Abstract
Purpose
To establish whether women over 65 years of age with newly diagnosed with breast cancer (BC) receive adjuvant chemotherapy less frequently than younger postmenopausal women and whether comorbidity influences this potential undertreatment.
Materials and methods
In a single-site, retrospective, comparative study, postmenopausal early stage BC patients treated between 01/2001 and 12/2005 at a major German university hospital were analyzed in two age Groups A and B (≥65 vs. <65 years) for initiation and completion of guideline-recommended adjuvant chemotherapy. Risk stratification was based on the 2005 St. Gallen Consensus Conference criteria. Comorbidity was parametrized using the Charlson Comorbidity Index (CCI).
Results
Analysis included 634 patients, 380 in Group A and 254 in Group B. Mean age (range) was 73 (65–94) and 61 (55–64) years, respectively. The proportion of patients from Group A given ≥3 cycles of chemotherapy was significantly decreased as compared to Group B. 52 % of patients with CCI <3 but only 20 % with CCI ≥3 were recommended to undergo chemotherapy (p < 0.001). Median follow-up [95 % confidence interval (CI)] was 85 (82–88) months. DFS was significantly shorter in patients aged ≥65 years as compared to younger postmenopausal patients (HR, 0.598; 95 % CI, 0.358–0.963; p = 0.048).
Conclusions
Despite being high-risk patients, older women with early stage BC were often not given guideline-recommended chemotherapy. Higher recurrence rates compared with younger postmenopausal women suggest that older patients are undertreated. Treatment needs to be adapted to general health and tumor biology rather than age. More trials in elderly BC patients are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One in eight women develops breast cancer (BC) in the course of her lifetime, with advanced age being one of the major risk factors (Siegel et al. 2015). Half of all women newly diagnosed with BC every year are over 65 years of age (Marshall et al. 2010; National Cancer Institute (NCI) 2014). This risk presents a challenge to society as a whole, especially in view of an aging population.
Modern systemic adjuvant treatment has resulted in a continuous increase in life expectance for patients with BC. Over the last decade, numerous new drugs and combinations of drugs have become clinically established based on large prospective multicenter studies. However, the high proportion of older BC patients has been underrepresented in clinical studies (Lewis et al. 2003; Van Ewijk et al. 2015). As a result of a lack of evidence regarding optimal treatment, this patient group often does not receive guideline-based treatment (Schonberg et al. 2010; Yardley 2015).
With increasing age, patients also potentially develop more comorbidities (Extermann et al. 1998). This limits the choice of potential treatment options and negatively affects treatment outcome (Yancik et al. 2001; Bouchardy et al. 2007). The Charlson Comorbidity Index (CCI) represents a standardized and validated tool that enables systematic ascertainment of comorbidities and their effect on mortality. It comprises 22 comorbidities that are assigned severity-based scores. The total score correlates negatively with survival (Charlson et al. 1987).
In this study, we compared the frequency of adjuvant chemotherapy in older patients (>65 years) with younger postmenopausal patients. Against the background of the existing comorbidities, we analyzed whether or not recommended chemotherapy had been initiated and completed as recommended. We also investigated the differences between younger and older postmenopausal patients with respect to disease-free survival (DFS) as well as the effect of classical breast cancer risk factors on prognosis.
Methods
Study design and ethics
The study was a single-site, retrospective, comparative analysis of patient data extracted from the medical records of BC patients treated at Tuebingen University Women’s Hospital, Tuebingen, Germany. Ethical approval was obtained in advance from the Ethics Committee of the Medical Faculty of the University of Tuebingen (approval no. 243/2011A).
Patients
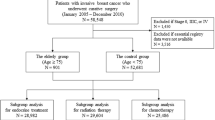
Included in the analysis were patients with early stage, primary invasive BC treated at our hospital between January, 2001 and December 2005. Elderly patients aged ≥65 years (Group A) were compared to a younger group of postmenopausal patients aged 55 to <65 years. Patients who were premenopausal, had additional cancers, were DCIS-only, or had metastases, recurrences, or bilateral BC were not included into the analysis.
Data collection
Patient data, tumor characteristics, details of the treatment administered, and survival data were gleaned from the tumor registry of the Tuebingen Comprehensive Cancer Center (CCC) and the patients’ medical records and transferred to a database created with Microsoft Access 2010 (Microsoft Corporation, Redmond, WA, USA).
Risk stratification
To reflect the reality of treatment during the study period, patients were assigned to one of the three risk categories (low, intermediate, and high risk) based on the 2005 St. Gallen Consensus Conference (Goldhirsch et al. 2005), as summarized in Table 1.
