Abstract
Purpose
Decisions on the type of adjuvant treatment in older breast cancer patients are challenging. Side effects of chemotherapy have to be weighed against life expectancy, comorbidities, functional status, and frailty on the basis of studies usually excluding patients over 69 years. To aid this decision, we analyzed a database of 6000 unselected patients and of those evaluated elderly primary breast cancer patients with hormone receptor-negative tumors from 1963 until 2003 in respect of survival data depending on adjuvant treatment.
Methods
A total of 131 elderly (i.e., >65 years) patients were observed retrospectively for a median of 72 months. Patients received breast-conserving therapy or mastectomy and adjuvant radiotherapy, chemotherapy, and endocrine therapy. Data were collected from a hospital-intern database.
Results
Median age at diagnosis was 72 years. Mostly, tumors were small (81 % T1, 17 % T2) but of unfavorable grading (40 % G2, 35 % G3). Lymph nodes were positive in 42 %. Mastectomy was performed in 65 %. While 42 % of patients received radiotherapy, only 10 % were treated with chemotherapy. Patients with G2 and G3 tumors (p = 0.027), younger women (p = 0.012), and patients with positive lymph node status (p < 0.0001) more likely received chemotherapy. Recurrence-free survival was longer in patients without chemotherapy (37 vs. 29 months, p = 0.234). Overall survival was non-significantly shorter in patients who received chemotherapy (59 vs. 81 months, p = 0.131).
Conclusions
In this analysis, adjuvant chemotherapy was not associated with improved survival, presumably caused by an a priori poor prognosis of these patients. For an aging society more data are urgently needed to help selecting and personalizing adjuvant treatment within subgroups of breast cancer in older women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Mean age of initial diagnosis of early breast cancer in Germany is 63.5 (median 64.1 years, standard deviation 14.0 years). At the same time, age-specific incidence for mammary carcinoma is greatest for women over the age of 84, with 420 newly diagnosed patients in every 100,000 women of the same age group [1]. Therefore, demographic change will increase the number of older breast cancer patients in future years [2].
Moreover, tumor characteristics change with increasing life expectancy. Tumors tend to be bigger at first diagnosis in elderly patients: one of the largest cancer registries in Germany reports a median age at initial diagnosis of women with pT1 tumors of 60 years while women suffering from large pT4 tumors at first diagnosis are over 70 years old [1].
Most older breast cancer patients suffer from well differentiated (G1) tumors with positive hormone receptor status showing typically low metastatic potential associated with a comparably good prognosis [3, 4]. Almost 20 % of tumors found in older patients are hormone receptor-negative, but even in triple-negative tumors the prognosis improves with patients’ age at first diagnosis [5, 6]. Nevertheless, chemotherapy remains the only systemic therapeutic option in these patients.
With age-dependent altering tumor characteristics and increasing age of the patients at first diagnosis, selection of adjuvant treatment becomes more difficult. Especially so in the case of chemotherapy in which significant side effects have to be weighed against comorbidities. Thus, treatment decisions are mostly based on studies that usually exclude patients over 69 years [7, 8].
To aid this decision, we retrospectively analyzed a longitudinal dual center database of 6000 unselected patients and evaluated primary breast cancer patients over the age of 65 with hormone receptor-negative tumors for recurrence-free and overall survival depending on their adjuvant treatment options.
Patients and methods
Patient collective
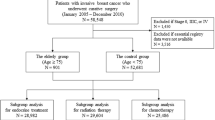
Patient data were retrieved retrospectively from a database of 6.096 breast cancer patients at the Department of Obstetrics and Gynaecology, Ludwig-Maximilians-University, Munich, and the University Hospital in Berlin-Charlottenburg, Germany, between 1963 and 2003 as published before [9]. In this database, 131 patients met the inclusion criteria:
-
1.
age over 65,
-
2.
primary tumor of stages I–III,
-
3.
histologically negative hormone receptor status (i.e., estrogen and progesterone receptor negative).
All patients were registered in the database upon first diagnosis. The median observation period was 72 months (range 3–276 months).
Surgical therapy
Patients received surgical therapy of the primary tumor according to clinical guidelines of that time period. 34 % received breast-conserving therapy, whereas 66 % had a mastectomy (65.6 %) [10]. In all cases a histological R0 resection was achieved.
In 84 % of the patients a dissection of axillar lymph nodes of levels I and II was performed while 16 % of the patients did not undergo lymph node removal due to advanced age. If macroscopic metastasis was present, level III lymph nodes were also removed.
