Abstract
Purpose
Treatment options for patients with relapsed and refractory multiple myeloma have improved since the introduction of immune-modulating agents such as lenalidomide and thalidomide. However, almost all patients relapse and suffer from an increasing amount of adverse events due to multiple lines of therapy that eventually lead to a reduced quality of life.
Methods
In this bicentric retrospective analysis, 58 patients who had been treated with either bendamustine monotherapy (62 % of the patients) or combined steroid therapy were included. Further inclusion criteria were at least relapsed disease. Patients had previously been treated with a mean of four lines of therapy (range 1–10). They received a median of three cycles of treatment. Dosage varied from 60 to 300 mg/m2 (median 120 mg/m2) and was administered intravenously on day 1 and 2 of a 28-day cycle.
Results
Observed toxicity was mild and most commonly led to hematological side effects such as thrombopenia and anemia. Response rates were as follows: no complete response, 20 % partial response, 39 % minimal response, 27 % stable disease and 14 % progressive disease. Median overall survival (OS) was 17 months. Median event-free survival was 7 months. Patients who had not received a concomitant steroid had a median OS of 17 months compared to 13 months median OS for patients who had received a concomitant steroid.
Conclusion
Bendamustine monotherapy is an effective treatment option for heavily pre-treated myeloma patients due to its favorable response rate and mild toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
OS for patients with multiple myeloma has increased due to novel agents such as lenalidomide (Revlimid), bortezomib (Velcade) and autologous stem cell transplantation. However, almost all patients suffer from relapsed disease or develop refractory disease that still limits the OS to 3–5 years. Besides, patients who receive up to ten lines of treatment experience an increasing amount of adverse events that eventually lead to a reduced quality of life. Therefore, we examined bendamustine as a treatment option for heavily pre-treated patients regarding OS and EFS as primary endpoints. Secondary endpoints included overall response rate (ORR) and toxicity.
Bendamustine is a bifunctional alkylating agent causing intra- and inter-strand cross-links between DNA bases and shows only partial cross-resistance with other alkylating agents. While the mechlorethamine group is similar to other alkylators, the benzimidazole ring has structural homogeny with some purine analogs, suggesting that bendamustine may have purine analog in addition to alkylator activity (Kalaycio 2009). It has been used for therapy of lymphoma and myeloma and is known for its mild toxicity, most frequently including anemia, thrombopenia and leukopenia.
In this retrospective two-center analysis, we identified 58 patients with relapsed multiple myeloma who had received a bendamustine monotherapy or a concomitant steroid. Bendamustine was administered intravenously on day 1 and 2 of a 28-day cycle. Exclusion criteria were other combination partners, prior bendamustine treatment and first-line treatment.
Patients and methods
This is a retrospective study in patients with relapsed or refractory multiple myeloma. We investigated patients who had been treated at the University Hospital Bonn and the Klinikum Chemnitz, both Germany, between January 2001 and August 2011. A total of 58 patients with refractory or relapsed myeloma were reported. Bendamustine is an approved treatment option for myeloma in Germany, and hence a formal consent is not required.
We observed heavily pre-treated patients with relapsed or refractory multiple myeloma who had received a median of four previous lines of therapy before bendamustine treatment was started. Exclusion criteria were other combination partners, prior bendamustine treatment and first-line treatment. All patients were 18 years of age or older.
Bendamustine was administered intravenously on day 1 and 2 of a 28-day cycle. Median dosage was 120 mg/m2 (60–300 mg/m2). Treatment was stopped when disease progression occurred under treatment. Thirty-eight percent of patients (n = 22) received concomitant steroid therapy which was administered at a median dose of 40 mg on day 1–4 and 9–12.
Primary endpoints were OS and EFS. Secondary endpoints included assessment of ORR [defined as complete response (CR) + partial response (PR) + minimal response (MR)], stable disease (SD), progressive disease (PD), toxicity and the influence of predictors on OS and EFS under bendamustine treatment.
