis on progression-free survival (PFS) and overall survival (OS) was performed using the Kaplan–Meier and log-rank tests, and multivariate analysis was conducted using the Cox proportional hazards regression model. The toxicity was evaluated according to National Cancer Institute Common Toxicity Criteria. The univariate analysis showed that PFS and OS were both worse for patients with high NLR than for those with low NLR before chemotherapy (median PFS 28 and 44 months, respectively, P = 0.001; median OS 30 and 48 months, P = 0.001). Multivariate analysis showed that NLRs before chemotherapy were independent prognostic factors of OS but not for progression-free survival. NLR may serve as a potential biomarker for survival prognosis in patients with stage III–IV gastric cancer receiving neoadjuvant chemotherapy. The FOLFOX 4 demonstrated an acceptable toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most common malignancies and the second leading cause of cancer-related death in the world [1, 2]. Patients with localized disease have a higher 5-year survival rate (82 %) compared to patients with regional (24 %) or distant metastases (3 %) [2]. Chemotherapy in advanced gastric cancer is an important issue because the majority of patients with gastric cancer develop metastases during the course of their disease. Unresectable gastric disease diagnosed in more than two-thirds of patients [3]. Therefore, systemic cytotoxic chemotherapy is a major therapeutic strategy for advanced gastric carcinoma. The combination of third-generation chemotherapeutic agents including paclitaxel, docetaxel, and oxaliplatin with 5-fluorouracil has improved therapeutic response rates and overall survival (OS) by approximately 20–30 % and 4–6 months, respectively. However, this treatment can result in clinically significant adverse effects. In order to improve survival outcome and administer the most effective treatment, there is a requirement for more sensitive tumor markers than those currently available [3]. Recently, novel immunological and histological biomarkers have been identified [4, 5]. However, these largely depend on specimens obtained after resection of the primary tumor, and this limits their use in clinical practice prior to surgery.
Progression of cancer depended on systemic inflammatory response [6, 7]. Borsig et al. [7] reported that the ability of a tumor to invade and metastasize was dependent on the intrinsic characteristics of the tumor cells, as well as the tumor microenvironment. Peripheral blood tests at the time of diagnosis and treatment can reflect inflammatory conditions within the tumor. Evaluation of peripheral blood parameters including C-reactive protein (CRP), leukocytes, neutrophil, lymphocyte, monocyte, and platelet counts, as well as neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR), has been proposed as prognostic factors for patients with various types of malignancies.
In patients with advanced gastric cancer, high preoperative NLR has been identified as a useful and convenient predictor of survival [8–12],
The aim of the present study was to evaluate the prognostic significance of pre-chemotherapy NLR in peripheral blood samples from patients with stage III–IV gastric cancer receiving neoadjuvant chemotherapy.
Patient selection
Seventy patients with histopathologically confirmed advanced unresectable gastric adenocarcinoma (stages III–IV). Tumors were staged according to the criteria of the American Joint Committee on Cancer (AJCC) TNM stage classification, seventh edition for gastric cancer [13]. From July 2010 to July 2014 in Tanta University Hospital patients with stage III–IV gastric cancer were studied prospectively. Eligibility for the study required patients to have an Eastern Cooperative Oncology Group performance status of 0–2, and available plasma and measurable tumor focus evaluated by multi-detector spiral CT scanning.
Treatment protocols and dose modification
On day 1, oxaliplatin (85 mg/m2) was administered by intravenous infusion in 500 ml of n dextrose 5 % over a period of 2 h. On day 1 and 2, leucovorin (200 mg/m2) was administered as intravenous infusion in 500 ml of normal saline over 2 h, immediately followed by 5-FU (400 mg/m2) given as a 10-min i.v. bolus, followed by 5-FU (600 mg/m2) as a continuous 22-h infusion, with a light shield every 2 weeks.
Dose modifications of oxaliplatin or 5-FU were made for hematologic, gastrointestinal, or neurological toxic effects based on the most severe grade of toxicity that had occurred during the previous cycle. Treatment could be delayed for up to 2 weeks if symptomatic toxicity persisted or if the absolute number of neutrophil was <1,500/μl or platelets count was <100,000/μl. The dosage of 5-FU was reduced by 25 % for subsequent courses after the occurrence reduced by 25 % for subsequent courses after the occurrence of National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade 3 diarrhea, stomatitis, or dermatitis. The dose of oxaliplatin was reduced by 25 % in subsequent cycles if there were persistent paresthesias between cycles or paresthesias with functional impairment lasting >7 days. Treatment was continued until there were signs of disease progression, development of unacceptable toxic effects, or the patient refused further treatment.
