Abstract
Purpose
In patients undergoing allogeneic stem cell transplantation, conditioning regimens containing alemtuzumab instead of anti-thymocyte globulin (ATG) may result in an earlier platelet engraftment and a reduced number of platelet transfusions.
Methods
We performed a retrospective, single-center, case–control study analyzing time to engraftment and transfusion needs using alemtuzumab in comparison with ATG as part of conditioning protocol.
Results
Median values for time to platelet engraftment, number of transfused platelet concentrates and number of transfused red cell concentrates were 12 versus 19.5 days (p < 0.001), 2 versus 14 (p < 0.001) and 6 versus 14.5 (p = 0.003) in the alemtuzumab and ATG group. Time to leukocyte engraftment did not differ with median 15 days in both groups. Patients in the ATG group showed a significant higher decrease in platelet count during conditioning (68 vs. 29 %, p = 0.001), leading to significant lower median platelet counts at the day of stem cell infusion (38 vs. 95.5 Gpt/l, p = 0.008), and higher values for median C-reactive protein after first antibody infusion (69.0 vs. 43.6 mg/l, p = 0.001) compared with alemtuzumab group. Test for significance was done by using Wilcoxon rank-sum test. Subgroup analysis considering the type of ATG used (Thymoglobulin vs. ATG Fresenius) revealed that differences between alemtuzumab and ATG group were more due to effects of ATG Fresenius than Thymoglobulin.
Conclusions
The use of alemtuzumab in comparison with ATG as part of the conditioning regimen may be an approach to reduce the number of transfused platelet and red cell concentrates after allogeneic stem cell transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients undergoing allogeneic stem cell transplantation may require intensive blood component support. Aside from rare transfusion-related complications, including transmission of viral and bacterial infections, transfusion-associated graft-versus-host disease, acute lung injury and alloimmunization to HLA class I expressed on platelets, platelet transfusions are cost-intensive, and there is an increasing demand for platelet transfusions.
During the last years, several studies have revealed that the need for platelet transfusions can be reduced by restrictive transfusion regimens and the avoidance of prophylactic transfusions (Wandt et al. 2012; Stanworth et al. 2013) in hematooncologic patients after intensive chemotherapy. This includes lowering of the threshold for prophylactic platelet transfusion, determination of the optimal dose (Slichter et al. 2010) (PLADO trial) and assessment of the feasibility of a therapeutic transfusion regimen.
Another interesting approach may be to apply modified conditioning regimens which result in an earlier platelet engraftment and a reduced number of platelet transfusions needed after intensive chemotherapy and allogeneic transplantation. Such a regimen leading to earlier platelet engraftment may be the use of the humanized monoclonal antibody against CD52 alemtuzumab as part of the conditioning regimen.
Alemtuzumab (Campath-1H) is the humanized derivate of the Campath-1G antibody developed by Waldmann and Hale (2005). Polyclonal antibodies like anti-thymocyte globulin (ATG) have been used in combination with standard cyclosporine/methotrexate (CsA/MTX) prophylaxis to reduce the incidence and severity of graft-versus-host disease (GvHD), especially in volunteer unrelated donor transplantation, for years (Weiden et al. 1978; Ramsay et al. 1982; Theurich et al. 2012; Finke et al. 2009). Alemtuzumab is used for prophylaxis of GvHD in vivo since 1998 (Hale et al. 1998; Kottaridis et al. 2000). The optimal dose and schedule of alemtuzumab in the allogeneic stem cell transplantation (allo-SCT) setting has not been defined yet (Poiré and van Besien 2011). High doses as 100 mg alemtuzumab infused over 5 days before transplant were found to be highly efficient in reducing GvHD (Perez-Simon et al. 2002; Delgado et al. 2008; Peggs et al. 2007; Van Besien et al. 2009), but possibly leading to impaired immune reconstitution (Penack et al. 2008; Juliusson et al. 2006), an increased rate of infections (Avivi et al. 2004; Chakrabarti et al. 2002; Myers et al. 2005; Park et al. 2009) and in some studies increased relapse rates (Kröger et al. 2005; Soiffer et al. 2011). During the last years, several studies using significant reduced doses of alemtuzumab have been successfully carried out when combined with CsA/MTX treatment after allo-SCT (Chakraverty et al. 2010; Bertz et al. 2009). In a previous study of our group analyzing GvHD and overall survival (OS) in patients after allo-SCT, we found no evidence for a reduced OS when using low doses of alemtuzumab as part of the conditioning regimen (Busemann et al. 2013).