Investigator-evaluated response was assessed according to the International Myeloma Working Group uniform response criteria for multiple myeloma (Durie et al. 2006). ORR was calculated as CR + PR + MR as recommended by the American Society of Hematology/US Food and Drug Administration Workshop on Clinical Endpoints in Multiple Myeloma (Anderson et al. 2008). OS was calculated from the first date of bendamustine treatment to death. EFS was defined as time from first day of treatment to disease progression or death due to any cause. Predictors for OS and EFS included Salmon and Durie stage of disease, Calcium level, hemoglobin level, thrombocyte level and creatinine level before first day of treatment, among others.
Adverse events were graded according to CTC AE criteria version 3.0.
For OS and EFS, the Kaplan–Meier procedure was used to characterize the survival function. Kaplan–Meier curves for OS and EFS were further evaluated using the log-rank test to evaluate the association between dosage, concomitant therapy, Ig subtype and number of prior lines of therapy and OS and EFS. Cox regression was used to show the association between relevant prognostic factors and OS and EFS. For further evaluation, we added the hazard ratio to show the strength of association between prognostic factors and the OS and EFS. Prognostic factors included sex, primary refractory versus relapsed and refractory disease, Salmon and Durie stage (IIa and IIIa vs. IIIb), LDH level, calcium level, hemoglobin level (>/<7 g/dl), platelet level (>/<100,000/µl) and creatinine level before first day of bendamustine therapy as well as the number of prior lines of therapy. We used the IBM SPSS Statistics version 22 for statistical analysis.
Results
Baseline demographic and disease-related characteristics are shown in Table 1. The mean/median time from diagnosis to bendamustine treatment was 3.5 years (range 0.1–12). Of all patients, 22 patients received a concomitant steroid therapy. Patients received a median of three (range 1–8) cycles of treatment. Most of the patients (n = 40) had a Salmon and Durie stage IIIa disease, one suffered from a stage IIa and 14 from a stage IIIb disease. Patients had received a median of four previous lines of therapy (range 1–10). Overall, 29 patients (50 %) had received prior treatment with VAD (vincristine, adriamycin, dexamethasone), thirteen (22 %) with VMCP (vincristine, melphalan, cyclophosphamide, prednisone), ten (17 %) with VCAP (vincristine, cyclophosphamide, adriamycin, prednisone), 16 (28 %) with lenalidomide and 30 (52 %) with bortezomib. Twenty patients (34 %) had previously undergone autologous stem cell transplantation. Ten of those had received stem cell transplantation twice. The median dose of bendamustine was 120 mg/m2 (range 60–300 mg/m2). All patients were investigated with regard to efficacy and toxicity.
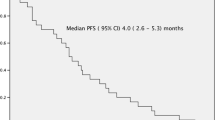
The median OS was 17 months (Fig. 1a), while median EFS was 7 months (Fig. 2a). Comparing monotherapy to treatment with concomitant steroids, median OS was 17 versus 13.5 months (p = 0.85) and median EFS was 7 months for both groups (p = 0.6). As we differentiated according to bendamustine dosage, OS for patients who had received <120 mg/m2 was 16.7 versus 15 months (p = 0.58) for those who had been treated with a dose of 120–300 mg/m2. Compared to non-responders, those patients who had at least achieved SD, OS did not improve significantly. Median OS for patients with IgG subtype was 21.8 versus 13.1 months for patients with IgA subtype (p = 0.18). Regarding number of prior lines of therapy, OS varied from 16.8 months for less than or three prior lines and 14 months for more than three lines of prior treatment (p = 0.83).