Charlson Comorbidity Index (CCI)
As shown in Table 2, the CCI divides comorbidities into four categories based on severity, assigning them scores of 1, 2, 3, and 6. CCI total scores were prospectively recorded for patients >65 and, therefore, available for all patients of Group A.
Statistical methods
Statistical analysis utilized PASW Statistics 21 (SPSS Inc., Chicago, IL, USA). Continuous variables were analyzed using mean and standard deviation (SD). Categorical variables were reported as frequency distributions and compared by the Chi squared test. Survival was analyzed in terms of time from primary diagnosis to either disease recurrence (local or distant recurrence), i.e., disease-free survival (DFS), or the patient’s death from any cause, i.e., overall survival (OS). If neither event occurred, the data were censured at the date of last follow-up. The influence of risk group assignment and treatment received was assessed by univariate analysis based on the hazard ratio (HR) and 95 % confidence interval (CI). Kaplan–Meier curves were constructed and compared by log-rank test. The two-sided significance level was set at p < 0.05.
Results
Patient characteristics
In total, 634 patients were included in the analysis, of whom 380 were ≥65 years old and hence assigned to Group A, and 254 were younger than 65 years. Mean age (SD, range) was 73 (6.31, 65–94) years in Group A and 61 (2.27, 55–64) years in Group B.
As shown in Table 3, Group A patients predominantly had invasive ductal tumors (71 %), ≤2 cm in size (51 %), grade G2 (83 %). Most patients were node negative (67 %) and HER2 negative (76 %). Accordingly, the majority of patients (79 %) were in the intermediate risk category. Hormone receptor status was mostly positive for estrogen receptor (ER; 89 %) and progesterone receptor (PR; 71 %).The comparison group of younger patients, Group B, had a similar proportion of patients (76 %, p = 0.139) with invasive ductal carcinoma. However, there were significantly more high grade tumors (p = 0.029) and ER-negative carcinomas (p = 0.004). In Group B, tumors <2 cm were less frequent than in Group A (66 %, p < 0.001). Nodal involvement, HER2 status, and PR status did not differ significantly between Groups A and B. Similarly to Group A, most (80 %) patients in Group B were in the intermediate risk category.
Chemotherapy
Compared with the younger postmenopausal patients in Group B, the older Group A patients in both the intermediate and high risk categories received chemotherapy less frequently (Table 4). Only 18 % of Group A patients compared with 56 % of Group B patients received ≥3 cycles of chemotherapy (p < 0.001). This difference was particularly marked in the intermediate risk category (16 vs. 55 %, p < 0.001).
As shown in Table 5, 111 patients from Group A received a recommendation to undergo chemotherapy but only 67 patients completed ≥3 cycles, while 6 patients discontinued chemotherapy earlier and 38 patients did not even start treatment.
Recommendation for chemotherapy was significantly associated with the presence of comorbidity in both intermediate and high risk patients, as shown in Table 6. Whereas 52 % of patients aged ≥65 years with a CCI <3 were recommended to undergo chemotherapy, only 20 % of elderly patients with a CCI ≥3 were advised to do so (p < 0.001).
Survival analysis
Median follow-up was 85 (95 % CI, 82–88) months. Figure 1 compares DFS in Groups A and B. DFS was significantly shorter in the older patients of Group A (HR, 0.598; 95 % CI, 0.358–0.963; p = 0.048).
Figure 2 shows a comparison of DFS (panel A) and OS (panel B) in low/intermediate risk patients as versus high risk patients from Group A. Both DFS (HR, 0.112; 95 % CI, 0.049–0.256; p < 0.001) and OS (HR, 0.218; 95 % CI, 0.111–0.426; p < 0.001) were found to be significantly decreased in high-risk elderly patients.
Discussion
The present analysis revealed that among postmenopausal patients with early stage primary BC treated at our hospital during 2001–2005 those aged ≥65 years (Group A) received adjuvant chemotherapy significantly less frequently than younger postmenopausal patients (Group B). At the same time, DFS was significantly shorter in the group of older women.
Several retrospective studies investigating the use of adjuvant chemotherapy in elderly patients have reported different treatment rates in the range from 5 to 32 % (Vlastos et al. 2001; Woodard et al. 2003; Brunello et al. 2005; Hawfield et al. 2006; Peters et al. 2015). However, these studies are not readily comparable due to differences in age distributions and patient characteristics. Nonetheless, in all studies, advanced age was a frequent reason to dispense with adjuvant chemotherapy, independently of comorbidities and prognostic factors.