Our initial clinical observations showed that recovery of platelets in patients transplanted from matched unrelated donors (MUD) was more rapid using alemtuzumab instead of ATG, substantially reducing platelet transfusion requirements.
So we performed a retrospective, single-center, case–control study at our institution to evaluate the time to engraftment and transfusion needs using alemtuzumab in comparison with ATG-containing protocols.
Patients and methods
In total, 44 patients who underwent allogeneic stem cell transplantation from MUD at the Department of Hematology and Oncology of the University of Greifswald during the period from 2001 to 2012 were analyzed retrospectively. A matched-pair analysis was carried out regarding age, diagnosis and conditioning regimen.
Since all patients had fulfilled common criteria to qualify for allogeneic stem cell transplantation, their bleeding risk profiles were comparable. In detail, no patient had dedicated risk factors for severe bleeding such as plasmatic coagulopathy, neither inborn nor hepatopathy-related. No patient had uncontrolled hypertonic disease or suffered from relevant vascular diseases.
One group of patients was treated with alemtuzumab (manufacturer Genzyme, USA) 10 mg at day −2 (respectively, 20 mg in case of mismatch transplantation); this group was called the Campath group. The other group was treated with an alternative immunosuppressive regimen containing ATG and called the ATG group. The ATG group was either treated with either ATG Fresenius (10 mg/kg for 3 consecutive days, manufacturer Fresenius, Germany) or Thymoglobulin (2 mg/kg for 3 consecutive days, manufacturer Genzyme, USA). Additionally, all patients received cyclosporin A, starting on day −3 (initial dose 3 mg/kg cont. i.v.), in conjunction with either short-course methotrexate (15 mg/m2 day +1, 10 mg/m2 day +3 and +6) or mycophenolate mofetil (MMF, CellCept 15 mg/kg bid p.o.) if recommended by the conditioning protocol. Briefly, CsA/MTX was preferred after myeloablative conditioning and CsA/MMF after reduced intense protocols.
Platelet engraftment was defined as the first day of transfusion-independent increase in platelet count above the threshold of 20 Gpt/l. Leukocyte engraftment was defined in the same way as the first day of increase in leukocyte count above the threshold of 1.0 Gpt/l. Platelet and leukocyte engraftment was noticed as day after transfusion of the first allogeneic stem cells for every patient analyzed. For patients who never fell below a platelet count of 20 Gpt/l (respectively, 1 Gpt/l for leukocyte count), engraftment was defined as day 0; for patients who never engrafted during observation period, engraftment was defined as day 40 (end of observation period). Furthermore, the number of transfused platelet and red cell concentrates from the day of transplantation (day 0) to day 40 after transplantation was noticed. In general, a platelet concentrate was transfused if the platelet count fell below 10 Gpt/l during post-transplantation period (respectively, below 20 Gpt/l in patients with fever or an increased bleeding risk) or in a situation of clinically relevant bleeding. Red cell concentrates were transfused if hemoglobin level fell below 5.0 mmol/l, in case of a relevant bleeding or under conditions when a higher hemoglobin level had to be maintained for clinical reasons.
For the Campath and ATG group, median values were calculated for leukocyte engraftment, platelet engraftment and the number of transfused platelet and red cell concentrates. In addition, these values were calculated for ATG subgroups (ATG Fresenius or Thymoglobulin). According to our clinical observations, we also calculated the median platelet count at day 0 before transfusion of the stem cell product. Looking at the baseline characteristics of our study population, we did not see any significant differences in platelet counts before conditioning between Campath and ATG group. Nevertheless, may slight imbalances in this parameter have an influence on the median platelet count at day 0. For this reason, we calculated the relation T TX/T C between the median platelet count at day 0 (T TX) and the median platelet count before conditioning (T C) for both groups. So the median decrease in platelet count during conditioning therapy can be calculated by 1 − T TX/T C. Additionally, the median value for C-reactive protein (CRP) at day 0 and on the first day after start of the antibody application was calculated. Subgroup analysis was also performed for all these values.
The results obtained in both groups were analyzed by the Wilcoxon rank-sum test (respectively, Kruskal–Wallis test for subgroup analysis) and calculated with the statistical software package SPSS. Statistical significance was assumed if significance level p was below 0.05.
