Abstract
Purpose
Dysregulation of microRNA (miRNA) metabolism has been observed in a variety of human cancers, but the expression patterns of the enzymes responsible for generating miRNAs remain largely unexplored. In this study, we investigated the expression profiles of the two most important enzymes of the miRNA machinery, Drosha and Dicer, which were closely correlated with nasopharyngeal carcinoma (NPC) and patient survival.
Methods
Dicer and Drosha mRNA levels were detected by quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) using 24 NPC tissues, 7 normal nasopharyngeal epithelium samples (NPE) and NPC cell lines. In addition, protein levels were detected by immunohistochemistry (IHC) using an NPC tissue microarray (TMA), which include 251 NPC and 105 NPE cases. For some NPC patients can not be contacted, the survival data were available only for 146 patients. Kaplan–Meier analysis was performed, and the chi-square and log-rank tests were used to detect significance levels using SPSS 15.0 software.
Results
The mean level of Dicer and Drosha mRNA were significantly down-regulated in NPC tissue specimens and cell lines when compared with controls. The low levels of Dicer and Drosha protein were frequently seen in NPC, and the low expression of Dicer and Drosha protein was significantly correlated with shorter progression-free survival (PFS) and overall survival (OS) of NPC patients.
Conclusions
We observed that Drosha and Dicer expression was dysregulation in NPC compared with healthy control samples and was significantly correlated with shorter PFS and OS of NPC patients. Therefore, we hypothesise that the expression levels of Dicer and Drosha could be used as potential prognostic biomarkers for NPC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MicroRNAs (miRNAs) are a class of small, evolutionarily conserved, endogenous non-coding RNAs which act as negative regulators of gene expression by inhibiting translation or promoting RNA degradation. miRNAs have been implicated in several key developmental and biological phenomena, including cell differentiation, proliferation and apoptosis (Bushati and Cohen 2007). According to the miRNA database (miRBase), 721 different human miRNAs have been described to date (miRNA database miRBase http://www.microrna.sanger.ac.uk/sequences/) and are thought to play important roles in the post-transcriptional regulation of up to 30% of all human genes. There is increasing coherent evidence indicating that miRNAs play an important role in cancer development (Esquela-Kerscher and Slack 2006).
miRNAs are transcribed by RNA polymerase II to generate primary miRNA transcripts, which are processed into ~70-nt hairpin structured pre-miRNAs by Drosha, a RNase III endonuclease located in the nucleus (Lee et al. 2003). After being transported to the cytoplasm by Exportin 5, pre-miRNA is further processed by another RNase III endonuclease, Dicer, to generate the ~22-nt mature miRNA (Macrae et al. 2006). Given the roles played by Drosha and Dicer, it has been hypothesised that the global down-regulation of miRNA expression in tumours may be driven by alterations in the expression levels or activity of these two enzymes. In fact, down-regulation of Dicer (Grelier et al. 2009; Chiosea et al. 2008) and both Drosha and Dicer expression levels has been associated with aggressive clinical behaviour in lung, breast (Dedes et al. 2011) and ovarian cancers (Merritt et al. 2008), and some studies have suggested a potential association between lower levels of Drosha and Dicer and reduced survival in neuroblastoma (Lin et al. 2010).
Nasopharyngeal carcinoma (NPC) is endemic in southern China, Southeast Asia and North Africa. Recently, Sengupta et al. (2008) identified eight differentially expressed miRNAs in NPC, and another study (Chen et al. 2009) identified 35 miRNAs whose expression levels were significantly altered in NPC tissues. However, the dysregulation of microRNA-processing enzymes Drosha and Dicer in NPC has not been investigated to date.
In this study, we therefore investigated the expression levels of the miRNA-processing enzymes Dicer and Drosha in NPC cell lines and clinical specimens of NPE and NPC. Both Dicer and Drosha were found to be down-regulated in NPC cell lines and the vast majority of NPC samples compared with normal tissues, and the low expression of Dicer and Drosha were significantly correlated with shorter progression-free survival (PFS) and overall survival (OS) of NPC patients.
Materials and methods
Cell lines and tumour samples
The normal NP cell line NP69 was kindly provided by The Chinese University of Hong Kong; the NPC cell lines HNE1, HNE2, CNE1, CNE2, 5-8F and 6-10B were kindly provided by the Cancer Center of Sun Yet-Sen University (Guangzhou, China).