Before each treatment courses, a physical examination, routine hematology, biochemistry, and chest X-ray were carried out. Computed tomography scans to define the extent of the disease, and the responses were carried out after four cycles of chemotherapy, or sooner if there was evidence of any clinical deterioration. Patients were assessed before initiating each 2-week cycle using the NCI-CTC [14], except in the case of neurotoxicity. For the neurotoxicity, an oxaliplatin-specific scale [15] was used: grade 1, paresthesias or dysesthesias of short duration, but resolving before the next dosing; grade 2, paresthesias persisting between doses (2 weeks); and grade 3, paresthesias interfering with function.
In accordance with the RECIST guidelines [16], response to therapy was categorized into four groups: complete response (CR), partial response (PR), stable disease (SD), and progression of disease (PD), with CR and PR confirmed for 4 weeks.
For tumor response assessment, objective responses after three cycles of treatment were evaluated on the basis of computed tomography (CT) scans.
Blood sample analysis
Venous blood samples were taken from patients admitted to the oncology outpatient clinic before neoadjuvant FOLFOX 4.
WBC differential counts were analyzed by XE-2100 hematology analyzer (Sysmex, Kobe, Japan), and CEA were evaluated by Architect i2000 (Abbott Laboratories, USA). The NLR was calculated from the differential count by dividing the neutrophil measurement by the lymphocyte measurement. An NLR 3 was considered as elevated.
Statistical analysis
The progression-free survival (PFS) and OS were calculated from the date of initiation of therapy to the date of disease progression and death, respectively. The association of each marker with survival was analyzed using Kaplan–Meier plots, the log-rank test, and its associated 95 % confidence interval (CI) was calculated [17].
Multivariate analyses were carried out using the Cox proportional hazards model. Variables with P < 0.05 on univariate analysis were entered into multivariate analyses. All the tests were two-sided, and P < 0.05 was considered statistically significant. Analyses were done using SPSS version 21.0 (SPSS Inc, Chicago, IL).
Patient characteristics
Table (1) shows the characteristics of the 70 patients: 47 were male and 23 were female, with a median age of 53 years (range 30–70 years). The median number of chemotherapy cycles was three (range 1–5). All 70 patients underwent gastrectomy; 41 (58.6 %) underwent total gastrectomy; and 19 (41.4 %) underwent subtotal gastrectomy. Clinical TNM (tumor, node, and metastasis) classification based on the AJCC staging was as follows: 49 (70 %) patients had stage III disease and 21 (30 %) had stage IV disease. Thirty-nine patients were adenocarcinoma. Thirty-five patients were poorly differentiated. Only 23 patients had CEA more than 5 ng/ml.
Blood parameters
The median pre-chemotherapy white blood cell, neutrophil, and lymphocyte counts were 6,400, 3,900, and 1,550 per mm3, respectively. The median pre-chemotherapy NLR was 2.74 (range 1–6.5). An NLR value of 3 was used as the cutoff value to classify patients into high (>3) or low (≤3) NLR groups.
The overall response was 37.1 % with complete response in four (5.7 %) patients and partial response in 22 (31.4 %) patients (Table 2).
Prognostic variables for PFS and OS
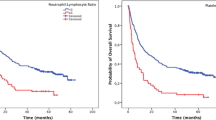
For the 70 patients, the median PFS was 30 months, and the median OS was 36 months (Fig. 1, 2). Factors predicting improved PFS were R0 resection, overall response, and preoperative NLR 3 or less (Table 3). Multivariate analysis identified overall response with hazard ratio 2.876 (95 % CI 1.228–6.737), p value = 0.015 as independent factors associated with worse PFS (Table 4), but NLR lost independent prognostic value.
As regards overall survival, factors predicting high OS were R0 resection, lower N stage, overall response, preoperative NLR 3 or less. Upon multivariate analysis; the factors with statistical significance with OS were R0 resection (P value = 0.038; 0.450; CI 0.212–0.957), overall response (P value = 0.041; 2.845; CI 1.043–6.762) and NLR (P value = 0.027; 3.259; CI 1.144–9.282) (Table 3). Median PFS and median OS were worse for patients with high NLR values than for those with low NLR values before chemotherapy (median PFS 28 and 44 months, respectively, P = 0.001; median OS 30 and 48 months, P = 0.001) (Figs. 3, 4).