Histological examinations
Tumor stage at first diagnosis was determined using the revised tumor-node-metastasis (TNM) classification of the American Joint Committee on Cancer (AJCC) [11]. Histological grading of the primary tumor was classified according to Bloom and Richardson [12]. Hormone receptor status was analyzed in specimens until 1986 in a radioimmunoassay (RIA) with a protein-binding capacity of greater than 3 fmol/ml being considered positive for the estrogen receptor and greater than 5 fmol/ml for the progesterone receptor. After 1987 the immune reactive score (IRS) according to Remmele and Stegner was used with greater than 10 % stained nuclei being considered positive [13].
Adjuvant therapies
For patients who received adjuvant radiotherapy a total dose of 50 Gy (separated into individual doses of 2 Gy each) was scheduled, as well as a boost of 10 Gy in cases of higher recurrence risk. Radiotherapy of the chest wall after mastectomy was indicated if more than three lymph nodes were involved or in case of T3 and T4 tumors.
Decisions on adjuvant treatment were made having obtained informed consent of the patient and, from 2003 onwards, were based on discussions in an interdisciplinary tumor board.
Targeted therapies (e.g., Trastuzumab) were not available outside of clinical trials in Germany before 2003, so none of the patients in this analysis received an anti-Her2 therapy.
Statistics
For statistical evaluation of the data, the log-rank test was used, with significance defined as p ≤ 0.05. The survival-rate curves were based on uni-variant survival estimation and created using the Kaplan–Meier method.
Multi-variant analyses were used for comparison of significance and independence of individual parameters with respect to survival, by using the Cox regression model. For data assessment we used the statistical software “SPSS” (“Statistical Package for the Social Sciences”, SPSS Inc., Chicago, Illinois, USA; Version 16.0).
Primary patient characteristics
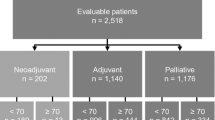
Median age at diagnosis was 72 years (range 65–94) (see Fig. 1). Most of the tumors were small (81 % pT1; 17 % pT2; 1 % pT3; 1 % pT4) and moderately to poorly differentiated (25 % G1; 40 % G2; 35 % G3).
The majority of the patients were nodal-negative (pN0 58 %; pN1 13 %; pN2 8 %; pN3 5 %; 16 % NX). According to the inclusion criteria, all patients had a negative hormone receptor status both for estrogen and progesterone receptor.
Adjuvant therapies
Overall, 60 % of the patients had any form of adjuvant therapy while 2 % rejected it at all. For the remaining 38 %, no further treatment had been recommended.
All patients who underwent breast-preserving surgery received postoperative radiotherapy; 13 % had radiotherapy of the thoracic wall after mastectomy. Radiotherapy was performed in 42 % of the patients.
To approximately 10 % of the patients adjuvant chemotherapy was administered while none of the patients over the age of 75 had any form of chemotherapy as part of their treatment of primary breast cancer. 12 % of the patients received endocrine therapy despite negative hormone receptor status. None of the patients underwent endocrine therapy subsequent to adjuvant chemotherapy.
Survival data
During the observation period of median 72 months, 58 % of the patients suffered any form of recurrence. Median recurrence-free survival (RFS) was 33 months. In 25 % local recurrences occurred and 21 % of the patients experienced distant metastases. Regional recurrences (in the ipsilateral lymph nodes) were recorded in 12 % of cases.
Recurrence-free patients had a 5-year overall survival (OS) of 95.4 % and a 10-year OS of 90.6 %. Survival rates after distant metastases were: 5-year OS 35.2 %, and 10-year OS 15.1 %. 54 % died of tumor-specific causes.
Results
Effect of adjuvant therapies on survival
Radiotherapy
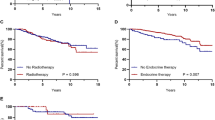
Patients who had adjuvant radiotherapy (42 %) did not survive significantly longer than women who did not receive radiotherapy.
44 % of patients who had radiotherapy died within 10 years due to tumor-related causes in comparison to 39 % of the patients in whom no adjuvant radiotherapy was performed (p = 0.619).
Endocrine treatment
For those 12 % of patients who received endocrine therapy in spite of negative receptor status, survival statistics were not different to patients without any endocrine therapy (p = 0.427) (Table 1).