a Kaplan–Meier plot of dosage-dependent (>/<120 mg/m2) OS. Median of OS was 17 months. b Kaplan–Meier plot of OS comparing monotherapy and concomitant steroid therapy. c Kaplan–Meier plot of OS comparing subtypes IgG and IgA. d Kaplan–Meier plot of OS comparing number of prior lines of therapy (>/≤3)
a Kaplan–Meier plot of dosage-dependent EFS (>/<120 mg/m2). Median event-free survival was 7 months. b Kaplan–Meier plot of EFS comparing monotherapy and concomitant steroid therapy. c Kaplan–Meier plot of EFS comparing subtypes IgG and IgA. d Kaplan–Meier plot of EFS comparing number of prior lines of therapy (>/≤3)
Regarding relevant prognostic factors for OS such as age, creatinine, thrombocytopenia and anemia that have been described previously, we could not find significant differences in OS or EFS as shown in Table 2 using Cox regression. To further evaluate the strength of association between these prognostic factors and OS and EFS, we added the hazard ratio (HR). An association between reduced OS and severe thrombocytopenia, severe anemia, primary refractory disease, prior autologous stem cell transplantation can be shown, while stage IIa and IIIa disease is associated with an increased OS in contrast to stage IIIb disease, as expected. Creatinine level, LDH level, age and sex were not associated with reduced or increased OS in this analysis (Table 2).
Forty-four patients were evaluable regarding response rate as shown in Table 3. The ORR (CR + PR + MR) was 59 %. No CR was observed. Of those patients, nine (20 %) were reported with PR, one of whom achieved a very good PR. Seventeen (39 %) patients achieved MR. SD was observed in twelve (27 %) patients. Six patients (14 %) suffered from PD during bendamustine treatment. Comparing those who had received a monotherapy to those who had received a concomitant steroid, response rates were both 59 %. The ORR for those who had received more or <120 mg/m2 was 53 versus 64 %. Regarding Ig subtype, response rates were 57 % with IgG subtype and 64 % with IgA subtype. Patients who had received three or less prior lines of therapy had an ORR of 65 %, while patients who had received more than three lines had an ORR of 54 %.
As shown in Table 4, there was no significant difference in patients who had at least achieved MR regarding dosage, combined steroid therapy, Ig subtype and number of prior lines of therapy compared to those patients who had suffered from SD or PD. There was also no significant difference regarding OS and EFS between patients who had at least achieved MR and patients who had SD or PD.
As expected and shown in Table 5, hematological side effects were reported most frequently. Anemia with hemoglobin level <6.5 g/dl (grade 4) was reportedly seen in 53 % of patients (n = 30). Of these patients, only six developed grade 4 anemia during or after bendamustine treatment. Of all patients, 79 % suffered from anemia. There was no significant difference regarding anemia between monotherapy and concomitant steroid treatment. There was also no association between dosage and grade of anemia. Leukopenia could be observed in 60 % (n = 35) of patients. Most of them (74 %) were mild to moderate, and no association with combined steroid therapy and dosage was observed. Thrombocytopenia was observed in 40 % (n = 23) of patients. Eleven patients had mild-to-moderate thrombocytopenia, whereas twelve patients suffered from severe thrombocytopenia with platelet counts of <50,000/µl. Dosage and concomitant steroid therapy were not associated with grade of thrombocytopenia. Erythrocyte concentrate and GCSF support were administered as required and were reported for two patients. Two patients suffered from a mild allergic reaction during the second cycle of bendamustine treatment. Allergic reactions included generalized exanthema and mild bronchospasm. Allergic reactions were not dose dependent. Other adverse events included mild fatigue, nausea and vomitus and were reportedly observed in three patients. In one patient, worsening in neuropathic pain was observed. No grade 3/4 non-hematological side effects were observed. No thromboembolic events were documented.
Discussion
In this retrospective study, bendamustine monotherapy and combined steroid therapy in heavily pre-treated myeloma patients resulted in a median OS of 17 months and median EFS of 7 months. ORR was estimated at 59 % (CR + PR + MR). However, just one vgPR and no immunofixation-negative CR, which might be considered a key determinant in long-term outcome of salvage therapy, were observed (Niesvizky et al. 2008). SD was considered a valid therapeutic goal and was achieved in 27 % (n = 12) of patients. Reported toxicity was mild, mainly including hematological side effects such as anemia, thrombocytopenia and leukopenia.