For quantitative real-time PCR (qRT-PCR), 7 NPE samples and 24 NPC biopsies were collected from Xiangya Hospital (Changsha, PR China) in 2010. All tissue samples were snap frozen in liquid nitrogen and stored at −80°C until laser-capture micro-dissection (LCM).
For preparation of the NPC tissue microarray (TMA), nasopharyngeal biopsy specimens were collected in the Ear, Nose and Throat (ENT) department at Xiangya Hospital (Changsha, PR China) from January 2002 to October 2004. All biopsies were immediately fixed in 4% buffered paraformaldehyde, routinely processed and embedded in paraffin.
The samples were fully encoded and examined under a protocol approved by the Institutional Review Board of Human Subjects Research Ethics Committee. All of the individuals participating in this project signed the informed consent form.
LCM and RNA isolation
Eight-micrometre-thick frozen sections of fresh NPC and NPE were prepared using a Leica CM 1900 cryostat (Lecia, Germany) at −25°C. The sections were placed on membrane-coated glass slides (2.0 μm, 50 pieces, PEF Membrane; Lecia, German), fixed in 75% alcohol for 30 s and stained with 0.5% violet-free methyl green (Sigma, USA). After staining, the sections were air-dried and micro-dissected using a Leica AS LMD system (Lecia, German). LCM caps were stored at −80°C until further use.
Total RNA was isolated using TRIZOL reagent (Invitrogen, USA) according to the manufacturer’s instructions. RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA) and treated with DNase I according to the manufacturer’s instructions (Qiagen, Valencia, CA). The integrity and quality of RNA were confirmed by agarose gel electrophoresis and absorbance at 260 nm. Then, total RNA was reverse transcribed to cDNA using SuperScript™ First-Strand Synthesis System with random hexamer primers (Promega, USA).
Real-time quantitative PCR
Real-time quantitative PCR was performed using cDNA; the following primers were used. Dicer: (forward) 5′-GTACGACTACCACAAGTACTTC-3′ and (reverse) 5′-ATAGTCACCTGCCAGACTGT-3′; Drosha: (forward) 5′-CATGTCACAGAATGTCGTTCCA-3′ and (reverse) 5′-GGGTGAAGCAGCCTCAGATTT-3′; β-actin: (forward) 5′-AGCGAGCATCCCCCAAAGTT-3′ and (reverse) 5′-GGGCACGAAGGCTCATCATT-3′. All reactions were run in triplicate, and expression levels were normalised against β-actin.
TMA construction and immunohistochemistry
Representative areas of 251 NPC and 105 various non-cancerous NPE specimens were marked on each haematoxylin-eosin (H&E) slide and tissue paraffin block, and the marked areas of the tissue paraffin blocks were sampled for the TMA. The TMAs were assembled with a tissue-arraying instrument (Zhang et al. 2009). Briefly, the instrument was used to create holes in a recipient paraffin block with defined array coordinates. A solid stylet was used to transfer the tissue cores into the recipient block. One to three 0.6-mm-diameter tissue cores were taken from each NPC and non-cancerous NPE. A series of 5-μm-thick sections were cut with a Leica microtome (RM 2135, Germany) and transferred to adhesive-coated slides using a paraffin tape-transfer system (Instrumedics Inc., USA). One slide from each recipient block was stained with H and E. The remaining slides were covered with thin paraffin and stored at 4°C before immunohistochemistry (IHC).
IHC studies were performed using the standard streptavidin/peroxidase staining method, as described previously (Zhang et al. 2009). The antibodies were rabbit anti-Dicer, 1:50 (Cell Signaling, USA) and rabbit anti-Drosha, 1:100 (Cell Signaling, USA). All known positive sections were taken as positive controls. Negative mouse serum and PBS were used instead of the first antibody as a negative control and blank control, respectively. A semi-quantitative scoring criterion for IHC was used in which both staining intensity and positive areas were recorded. The tissue core numbers on each section were slightly different because of additional losses suffered from block trimming and staining procedures.