The most common toxicities were hematologic. The National Cancer Institute Common Toxicity Criteria grade 3 and 4 neutropenia, leucopenia, anemia, and thrombocytopenia were recorded in 25/70 (35.7 %), 13/70 (18.6 %), three out of 70 (4.3 %), and 7 out of 70 (10 %) patients, respectively. Two out of 70 patients experienced febrile neutropenia. No NCI-CTC grade 4 gastrointestinal toxicity was observed, while grade 3 diarrhea, nausea, and vomiting were recorded in 4.3, 4.3, and 2.8 % of the patients, respectively. Neurotoxicity was moderate and was observed in 30 % (grade 1 & 2 in 25.7 % and grade 3 in 4.3 %) of the patients (Table 5).
Discussion
Gastric cancer is the most common cause of cancer-related death after lung cancer. Multimodal therapy, including radiotherapy, adjuvant chemotherapy, and targeted therapy, has greatly improved the survival of patients with advanced gastric cancer. Neoadjuvant chemotherapy is well established [18].
The FOLFOX regimen has oxaliplatin combined with calcium folinate and fluorouracil. Since 2001, the FOLFOX program had become one of the most common treatments for advanced gastric cancer. Ji and colleagues [19] treated 15 patients with advanced (IIIB or IV TNM staging) gastric cancer who received oxaliplatin-based combination chemotherapy (OXA 130 mg/m2 d1, 5-FU 400 mg/m2, 5-FU 2.5 g/m2 continuous infusion, LV 200 mg/m2 d1, q3 W); they found that seven cases had partial remission (46.7 %) and six had stable disease (40 %). Therefore, this chemotherapy was well tolerated by all patients who received it.
Yan et al. [20] treated 96 patients with locally advanced or metastatic cancer stomach with FOLFOX 4 regimen: oxaliplatin 85 mg/m2 iv in 2 h on D1, leucovorin 200 mg/m2 iv in 2 h on D1 and D2, 5-Fu 400 mg/m2 iv on D1 and D2, and then continuous infusion of it at a dose of 600 mg/m2 for 44 h; the overall response was 40 % which was nearly equal to that reported by us 37.1 %. This was also constant with that reported by many authors [21–23].
However, in order to select the need of neoadjuvant chemotherapy, accurate predictors that identify those patients who are more likely to benefit from neoadjuvant chemotherapy are needed.
However, to our knowledge, the prognostic significance of NLR in patients with advanced gastric cancer receiving neoadjuvant chemotherapy has rarely been studied. We analyzed the relationship between pre-chemotherapy and survival in patients with stage III–IV gastric cancer.
Our results showed that high pre-chemotherapy NLR independently predicted worse PFS and OS in patients with stage III–IV gastric cancer receiving neoadjuvant chemotherapy. The univariate analysis showed that PFS and OS were both worse for patients with high NLR than for those with low NLR before chemotherapy (median PFS 28 and 44 months, respectively, P = 0.001; median OS 30 and 48 months, P = 0.001) and this is constant with other authors [24–26].
Although high pre-chemotherapy NLR lost its independent prognostic significance for PFS, but retained it with OS in multivariate analysis, it still provided important information on NLR for clinical practice and this was different from that reported by Lee et al. [24] who reported statistical significance of NLR with PFS but not with OS in multivariate analysis.
The association between elevated NLR and poor survival in patients with various types of cancer has not been clearly defined until now. It is possible that pre-treatment neutrophil and lymphocyte numbers indicate the level of inflammation within the tumor and thus predict prognosis.
FOLFOX regimen used in this study demonstrated an acceptable tolerability. Grade 3/4 neutropenia was the most common hematologic toxicity occurring in 35.7 % of the patients, but febrile neutropenia was detected in only 2.9 % of the patients.
In a number of trials with oxaliplatin-based therapies, neurotoxicity was the most frequent side effect that led to treatment discontinuation. However, in our study, neurotoxicity was restricted to a limited number of patients (4.3 %). The FOLFOX regimen was tolerable with mild toxicity as reported by many authors [27, 28].
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9.
Carlomagno C, Matano E, Bianco R, Cimminiello C, Prudente A, Pagliarulo C, et al. Adjuvant FOLFOX-4 in patients with radically resected gastric cancer: tolerability and prognostic factors. Exp Ther Med. 2010;1:611–7.
Shirai O, Ohmiya N, Taguchi A, Nakamura M, Kawashima H, Miyahara R, et al. P53, P21 and P73 gene polymorphisms in gastric carcinoma. Hepatogastroenterology. 2010;11:1595–601.