Chemotherapy
Recurrence-free survival was insignificantly shorter in those elderly patients who were treated with chemotherapy, compared to those who did not receive chemotherapy (37 vs. 29 months, p = 0.234). Patients who had adjuvant chemotherapy had a non-significantly shorter OS than patients without chemotherapy (59 vs. 81 months, p = 0.131) (see Fig. 2). After 10 years, 92.3 % of the women who had undergone chemotherapy had died, compared with 69.5 % of those who had not undergone adjuvant chemotherapy. Chemotherapy had no effect on OS with regard to non-tumor-related death (p = 0.914).
In the first 10 years, 77 % of the patients with chemotherapy died of a tumor-specific cause compared to 38 % of the patients without chemotherapy (p = 0.012) (see Fig. 3).
Characteristics of elderly patients with hormone receptor-negative breast cancer who received chemotherapy
All patients who received chemotherapeutic agents were under the age of 76 and had a positive nodal status as well as moderately to poorly differentiated tumors (G2 and G3). In multivariate analysis the decision towards adjuvant chemotherapy significantly depended on patients’ younger age (p = 0.012), tumor grade (p = 0.027) and positive nodal status (p = 0.0001).
After chemotherapy 8 % of the patients remained recurrence free while this was the case in 46 % of the patients who had no chemotherapy (p = 0.004).
Discussion
In this retrospective database analysis of elderly, surgically treated, hormone receptor-negative breast cancer patients of two German university hospitals over a 40-year time period until 2003 we could demonstrate that adjuvant chemotherapy was recommended for patients that were younger than 75 years and had advanced disease or poorer tumor grading. These patients showed non-significantly shorter survival compared to patients without chemotherapy.
Possible reasons for these findings might be that the prognosis of these patients was so poor they did not benefit from chemotherapy whatsoever, at least not from the cytostatic regimens available at the time of their diagnosis.
Most of the patients who received chemotherapy underwent mastectomy and consecutively were not treated with radiotherapy. This fact might have affected recurrence-free survival in this group as well due to withholding of the known protective effect of adjuvant radiotherapy regarding disease recurrence [14, 15].
In the whole patient collective, more than half of the patients suffered a tumor recurrence, in the group to whom chemotherapy was administered this was observed in over 90 % explaining the poor survival data of this group.
As a positive lymph node status is known to be the strongest prognostic parameter in breast cancer [16] it is not surprising that all of the patients who received chemotherapy were nodal positive while in only 23 % of the women without chemotherapy lymph node metastases were present.
On the other hand, as this is a retrospective analysis in a non-randomized patient group, recurrence-free and overall survival rates for a matching cohort without chemotherapy remain unclear. Possibly, the patients would have suffered recurrences or died even earlier if not treated with cytostatic agents.
Effectiveness of chemotherapeutic regimens has been doubted by some authors [17] and according to the presented data a significant benefit could not be shown either, since only 8 % of patients with chemotherapy did not suffer a tumor recurrence compared to 46 % without chemotherapy. Nevertheless, cytostatic agents become more effect-focused at the same time trying to minimize side effects. Together with newer therapeutic regimens (such as PARP inhibitors, antiangiogenetic drugs and anti-Her2 agents) more individualized tumor therapies are available. Moreover, recently diagnostic tests have been developed that can assist the decision-making process of adjuvant therapy and for or against chemotherapy [18, 19].
However, following a recent diagnosis of cancer, older women often receive less than standard therapy [20]. There are trends to decreased surgical rates, less frequent adjuvant radiation therapy following breast-conserving therapy and increased rates of primary endocrine therapy [21].
In a recent analysis in breast cancer patients, elderly guideline-adherently treated patients showed a better outcome than patients not treated conform to guidelines. Even compared to non-elderly study participants outcome of these patients was not significantly inferior [22].
During most of the 40 years that are included in these analyses, tools that help in deciding on adjuvant therapeutic options were not available. Even now, online implements are generated from and validated in databases that primarily include women age 69 and younger [23].
As for other tumor subgroups decisions on adjuvant therapy in hormone receptor-negative elderly breast cancer patients will depend on life expectancy, comorbidities, functional status and frailty [24]. However, specific side effects of chemotherapeutic agents and the intention not to harm often restrict chemotherapy to those patients with an especially challenging tumor biology that nowadays goes beyond mere receptor negativity [25, 26]. That kind of selection effect could be demonstrated in the data presented here.
In the study population from 1963 to 2003, Her2-data were not available. In a contemporary setting, a significant proportion of these patients might profit from targeted Her2 therapy [27], which, however, still has to be accompanied with chemotherapy.