Similar OS and EFS (17/7 months) were reported by Michael et al. (2010) for bendamustine monotherapy in relapsed or refractory myeloma for patients who had received a median of two lines of previous therapy (Michael et al. 2010). There are some studies which have investigated bendamustine and combination partners in relapsed or refractory myeloma. For bendamustine in combination with dexamethasone and bortezomib, OS of 50 months was reported with a less heavily pre-treated collective of patients (two lines of previous treatment) without severe hematological toxicities due to previous treatments (Pönisch et al. 2013). Lentzsch et al. (2012) conducted a phase I/II trial to investigate the efficacy of a bendamustine, lenalidomide and dexamethasone combination in heavily pre-treated patients (three lines of previous therapy). PFS was 6.1 months, and OS after 1 year was 93 %.
Another study conducted by Ludwig et al. (2014) investigating bendamustine, bortezomib and dexamethasone (BVD) combination showed an OS of 25.6 months and PFS of 9.7 months in patients with two prior therapy lines.
Regarding response rates, similar results (ORR 43 %) were achieved in comparably pre-treated patients (five previous lines of therapy, baseline hemoglobin 9.8 mg/dl), who were treated with thalidomide, bendamustine and dexamethasone as reported by Grey-Davies et al. (2012). However, OS was estimated at 13 versus 17 months in our study. A superior response rate of 76 % was reached (PR 28 %, vgPR 24 %, MR 24 %) with a bendamustine, lenalidomide and dexamethasone combination (Pönisch et al. 2013). An ORR of 48 % in 40 heavily pre-treated patients (six previous lines of therapy) was reported by Berenson et al. (2013) in a phase I/II trial assessing bendamustine plus bortezomib in patients with relapsed or refractory multiple myeloma. OS was estimated at 13.3 months. Rodon et al. (2015) conducted a phase II study of BVD in elderly patients (>65 years) with one previous line of therapy. ORR was 67 % (CR 10.9 %, VGPR 16.5 %, PR 39.7 %), and median OS was estimated at 23 months. A response rate of 71.5 % (16 % CR, 18.5 % VGPR, 37 % PR) in 75 patients who had a median of one line of previous therapy was reported by Offidani et al. (2013) in a phase II study with the BVD regimen.
As expected, most side effects under bendamustine treatment were hematological such as grade 3/4 anemia (71 %) and grade 1/2 leukopenia (45 %). In the study Michael et al. conducted, grade 1/2 anemia was reported for 82 % of patients, grade 3/4 neutropenia in 41 % as well as grade 3/4 thrombopenia in 27 %. Still it has to be considered that patients in this study were less heavily pre-treated and therefore did not show a baseline grade 3/4 anemia. Combination therapies resulted in an increase in adverse events. 38 % of patients receiving bendamustine, lenalidomide and dexamethasone showed grade 3/4 thrombocytopenia and 62 % suffered from grade 3/4 neutropenia with 41 % receiving G-CSF for prolonged grade 3/4 neutropenia (Pönisch et al. 2013). As reported by Berenson et al. (2013), severe hematological side effects (grade 3/4 leucopenia, neutropenia, lymphopenia, thrombocytopenia and anemia) occurred in 83 % of patients with the bendamustine and bortezomib regimen. In the BVD regimen, severe adverse events were reported for 65 % of patients mostly due to infections (23.2 %). Few severe hematological side effects were reported (20.5 % neutropenia, 9.5 % thrombocytopenia, 5.5 % anemia) (Rodon et al. 2015). In the study conducted by Offidani et al. (2013) with the BVD regimen, common severe adverse events were thrombocytopenia (30.5 %), neutropenia (18.5 %), infections (12 %) and neuropathy (8 %).
Because of the small group of patients and the retrospective nature of this study, results should be viewed critically. Furthermore, not all patients had been previously exposed to immune-modulating agents or proteasome inhibitors which might be considered an important factor regarding response rate.
With the use of a validated questionnaire, a better quality of life has been reported for bendamustine first-line treatment and might also be suggested for salvage therapy (Pönisch et al. 2006). Regarding OS, a better outcome for combined steroid therapy could not be reported. In contrast, patients who had received concomitant steroids showed an OS of 13 versus 17 months for patients who had been treated with bendamustine monotherapy like it had previously been reported by Michael et al. (2010).