To reflect the reality of treatment during the study period, this study divided patients into three risk categories—low, intermediate, and high risk—based on the 2005 St. Gallen Consensus Conference (Goldhirsch et al. 2005). There was no significant difference between the Group A and the Group B patients with regard to patient assignment to one of the three risk categories. As in other studies, older patients more frequently had larger tumors, which, however, tended to be less aggressive, i.e., G3 or hormone receptor-negative (Schonberg et al. 2010; Pappo et al. 2007; Diab et al. 2000). These observations indicate that diagnosis of BC is often delayed in elderly patients.
The observation that older patients aged ≥65 years received adjuvant chemotherapy less frequent than younger postmenopausal patients was noted across all risk categories. Similarly, the presence of comorbidities was associated with the absence of adjuvant chemotherapy in all risk categories. However, due to the retrospective nature of our study, we were unable to investigate in detail any additional factors that may have influenced the decision to proceed or not to proceed with chemotherapy.
Comorbidity was not associated with increased discontinuation of chemotherapy in our analysis (data not shown). Similar results were reported by Klepin et al. (2014), who showed that both chemotherapy tolerance and DFS were not negatively affected by comorbidity. However, when interpreting the data published by Klepin et al., it should be noted that patients in their study had an excellent performance status. As regards our own study, those patients who actually started chemotherapy presumably had a better performance status than did those for whom chemotherapy was not a treatment option. In contrast, other retrospective studies found a high CCI to be associated with an increased discontinuation rate, dose reduction, and grade 3–4 toxicity (Garg et al. 2009; Zauderer et al. 2009).
High-risk elderly patients aged ≥65 years (Group A) experienced a significantly worse outcome than did intermediate or low-risk patients. Hence, high-risk patients seem to require more aggressive or more effective treatment. Our finding that DFS was significantly longer in the younger postmenopausal patients (Group B) indirectly suggests that the older patients were potentially undertreated. A multicenter cohort study by Van Ewijk et al. (2015) showed guideline-adherent treatment to be associated with an improved prognosis. In a prospective randomized study, Muss and colleagues (Muss et al. 2009) compared capecitabine with standard polychemotherapy in older patients with early stage BC. They observed that patients treated with capecitabine alone had significantly shorter survival than patients receiving aggressive chemotherapy. In particular, hormone receptor-positive, node-negative women appeared to derive the greatest benefit from chemotherapy. Of note, while quality of life was decreased during treatment with the more aggressive regimen due to its higher toxicity, quality of life 1 year after treatment was the same in both arms of the study (Kornblith et al. 2011).
Limitations of this study include its retrospective nature and the fact that the data were collected during the 2001–2005 period. While this enabled a longer follow-up, modern and in particular targeted treatments, were not available yet at the time of data collection. A direct analysis of the extent to which chemotherapy might benefit older patients in terms of a better prognosis cannot be performed because other factors that also determine prognosis have a decisive influence on the decision whether or not to recommend chemotherapy. Although appropriate in this situation, a multivariate analysis cannot meaningfully be performed due to the great number of factors to be investigated and the limited number of cases available. We also did not compare survival in Groups A and B because older patients per se have a shorter life expectancy and BC-specific survival data were not available.
Conclusions
Our study showed that older BC patients with early stage disease often do not receive chemotherapy even if they are high-risk patients. The higher recurrence rate compared with younger postmenopausal women suggests that older patients are undertreated. Even though the actual impact of undertreatment on prognosis may be difficult to judge, no patient should be refused guideline-adherent treatment merely on the basis of age. Rather, treatment needs to be adapted to the patient’s general state of health and tumor biology.
In the future, predictive tests will be able to better estimate the actual benefit a treatment may provide and a larger number of targeted drugs with fewer adverse effects will come into use. Against this backdrop, there is a need for more clinical trials with a focus on the continually growing population of elderly BC patients.