Results
In total, 44 patients were included in the analysis. The HLA match was 10/10 (matched unrelated donor, MUD) in 26 cases (59 %) and 9/10 (mismatched unrelated donor, MMUD) in the remaining 18 cases (41 %). Baseline characteristics and demographic data of our study population are given in Table 1.
Although there are no serious imbalances in the distribution between both groups for the attributes listed in Table 1, a trend toward more aggressive conditioning regimens in the Campath group can be noticed. Nevertheless, Chi-square test shows no significant differences for distribution of conditioning regimens between both groups. Additionally, the distribution of MUD and MMUD is not significantly different between both groups using Chi-square test.
Differences in median age, median dose of CD34+ cells and median platelet count before conditioning are not significant between Campath group and ATG group using Wilcoxon rank-sum test.
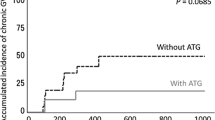
The results are given in Table 2. Leukocyte engraftment was not influenced by the application of alemtuzumab as part of the conditioning regimen; in both groups, leukocytes take in median 15 days to reach the threshold of 1 Gpt/l. In contrast, platelet engraftment was heavily influenced by the application of alemtuzumab instead of ATG. While platelets in the ATG group needed 19.5 days in median to rise above 20 Gpt/l, the median time for platelet engraftment was only 12 days in the Campath group. In addition, a median number of 14 platelet concentrates (range 1–31) had to be transfused in the ATG group compared with a median of 2 (range 0–12) in the Campath group. These results are significantly different as shown by the Wilcoxon rank-sum test with p values below 0.001 (Table 2).
Interestingly, the number of transfused red cell concentrates was also significantly higher in the ATG group (median 14.5; range 2–31) compared with the Campath group (median 6; range 2–24). Median CRP after first antibody application and platelet count at day 0 differed significantly in Campath and ATG group, with lower CRP values and higher platelet counts in the Campath group (43.6 vs. 69.0 mg/l and 95.5 vs. 38 Gpt/l). Likewise, Campath patients showed a much lower decrease in median platelet count during conditioning therapy compared with patients receiving ATG (29 vs. 68 %). Median CRP at day 0 did not differ significantly between both groups.
Analyzing our data considering the type of ATG used, we found different results (Table 3). While differences between the Campath and Thymoglobulin group are relatively small in terms of engraftment and application of blood component concentrates, comparison of Campath with ATG Fresenius group shows sharper distinctions for both platelet engraftment and number of transfused blood component concentrates. Therefore, the differences found in our first analysis seem to be more due to effects of ATG Fresenius than of Thymoglobulin.
Comparable differences were found for platelet count at day 0 and platelet decrease during conditioning therapy. Differences between Campath and Thymoglobulin group were small, while differences between Campath and ATG Fresenius group are impressive and clinically relevant.
Discussion
In this retrospective analysis, we could confirm our clinical experience, which shows a reduced consumption of blood component products and earlier platelet engraftment when using alemtuzumab instead of ATG for GvHD prophylaxis during conditioning therapy.
The difference in platelet engraftment between Campath and ATG group found in our study is in contrast to results from two other studies by Juliusson et al. (2006) and Norlin and Remberger (2010), who did not find any significant differences in platelet engraftment between patients that received alemtuzumab or ATG. In contrast, a study by Kröger et al. (2005) confirmed a significant earlier platelet engraftment using alemtuzumab instead of ATG as part of the conditioning regimen.
These varying results may be explained by the type of ATG used in the studies. While Juliusson and Norlin used Thymoglobulin in their studies, Kröger used ATG Fresenius. Our ATG group included both types of ATG, Thymoglobulin and ATG Fresenius. In our subgroup analysis, we found only small differences in platelet engraftment for Thymoglobulin (14 vs. 12 days), but huge differences for ATG Fresenius (23 vs. 12 days). Therefore, there is no major contradiction of our results to those of Juliusson, Norlin and Kröger. The direct comparison in our subgroup analysis suggests that differences in platelet engraftment between Campath and ATG group were more due to effects of ATG Fresenius than of Thymoglobulin.
But how can these differences in platelet engraftment be explained?
Patients treated with ATG showed a significant higher platelet decrease during conditioning therapy and lower platelet counts at day 0 before infusion of the stem cell product compared with patients treated with alemtuzumab. From our clinical experience, we observed that ATG induces more serious inflammatory side effects than low doses of alemtuzumab, including fever, hypotension and SIRS. Since these clinical parameters are hardly to objectivize in a retrospective analysis, we focused on CRP as a surrogate parameter.