Kim KH, Kwon HC, Oh SY, Kim SH, Lee S, Kwon KA, et al. Clinicopathological significance of ERCC1, thymidylate synthase and glutathione S-transferase P1 expression for advanced gastric cancer patients receiving adjuvant 5-Fu and cisplatin chemotherapy. Biomarkers. 2011;11:74–82.
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumors, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–71.
Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis–tracing the accessory. Oncogene. 2014;33(25):3217–24.
Hirashima M, Higuchi S, Sakamoto K, Nishiyama T, Okada H. The ratio of neutrophils to lymphocytes and the phenotypes of neutrophils in patients with early gastric cancer. J Cancer Res Clin Oncol. 1998;11:329–34.
Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;11:215–20.
Shimada H, Tajiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;11:170–6.
Aliustaoglu M, Bilici A, Ustaalioglu BB, Konya V, Gucun M, Seker M, et al. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol. 2010;11:1060–5.
Jung MR, Park YK, Jeong O, Seon JW, Ryu SY, Kim DY, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;11:504–10.
Ha TK, Kim HJ, Kwon SJ. Does the new UICC/AJCC TNM staging system (7th Edition) improve assessing prognosis in gastric cancer compared to the old system (6th Edition)? J Korean Gastric Cancer Assoc. 2009;9:159–66.
National Cancer Institute NIoH. US department of health and human services common terminology criteria for adverse events CTCAE, version 4. Washington DC: National Cancer Institute; 2009.
Caussanel JP, Lévi F, Brienza S, Misset JL, Itzhaki M, Adam R, et al. Phase I trial of 5-day continuous venous infusion of oxaliplatin at circadian rhythm modulated rate compared with constant rate. J Natl Cancer Inst. 1990;82:1046–50.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RESIST guideline (version 1.1). Eur J Cancer. 2009;11:228–47.
Bland JM, Altman DG. The log rank test. BMJ. 2008;328(7447):1073.
Schuhmacher C, Reim D, Novotny A. Neoadjuvant treatment for gastric cancer. J Gastric Cancer. 2013;13(2):73–8.
Ji JF, Yu Z, Zhong XN, Wu XJ, Wu QZ, Bu D, et al. Oxaliplatin-based regimen as neoadjuvant chemotherapy for Chinese patients with advanced gastric cancer: preliminary results of a phase II study. J Clin Oncol. 2004;22(14S):4184.
Yan D, Dai H. FOLFOX regimen in the patients with locally advanced or metastatic gastric cancer. Zhonghua Zhong Liu Za Zhi. 2009;31(3):217–9.
De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005;92(9):1644–9.
Oh SY, Kwon HC, Seo BG, Kim SH, Kim JS, Kim HJ. A phase II study of oxaliplatin with low dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) as first line therapy for patients with advanced gastric cancer. Acta Oncol. 2007;46(3):336–41.
Zhu X, Leaw J, Gu W, Qian Y, Du H, Wang B, et al. Phase II clinical trial of advanced and metastatic gastric cancer based on continuous infusion of 5-fluorouracil combined with epirubicin and oxaliplatin. J Cancer Res Clin Oncol. 2008;134(9):929–36.
Lee S, Oh SY, Kim SH, Lee JH, Kim MC, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;22(13):350.
Jin H, Zhang G, Liu X, Liu X, Chen C, Yu H, et al. Blood neutrophil-lymphocyte ratio predicts survival for stages III–IV gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol. 2013;24(11):112.
Yuan D, Zhu K, Li K, Yan R, Jia Y, Dang C, Yuan D. The preoperative neutrophil-lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. J Surg Oncol. 2014;110(3):333–40.
Seo HY, Kim DS, Choi YS, Sung HJ, Park KH, Choi IK, et al. Treatment outcomes of oxaliplatin, 5-FU, and leucovorin as salvage therapy for patients with advanced or metastatic gastric cancer: a retrospective analysis. Cancer Chemother Pharmacol. 2009;63(3):433–9.
Kim YJ, Goh PG, Kim ES, Lee SY, Moon HS, Lee ES, et al. Comparison of the toxicities and efficacies of the combination chemotherapy regimens in advanced gastric cancer patients who achieved complete response after chemotherapy. Korean J Gastroenterol. 2011;58(6):311–7.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
el Aziz, L.M.A. Blood neutrophil–lymphocyte ratio predicts survival in locally advanced cancer stomach treated with neoadjuvant chemotherapy FOLFOX 4. Med Oncol 31, 311 (2014). https://doi.org/10.1007/s12032-014-0311-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0311-2