In conclusion, we can recommend bendamustine monotherapy as an effective salvage treatment option in heavily pre-treated patients, especially for those who are not eligible for combination therapies due to its mild toxicity and convincing response rates.
References
Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P (2008) Clinically relevant end points and new drug approvals for myeloma. Leukemia 22(2):231–239. doi:10.1038/sj.leu.2405016
Berenson JR, Yellin O, Bessudo A, Boccia RV, Noga SJ, Gravenor DS et al (2013) Phase I/II trial assessing bendamustine plus bortezomib combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. Br J Haematol 160(3):321–330. doi:10.1111/bjh.12129
Durie BGM, Harousseau J-L, Miguel JS, Bladé J, Barlogie B, Anderson K et al (2006) International uniform response criteria for multiple myeloma. Leukemia 20(9):1467–1473. doi:10.1038/sj.leu.2404284
Grey-Davies E, Bosworth JL, Boyd KD, Ebdon C, Saso R, Chitnavis D et al (2012) Bendamustine, thalidomide and dexamethasone is an effective salvage regimen for advanced stage multiple myeloma. Br J Haematol 156(4):552–555. doi:10.1111/j.1365-2141.2011.08887.x
Kalaycio M (2009) Bendamustine: a new look at an old drug. Cancer 115(3):473–479. doi:10.1002/cncr.24057
Lentzsch S, O’Sullivan A, Kennedy RC, Abbas M, Dai L, Pregja SL et al (2012) Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood 119(20):4608–4613. doi:10.1182/blood-2011-12-395715
Ludwig H, Kasparu H, Leitgeb C, Rauch E, Linkesch W, Zojer N et al (2014) Bendamustine–bortezomib–dexamethasone is an active and well-tolerated regimen in patients with relapsed or refractory multiple myeloma. Blood 123(7):985–991. doi:10.1182/blood-2013-08-521468
Michael M, Bruns I, Bölke E, Zohren F, Czibere A, Safaian NN et al (2010) Bendamustine in patients with relapsed or refractory multiple myeloma. Eur J Med Res 15(1):13–19
Niesvizky R, Richardson PG, Rajkumar SV, Coleman M, Rosiñol L, Sonneveld P et al (2008) The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 APEX trial in relapsed multiple myeloma. Br J Haematol 143(1):46–53. doi:10.1111/j.1365-2141.2008.07303.x
Offidani M, Corvatta L, Maracci L, Liberati AM, Ballanti S, Attolico I et al (2013) Efficacy and tolerability of bendamustine, bortezomib and dexamethasone in patients with relapsed-refractory multiple myeloma: a phase II study. Blood Cancer J 3(11):e162. doi:10.1038/bcj.2013.58
Pönisch W, Mitrou PS, Merkle K, Herold M, Assmann M, Wilhelm G et al (2006) Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone—a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO). J Cancer Res Clin Oncol 132(4):205–212. doi:10.1007/s00432-005-0074-4
Pönisch W, Heyn S, Beck J, Wagner I, Mohren M, Hoffmann FA et al (2013) Lenalidomide, bendamustine and prednisolone exhibits a favourable safety and efficacy profile in relapsed or refractory multiple myeloma: final results of a phase 1 clinical trial OSHO-#077. Br J Haematol. doi:10.1111/bjh.12361
Rodon P, Hulin C, Pegourie B, Tiab M, Anglaret B, Benboubker L et al (2015) Phase II study of bendamustine, bortezomib and dexamethasone as second-line treatment for elderly patients with multiple myeloma: the Intergroupe Francophone du Myelome 2009-01 trial. Haematologica 100(2):e56–e59. doi:10.3324/haematol.2014.110890
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that no conflicts of interest exist. There is no conflict to study participants. There is no study sponsor.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethics approval for this retrospective study had been obtained according to the guidelines of the host institution.
Rights and permissions
About this article
Cite this article
Stöhr, E., Schmeel, F.C., Schmeel, L.C. et al. Bendamustine in heavily pre-treated patients with relapsed or refractory multiple myeloma. J Cancer Res Clin Oncol 141, 2205–2212 (2015). https://doi.org/10.1007/s00432-015-2014-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-2014-2