Abbreviations
- BC:
-
Breast cancer
- CI:
-
Confidence interval
- DFS:
-
Disease-free survival
- ER:
-
Estrogen receptor
- G:
-
Grade
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- OS:
-
Overall survival
- pN:
-
Pathologically confirmed nodal status
- PR:
-
Progesterone receptor
- pT:
-
Pathologically determined tumor size
References
Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G (2007) Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol 25(14):1858–1869
Brunello A, Basso U, Pogliani C, Jirillo A, Ghiotto C, Koussis H, Lumachi F, Iacobone M, Vamvakas L, Monfardini S (2005) Adjuvant chemotherapy for elderly patients (> or =70 years) with early high-risk breast cancer: a retrospective analysis of 260 patients. Ann Oncol 16(8):1276–1282
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Diab SG, Elledge RM, Clark GM (2000) Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 92(7):550–556
Extermann M, Overcash J, Lyman GH, Parr J, Balducci L (1998) Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 16(4):1582–1587
Garg P, Rana F, Gupta R, Buzaianu EM, Guthrie TH (2009) Predictors of toxicity and toxicity profile of adjuvant chemotherapy in elderly breast cancer patients. Breast J 15(4):404–408
Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2005) Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 16(10):1569–1583
Hawfield A, Lovato J, Covington D, Kimmick G (2006) Retrospective study of the effect of comorbidity on use of adjuvant chemotherapy in older women with breast cancer in a tertiary care setting. Crit Rev Oncol Hematol 59(3):250–255
Klepin HD, Pitcher BN, Ballman KV, Kornblith AB, Hurria A, Winer EP, Hudis C, Cohen HJ, Muss HB, Kimmick GG (2014) Comorbidity, chemotherapy toxicity, and outcomes among older women receiving adjuvant chemotherapy for breast cancer on a clinical trial: CALGB 49907 and CALGB 361004 (alliance). J Oncol Pract 10(5):e285–e292
Kornblith AB, Lan L, Archer L, Partridge A, Kimmick G, Hudis C, Winer E, Casey R, Bennett S, Cohen HJ, Muss HB (2011) Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907. J Clin Oncol 29(8):1022–1028
Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, Housman MG, Escarce JJ (2003) Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 21(7):1383–1389
Marshall SF, Clarke CA, Deapen D, Henderson K, Largent J, Neuhausen SL, Reynolds P, Ursin G, Horn-Ross PL, Stram DO, Templeman C, Bernstein L (2010) Recent breast cancer incidence trends according to hormone therapy use: the California Teachers Study cohort. Breast Cancer Res 12(1):R4
Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, Partridge AH, Dressler LG, Cohen HJ, Becker HP, Kartcheske PA, Wheeler JD, Perez EA, Wolff AC, Gralow JR, Burstein HJ, Mahmood AA, Magrinat G, Parker BA, Hart RD, Grenier D, Norton L, Hudis CA, Winer EP, Investigators C (2009) Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med 360(20):2055–2065
National Cancer Institute (NCI). Surveillance, epidemiology, and end results (SEER) programm. 2014. http://www.seercancer.gov/popdata. Accessed 24 Feb 2016
Pappo I, Karni T, Sandbank J, Dinur I, Sella A, Stahl-Kent V, Wasserman I, Halevy A (2007) Breast cancer in the elderly: histological, hormonal and surgical characteristics. Breast 16(1):60–67
Peters E, Anzeneder T, Jackisch C, Dimpfl T, Kunz G, Katalinic A, Waldmann A (2015) The treatment of primary breast cancer in older women with adjuvant therapy. Dtsch Arztebl Int 112(35–36):577–584
Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP (2010) Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol 28(12):2038–2045
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Van Ewijk R, Wockel A, Gundelach T, Hancke K, Janni W, Singer S, Kreienberg R, Blettner M, Schwentner L (2015) Is guideline-adherent adjuvant treatment an equal alternative for patients aged > 65 who cannot participate in adjuvant clinical breast cancer trials? A retrospective multi-center cohort study of 4,142 patients. Arch Gynecol Obstet 291(3):631–640
Vlastos G, Mirza NQ, Meric F, Hunt KK, Kuerer HM, Ames FC, Ross MI, Buchholz TA, Hortobagyi GN, Singletary SE (2001) Breast conservation therapy as a treatment option for the elderly. The M. D. Anderson experience. Cancer 92(5):1092–1100
Woodard S, Nadella PC, Kotur L, Wilson J, Burak WE, Shapiro CL (2003) Older women with breast carcinoma are less likely to receive adjuvant chemotherapy: evidence of possible age bias? Cancer 98(6):1141–1149
Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW (2001) Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 285(7):885–892
Yardley DA (2015) Taxanes in the elderly patient with metastatic breast cancer. Breast Cancer (Dove Med Press) 7:293–301
Zauderer M, Patil S, Hurria A (2009) Feasibility and toxicity of dose-dense adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat 117(1):205–210
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Wallwiener, C.W., Hartkopf, A.D., Grabe, E. et al. Adjuvant chemotherapy in elderly patients with primary breast cancer: are women ≥65 undertreated?. J Cancer Res Clin Oncol 142, 1847–1853 (2016). https://doi.org/10.1007/s00432-016-2194-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2194-4