Lower platelet counts at day 0 after ATG may be due to a direct anti-platelet effect of the polyclonal antibodies, the induction of a more serious inflammatory reaction or even both. Two studies from Rosenberg et al. (1975) and Lekas and Rosenberg (1976) from the 1970s already described a direct anti-platelet effect of ATG in vitro. A recent study from Cumpelik confirmed this observation, describing a binding of ATG to platelets leading to platelet activation and hypercoagulability (Cumpelik et al. 2015). Corresponding to our clinical observations, we found significant higher levels for the inflammatory marker CRP after application of ATG compared with alemtuzumab (Table 2). So differences in clinically observed inflammatory side reactions and probable direct anti-platelet effects of ATG leading to a lower platelet count at day 0 paralleled by an increased number of transfused platelet concentrates may explain our findings in part.
Additionally, our results may be explained by a possible stimulatory effect of alemtuzumab on thrombocytopoiesis and megakaryocytopoiesis as already demonstrated in vitro by Deutsch et al. (1993) and Nagler et al. (1997) and supported by our own unpublished experiments using in vitro cultured megakaryocytic colonies in the absence and presence of alemtuzumab (Dölken G, unpublished).
As expected, a less pronounced reduction in platelet counts during conditioning and early after stem cell transplant as well as the earlier platelet engraftment in the Campath group runs parallel with a lower number of transfused platelet concentrates. This effect is also clinically of major relevance, with a ten times higher platelet concentrate consumption in the ATG subgroup with the latest platelet engraftment (alemtuzumab vs. ATG Fresenius). A shorter time to platelet engraftment was also related to a reduced number of transfused red cell concentrates necessary; one might discuss a reduction in silent blood losses in the presence of higher platelet counts.
Beside a reduction in bleeding risks, the application of alemtuzumab may be an approach in reducing the number of and costs for blood products to be given after allogeneic stem cell transplantation.
As in every retrospective analysis, results may be biased by changes in concomitant therapies. Our analysis was performed with patients treated from 2001 to 2012, when ATG was mainly used during earlier times of this period, while alemtuzumab was first used in 2007 in our institution. During this time, concomitant therapies (e.g., antiinfectives) and treatment of complications improved continuously which might have influenced engraftment times and consumption of blood component products. To exclude this bias, a prospective randomized trial will be necessary to proof the use of alemtuzumab as an approach to reduce the number of blood components to be transfused after allogeneic stem cell transplantation.
References
Avivi I, Chakrabarti S, Milligan DW et al (2004) Incidence and outcome of adenovirus disease in transplant recipients after reduced-intensity conditioning with alemtuzumab. Biol Blood Marrow Transpl 2004(10):186–194
Bertz H, Spyridonidis A, Wäsch R, Grüllich C, Egger M, Finke J (2009) A novel GVHD-prophylaxis with low-dose alemtuzumab in allogeneic sibling or unrelated donor hematopoetic cell transplantation: the feasibility of deescalation. Biol Blood Marrow Transpl 2009(15):1563–1570
Busemann C, Neumann T, Schulze M et al (2013) Low-dose alemtuzumab vs. standard policy for prevention of graft-versus-host disease in unrelated and related allogeneic stem cell transplantation-a matched pair analysis. Ann Hematol 2013(92):945–952
Chakrabarti S, Mackinnon S, Chopra R et al (2002) High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood 2002(99):4357–4363
Chakraverty R, Orti G, Roughton M et al (2010) Impact of in vivo alemtuzumab dose before reduced intensity conditioning and HLA-identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood 2010(116):3080–3088
Cumpelik A, Gerossier E, Jin J et al (2015) Mechanism of platelet activation and hypercoagulability by antithymocyte globulins (ATG). Am J Transpl 2015(15):2588–2601
Delgado J, Pillai S, Benjamin R et al (2008) The effect of in vivo T cell depletion with alemtuzumab on reduced-intensity allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia. Biol Blood Marrow Transpl 2008(14):1288–1297
Deutsch VR, Nagler A, Slavin S, Condiotti R, Levine RF, Eldor A (1993) Effect of bone marrow T lymphocytes treated with CAMPATH 1G on megakaryocyte colony formation. Exp Hematol 1993(21):1427–1435