Nevertheless, there is evidence that adjuvant chemotherapy reduces the mortality rate in older patients with triple-negative breast cancer, except when tumors are very small or if life expectancy is less than 5 years [8]. A meta-analysis of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) showed a 6 % reduction in the 10-year mortality rate by adjuvant chemotherapy with cyclophosphamide, methotrexate, and 5-flurouracil (CMF) in patients between the ages of 50 and 69 with triple-negative tumors [28]. More pronounced effects concerning recurrence-free survival might be achieved with more advanced chemotherapy regimens [29]. Unfortunately, our data do not differentiate between various chemotherapeutic regimens, but patients who did receive chemotherapy did not experience an increase in non-tumor-related incidents, which seems to be reassuring with respect to an age-adapted recommendation of chemotherapy at all.
In our data adjuvant chemotherapy was not significantly associated with an improvement in the tumor-specific survival, presumably caused by an a priori poor prognosis of the treated patient group. For an aging society more clinical study results from prospectively randomized trials are urgently needed to help selecting and personalizing adjuvant treatment within subgroups of breast cancer in elderly women.
References
Schrodi S, Engel J, Schubert-Fritschle G (2013) Epidemiologie. In: Bauerfeind I (ed) Manual Mammakarzinome, Empfehlungen zur Diagnostik, Therapie und Nachsorge, vol 14. Zuckschwerdt Verlag, München, pp 1–10
Van Ewijk RJ, Schwentner L, Wockel A, Konig J, Kreienberg R, Blettner M (2013) Trends in patient characteristics, treatment and survival in breast cancer in a non-selected retrospective clinical cohort study of 2600 patients. Arch Gynecol Obstet 287(1):103–110. doi:10.1007/s00404-012-2544-7
Eppenberger-Castori S, Moore DH Jr, Thor AD, Edgerton SM, Kueng W, Eppenberger U, Benz CC (2002) Age-associated biomarker profiles of human breast cancer. Int J Biochem Cell Biol 34(11):1318–1330. doi:10.1016/S1357-2725(02)00052-3
Diab SG, Elledge RM, Clark GM (2000) Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 92(7):550–556
Liedtke C, Hess KR, Karn T, Rody A, Kiesel L, Hortobagyi GN, Pusztai L, Gonzalez-Angulo AM (2013) The prognostic impact of age in patients with triple-negative breast cancer. Breast Cancer Res Treat 138(2):591–599. doi:10.1007/s10549-013-2461-x
Anderson WF, Katki HA, Rosenberg PS (2011) Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst 103(18):1397–1402. doi:10.1093/jnci/djr257djr257
Albrand G, Terret C (2008) Early breast cancer in the elderly: assessment and management considerations. Drugs Aging 25(1):35–45. doi:10.2165/00002512-200825010-00004
Taylor WC, Muss HB (2010) Recent advances: adjuvant therapy for older women with breast cancer. Cancer J 16(4):289–293. doi:10.1097/PPO.0b013e3181eea208-201007000-00001
Rack B, Janni W, Gerber B, Strobl B, Schindlbeck C, Klanner E, Rammel G, Sommer H, Dimpfl T, Friese K (2003) Patients with recurrent breast cancer: does the primary axillary lymph node status predict more aggressive tumor progression? Breast Cancer Res Treat 82(2):83–92. doi:10.1023/B:BREA.0000003955.73738.9e
Martin JK Jr, van Heerden JA, Taylor WF, Gaffey TA (1986) Is modified radical mastectomy really equivalent to radical mastectomy in treatment of carcinoma of the breast? Cancer 57(3):510–518
Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL (2002) Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol 20(17):3628–3636
Le Doussal V, Tubiana-Hulin M, Friedman S, Hacene K, Spyratos F, Brunet M (1989) Prognostic value of histologic grade nuclear components of Scarff–Bloom–Richardson (SBR). An improved score modification based on a multivariate analysis of 1262 invasive ductal breast carcinomas. Cancer 64(9):1914–1921
Remmele W, Stegner HE (1987) Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8(3):138–140
Baral E, Ogenstad S, Wallgren A (1985) The effect of adjuvant radiotherapy on the time of occurrence and prognosis of local recurrence in primary operable breast cancer. Cancer 56(12):2779–2782