Survival analysis
For some NPC patients can not be contacted, the survival data were available only for 146 patients (20 were NPC relapses after radiation) and the median follow-up time was 51 months (3–96 months). Of the 146 NPC patients, 125 were men and 21 were women (mean age, 48 years). Using the tumour-node-metastasis (TNM) classification of malignant tumours, the clinical stages of the NPC samples were classified as follows: 11 cases of stage I, 26 cases of stage II, 48 cases of stage III, and 61 cases of stage IV. Of the patients included in the study, 104 had lymph node metastasis, and 42 patients were lymph node negative. No case showed distant metastasis. Overall survival (OS) was defined as the time from diagnosis to date of death. Progression-free survival (PFS) was defined as the time from diagnosis to date of first failure. The OS and PFS estimates over time were calculated using the Kaplan–Meier method, and differences were compared using the log-rank test. Results of the analysis were considered significant in a log-rank test if P < 0.05.
Data analysis
Statistical procedures were analysed using SPSS version 15.0. The best cut-off values for separating the two groups in terms of gene expression levels (Dicer, Drosha) were determined by the chi-square test. PFS and OS were estimated using the Kaplan–Meier method and compared by log-rank test. P < 0.05 was considered statistically significant.
Results
Down-regulation of Dicer and Drosha in NPC samples and cell lines
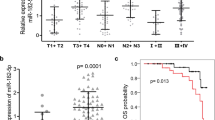
To explore the mRNA expression of Dicer and Drosha in a clinical setting, 24 NPC specimens and 7 normal NPE samples were obtained to analyse Dicer and Drosha mRNA expression in these tissues by qRT-PCR. All tissue specimens were purified by LCM before the RNA extraction. Figure 1a shows that samples were micro-dissected by the LCM system to remove lymphocytes and interstitial cells. Figure 1b and c show that the average mRNA expression levels of Dicer (P = 0.02) and Drosha (P = 0.01) were lower in NPC specimens than in NPE tissues.
Expression levels of Dicer and Drosha in NPC samples and cell lines. a Top panel representative photographs (X100) of a micro-dissected normal nasopharyngeal epithelium sample; bottom panel NPC epithelium stained by methyl green. b and c qRT-PCR analysis of the relative expression levels of Dicer and Drosha in 24 NPC specimens and 7 NPE specimens; data were normalised according to β-actin. Differences between groups were analysed using the chi-square test (Drosha: P = 0.01; Dicer: P = 0.02). d qRT-PCR analysis of the relative expression levels of Dicer and Drosha in the cell lines; data were normalised according to β-actin
We also examined Dicer and Drosha mRNA levels in a panel of NPC cell lines (Fig. 1d). Compared with NP69, both Dicer and Drosha mRNA levels were reduced in all NPC cell lines.
Protein expression of Dicer and Drosha using NPC-specific TMA
For the purpose of clinical correlative analyses, we used Student’s t-test to select a cut-off value that could best discriminate high- and low-protein expression groups for Dicer and Drosha. As shown in Table 1, low Dicer (159 of 231, 78.8%) and Drosha (153 of 231, 76.2%) protein expression levels were frequently seen in NPC; however, in NPE, low-Dicer protein expression was found at a frequency of 17.3% (18/104) and low-Drosha protein expression was found in 15.4% (16/104) of the samples. In addition, Dicer and Drosha protein expression levels were not correlated with age, gender or lymph node metastasis, but the protein expressions of Dicer (P = 0.001) and Drosha (P = 0.002) were significantly lower in stage III + IV than in stage I + II. Representative images can be seen in Fig. 2.
Lower expression of Dicer or Drosha protein is associated with shorter survival
Next, we examined the prognostic value of Dicer and Drosha protein expression. The Kaplan–Meier survival analyses showed that NPC patients with low expression levels of Dicer protein had a significantly shorter PFS (42.5% vs. 67.1%; P = 0.004) and OS (42.5 vs. 67.1%; P = 0.001) than those with high levels of Dicer (Fig. 3a). Similarly, low expression of Drosha protein was significantly associated with shorter PFS (40.5% vs. 69.4%, P = 0.02) and OS (40.5% vs. 69.4%; P = 0.01) compared with high expression of Drosha protein (Fig. 3b). These findings suggest that the protein expression levels of Dicer and Drosha can serve as important and independent predictors in NPC patients.
For the cox multivariate analysis, there were no difference regarding sex and age, and there was a trend to a higher chance of PFS or OS in the stage (I + II) versus stage (III + IV), but all the difference was non-significant. On the other hand, there were highly significant differences between low Dicer and Drosha expression and decreased PFS with hazard ratios (HR) of 0.453 (P = 0.027) and 0.462 (P = 0.032), respectively. A similar highly significant differences of association was noted between low Dicer and Drosha expression and decreased OS with hazard ratios (HR) of 0.417 (P = 0.019) and 0.429 (P = 0.024), respectively, (Table 2).