Finke J, Bethge WA, Schmoor C et al (2009) Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 2009(10):855–864
Hale G, Zhang MJ, Bunjes D et al (1998) Improving the outcome of bone marrow transplantation by using CD52 monoclonal antibodies to prevent graft-versus-host disease and graft rejection. Blood 1998(92):4581–4590
Juliusson G, Theorin N, Karlsson K, Frödin U, Malm C (2006) Subcutaneous alemtuzumab vs ATG in adjusted conditioning for allogeneic transplantation: influence of Campath dose on lymphoid recovery, mixed chimerism and survival. Bone Marrow Transpl 2006(37):503–510
Kottaridis PD, Milligan DW, Chopra R et al (2000) In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood 2000(96):2419–2425
Kröger N, Shaw B, Iacobelli S et al (2005) Comparison between antithymocyte globulin and alemtuzumab and the possible impact of KIR-ligand mismatch after dose-reduced conditioning and unrelated stem cell transplantation in patients with multiple myeloma. Br J Haematol 2005(129):631–643
Lekas N, Rosenberg JC (1976) Release, aggregation and lysis of human platelets by antilymphocyte globulin and antiplatelet serum. Thromb Haemost 1976(36):411–423
Myers GD, Krance RA, Weiss H et al (2005) Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath). Bone Marrow Transpl 2005(36):1001–1008
Nagler A, Condiotti R, Lubina A, Deutsch VR (1997) Enhancement of megakaryocytopoiesis by Campath-1G-treated natural killer cells. Bone Marrow Transpl 1997(20):525–531
Norlin AC, Remberger M (2010) A comparison of Campath and Thymoglobulin as part of the conditioning before allogeneic hematopoietic stem cell transplantation. Eur J Haematol 2010(86):57–66
Park SH, Choi SM, Lee DG et al (2009) Infectious complications associated with alemtuzumab use for allogeneic hematopoietic stem cell transplantation: comparison with anti-thymocyte globulin. Transpl Infect Dis 2009(11):413–423
Peggs KS, Sureda A, Qian W et al (2007) Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcomes. Br J Haematol 2007(139):70–80
Penack O, Fischer L, Stroux A et al (2008) Serotherapy with thymoglobulin and alemtuzumab differentially influences frequency and function of natural killer cells after allogeneic stem cell transplantation. Bone Marrow Transpl 2008(41):377–383
Perez-Simon JA, Kottaridis PD, Martino R et al (2002) Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood 2002(100):3121–3127
Poiré X, van Besien K (2011) Alemtuzumab in allogeneic hematopoetic stem cell transplantation. Expert Opin Biol Ther 2011(11):1099–1111
Ramsay NK, Kersey JH, Robison LL et al (1982) A randomized study of the prevention of acute graft-versus-host disease. N Engl J Med 1982(306):392–397
Rosenberg JC, Lekas N, Lysz K, Morrell R (1975) Effect of antithymocyte globulin and other immune reactants on human platelets. Surgery 1975(77):520–529
Slichter SJ, Kaufman RM, Assmann SF et al (2010) Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med 2010(362):600–613
Soiffer RJ, LeRademacher J, Ho V et al (2011) Impact of immune modulation with anti–T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood 2011(117):6963–6970
Stanworth SJ, Estcourt LJ, Powter G et al (2013) A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med 2013(368):1771–1780
Theurich S, Fischmann H, Shimabukuro-Vornhagen A et al (2012) Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults. Cochrane Database Syst Rev 9:CD009159
Van Besien K, Kunavakkam R, Rondon G et al (2009) Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol Blood Marrow Transpl 2009(15):610–617
Waldmann H, Hale G (2005) CAMPATH: from concept to clinic. Philos Trans R Soc Lond B Biol Sci 2005(360):1707–1711
Wandt H, Schaefer-Eckart K, Wendelin K et al (2012) Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet 2012(380):1309–1316
Weiden PL, Doney K, Storb R, Thomas ED (1978) Anti-human thymocyte globulin (ATG) for prophylaxis and treatment of graft-versus-host disease in recipients of allogeneic marrow grafts. Transpl Proc 1978(10):213–216
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Neumann, T., Schneidewind, L., Thiele, T. et al. Reduced platelet transfusions and earlier platelet engraftment using alemtuzumab-based conditioning regimen in allogeneic stem cell transplantation. J Cancer Res Clin Oncol 142, 1091–1097 (2016). https://doi.org/10.1007/s00432-016-2114-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2114-7