Nevin JE, Baggerly JT, Laird TK (1982) Radiotherapy as an adjuvant in the treatment of carcinoma of the breast. Cancer 49(6):1194–1200
Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, Vlastos G, Wallace AM, Hortobagyi GN, Nieto Y (2006) Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol 24(18):2910–2916. doi:10.1200/JCO.2005.03.1526
Morgan G, Ward R, Barton M (2004) The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clin Oncol (R Coll Radiol) 16(8):549–560
Bao T, Davidson NE (2008) Gene expression profiling of breast cancer. Adv Surg 42:249–260
Arpino G, Generali D, Sapino A, Del Matro L, Frassoldati A, de Laurentis M, Paolo P, Mustacchi G, Cazzaniga M, De Placido S, Conte P, Cappelletti M, Zanoni V, Antonelli A, Martinotti M, Puglisi F, Berruti A, Bottini A, Dogliotti L (2013) Gene expression profiling in breast cancer: a clinical perspective. Breast 22(2):109–120. doi:10.1016/j.breast.2013.01.016S0960-9776(13)00018-0
Trillsch F, Woelber L, Eulenburg C, Braicu I, Lambrechts S, Chekerov R, van Nieuwenhuysen E, Speiser P, Zeimet A, Castillo-Tong DC, Concin N, Zeillinger R, Vergote I, Mahner S, Sehouli J (2013) Treatment reality in elderly patients with advanced ovarian cancer: a prospective analysis of the OVCAD consortium. J Ovarian Res 6(1):42. doi:10.1186/1757-2215-6-421757-2215-6-42
Bastiaannet E, Liefers GJ, de Craen AJ, Kuppen PJ, van de Water W, Portielje JE, van der Geest LG, Janssen-Heijnen ML, Dekkers OM, van de Velde CJ, Westendorp RG (2010) Breast cancer in elderly compared to younger patients in the Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res Treat 124(3):801–807. doi:10.1007/s10549-010-0898-8
Van Ewijk R, Wockel A, Gundelach T, Hancke K, Janni W, Singer S, Kreienberg R, Blettner M, Schwentner L (2015) Is guideline-adherent adjuvant treatment an equal alternative for patients aged >65 who cannot participate in adjuvant clinical breast cancer trials? A retrospective multi-center cohort study of 4142 patients. Arch Gynecol Obstet 291(3):631–640. doi:10.1007/s00404-014-3438-7
de Glas NA, van de Water W, Engelhardt EG, Bastiaannet E, de Craen AJ, Kroep JR, Putter H, Stiggelbout AM, Weijl NI, van de Velde CJ, Portielje JE, Liefers GJ (2014) Validity of adjuvant! Online program in older patients with breast cancer: a population-based study. Lancet Oncol 15(7):722–729. doi:10.1016/S1470-2045(14)70200-1
Goodwin JS, Hunt WC, Samet JM (1993) Determinants of cancer therapy in elderly patients. Cancer 72(2):594–601
Brunello A, Basso U, Pogliani C, Jirillo A, Ghiotto C, Koussis H, Lumachi F, Iacobone M, Vamvakas L, Monfardini S (2005) Adjuvant chemotherapy for elderly patients (> or =70 years) with early high-risk breast cancer: a retrospective analysis of 260 patients. Ann Oncol 16(8):1276–1282. doi:10.1093/annonc/mdi257
Hurria A, Wong FL, Villaluna D, Bhatia S, Chung CT, Mortimer J, Hurvitz S, Naeim A (2008) Role of age and health in treatment recommendations for older adults with breast cancer: the perspective of oncologists and primary care providers. J Clin Oncol 26(33):5386–5392. doi:10.1200/JCO.2008.17.6891JCO.2008.17.6891
Kaufmann M, von Minckwitz G, Bergh J, Conte PF, Darby S, Eiermann W, Howell A, Kiechle M, Mauri D, Senn HJ, Viale G, Loibl S (2013) Breakthroughs in research and treatment of early breast cancer: an overview of the last three decades. Arch Gynecol Obstet 288(6):1203–1212. doi:10.1007/s00404-013-3069-4
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, Martino S, Perez EA, Muss HB, Norton L, Hudis C, Winer EP (2006) Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295(14):1658–1667. doi:10.1001/jama.295.14.1658
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jueckstock, J., Kasch, F., Jaeger, B. et al. Adjuvant therapeutic decisions in elderly breast cancer patients: the role of chemotherapy in a retrospective analysis. Arch Gynecol Obstet 292, 1101–1107 (2015). https://doi.org/10.1007/s00404-015-3728-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3728-8