Discussion
In this study, we determined the expression profiles of Dicer and Drosha, two key enzymes in microRNA biogenesis, at the mRNA and protein levels. We found a strong correlation between Dicer and Drosha protein expression and PFS as well as OS, which showed a highly significant, linear distribution in the Kaplan–Meier analysis (P < 0.01). If corroborated in future prospective studies, this indicates that a combination of Dicer and Drosha protein expression levels at diagnosis may serve as an NPC-specific biomarker for risk group assignment and selection of optimal therapy as well as a powerful predictor of clinical outcome.
Cumulative evidence suggests that miRNAs have major functions in the pathogenesis of tumours. Large-scale profiling has revealed a global alteration of miRNA expression patterns in human cancers (Murakami et al. 2006; Kida and Han 2008), especially in NPC (Chen et al. 2009). Biological characterisation has also identified several miRNAs that function as tumour suppressors or oncogenes. Our previous study showed that miRNA-141 is involved in a NPC-related gene network (Zhang et al. 2010). Recently, large studies showed globally lower miRNA levels were seen in tumour tissues compared with corresponding normal tissues, especially in poorly differentiated compared with well-differentiated tumour tissues (Lu et al. 2005). The same finding was observed in several cell lines (Gaur et al. 2007), indicating that distinct miRNA expression signatures might serve as diagnostic and prognostic markers for various types of human cancers (Schetter et al. 2008; Yu et al. 2008).
Both the intranuclear miRNA-processing enzyme Drosha and the extranuclear microRNA-processing enzyme Dicer play pivotal roles in the maturation of miRNAs. For example, Lee et al. (2003) demonstrated that in cells with silenced Dicer or Drosha expression, precursor and mature miRNA sequences were reduced. Loss of Dicer in mice disrupts embryonic stem-cell differentiation and is lethal during early development (Bernstein et al. 2003). Low levels of Dicer mRNA also affect normal cellular development and immune responses in pre-clinical models (Cobb et al. 2006; Iida et al. 2011). Subsequent functional experiments revealed that cells with Dicer and Drosha targeted by short hairpin RNAs could be driven towards transformation (Kumar et al. 2007; Kobayashi et al. 2008).
Lately, emerging evidence has linked the miRNA-processing machinery to cancer. Large studies have shown that the low expression of Dicer and Drosha was seen in various tumours, including breast, lung and ovarian cancer as well as neuroblastoma (Merritt et al. 2008; Dedes et al. 2011). At the same time, the low expression of Dicer and Drosha can predict prognosis and delineate risk groups (Lin et al. 2010). In contrast, the up-regulation of Dicer has also been reported in prostate adenocarcinoma (Chiosea et al. 2006) and colorectal cancer (Faber et al. 2011). As described above, aggressive tumours are thought to have decreased total miRNA levels, contributing to their poor differentiation. This would suggest a decreased rather than an increased expression of Dicer or Drosha in aggressive cancers. This is consistent with our finding in NPC. In our study, we found that low expression of either Dicer or Drosha in NPC was correlated well with global miRNA down-regulation (Chen et al. 2009). We also found that low expression of either Dicer or Drosha seemed to have a prognostic impact. These studies revealed that the dysregulation of Dicer and Drosha exist in many types of human cancers, which may affect tumour progression and prognosis by altering miRNA expression.
Although several studies have been conducted in different cancer types to elucidate the roles of Dicer and Drosha in carcinogenesis and their impact on prognosis, the contribution of Dicer and Drosha has not been addressed in NPC. We demonstrated that lower expression of Dicer and Drosha was responsible for global down-regulation of miRNAs in NPC. Importantly, low expression of Dicer and Drosha protein was significantly correlated with shorter PFS and OS of NPC patients. Thus, expression of Dicer and Drosha appeared to be another significant prognostic factor for NPC, and we can probably predict the prognosis of NPC by measuring both Dicer and Drosha in future.
References
Bernstein E, Kim SY, Carmell MA et al (2003) Dicer is essential for mouse development. Nat Genet 35(3):215–217
Bushati N, Cohen SM (2007) microRNA functions. Annu Rev Cell Dev Biol 23:175–205
Chen HC, Chen GH, Chen YH et al (2009) MicroRNA deregulation and pathway alterations in nasopharyngeal carcinoma. Br J Cancer 100(6):1002–1011
Chiosea S, Jelezcova E, Chandran U et al (2006) Up-regulation of dicer, a component of the MicroRNA machinery, in prostate adenocarcinoma. Am J Pathol 169(5):1812–1820
Chiosea SI, Barnes EL, Lai SY et al (2008) Mucoepidermoid carcinoma of upper aerodigestive tract: clinicopathologic study of 78 cases with immunohistochemical analysis of Dicer expression. Virchows Arch 452(6):629–635
Cobb BS, Hertweck A, Smith J et al (2006) A role for Dicer in immune regulation. J Exp Med 203(11):2519–2527
Dedes KJ, Natrajan R, Lambros MB et al (2011) Down-regulation of the miRNA master regulators Drosha and Dicer is associated with specific subgroups of breast cancer. Eur J Cancer 47(1):138–150
Esquela-Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6(4):259–269
Faber C, Horst D, Hlubek F, Kirchner T (2011) Overexpression of Dicer predicts poor survival in colorectal cancer. Eur J Cancer 47(9):1414–1419
Gaur A, Jewell DA, Liang Y et al (2007) Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res 67(6):2456–2468
Grelier G, Voirin N, Ay AS et al (2009) Prognostic value of Dicer expression in human breast cancers and association with the mesenchymal phenotype. Br J Cancer 101(4):673–683
Iida A, Shinoe T, Baba Y, Mano H, Watanabe S (2011) Dicer plays essential roles for retinal development by regulation of survival and differentiation. Invest Ophthalmol Vis Sci 52(6):3008–3017
Kida Y, Han YP (2008) MicroRNA expression in colon adenocarcinoma. JAMA 299(22):2628 author reply 2628-2629
Kobayashi T, Lu J, Cobb BS et al (2008) Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc Natl Acad Sci USA 105(6):1949–1954
Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T (2007) Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 39(5):673–677
Lee Y, Ahn C, Han J et al (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425(6956):415–419
Lin RJ, Lin YC, Chen J et al (2010) microRNA signature and expression of Dicer and Drosha can predict prognosis and delineate risk groups in neuroblastoma. Cancer Res 70(20):7841–7850
Lu J, Getz G, Miska EA et al (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834–838
Macrae IJ, Zhou K, Li F et al (2006) Structural basis for double-stranded RNA processing by Dicer. Science 311(5758):195–198
Merritt WM, Lin YG, Han LY et al (2008) Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med 359(25):2641–2650
Murakami Y, Yasuda T, Saigo K et al (2006) Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene 25(17):2537–2545
Schetter AJ, Leung SY, Sohn JJ et al (2008) MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA 299(4):425–436
Sengupta S, den Boon JA, Chen IH et al (2008) MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA 105(15):5874–5878
Yu SL, Chen HY, Chang GC et al (2008) MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell 13(1):48–57
Zhang W, Zeng Z, Zhou Y et al (2009) Identification of aberrant cell cycle regulation in Epstein-Barr virus-associated nasopharyngeal carcinoma by cDNA microarray and gene set enrichment analysis. Acta Biochim Biophys Sin (Shanghai) 41(5):414–428
Zhang L, Deng T, Li X et al (2010) microRNA-141 is involved in a nasopharyngeal carcinoma-related genes network. Carcinogenesis 31(4):559–566
Acknowledgments
We are grateful to the Ear, Nose and Throat (ENT) Department at Xiangya Hospital for providing NPC samples. Guiyuan Li has received the 111 project (111-2-12) research grants. Wenling Zhang has received the National Science Foundation of China (81071644). Wei Xiong has received the Program for New Century Excellent Talents in University (NCET-08-0562).
Conflict of interest
We declare that we have no financial and personal relationships with other people or organisations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the manuscript entitled “The microRNA-Processing Enzymes Drosha and Dicer can Predict Prognosis of Nasopharyngeal Carcinoma”.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiaofang Guo and Qianjin Liao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, X., Liao, Q., Chen, P. et al. The microRNA-processing enzymes: Drosha and Dicer can predict prognosis of nasopharyngeal carcinoma. J Cancer Res Clin Oncol 138, 49–56 (2012). https://doi.org/10.1007/s00432-011-1058-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-1058-1