Abstract
MicroRNAs (miR) are small noncoding RNAs that are predicted to regulate up to 30% of protein-encoding genes. miR maturation requires functional microRNA machinery, including the Dicer protein. We review our experience with mucoepidermoid carcinoma (MEC) and characterize the prognostic value of Dicer expression. Expression of Dicer was assessed in 78 MEC by immunohistochemistry. Dicer expression was scored semiquantitatively and relative to the internal controls: large excretory/striated ducts or basal/parabasal layers of normal squamous epithelium (mucosa). Dicer scores were then correlated with clinical and pathologic parameters. Dicer over- and/or under-expression were more commonly seen in high-grade MEC (83%) than in low/intermediate grade MEC (35%; p = 0.002) and in stage III/IV MEC (80%) than in stage I/II MEC (41%; p = 0.04). Abnormal Dicer expression correlates with high-grade and advanced stage, acting as a univariate predictor of poor disease-specific survival (DSS) in MEC. Age and stage were independent predictors of poor DSS on multivariate analysis. Abnormal immunoexpression of Dicer in aggressive MEC suggests a role for miR and miR machinery in tumor progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salivary gland malignancies are rare, accounting for about 6% of all head and neck cancers. They show great morphologic diversity even within a specific tumor type. Ancillary studies such as DNA content analysis, proliferation index and p53 expression have been applied to salivary gland neoplasms in an attempt to refine diagnosis and predict biologic behavior [15, 27, 31]. More recently, several gene array studies have expanded the list of biomarkers in salivary gland tumors [9, 10, 18, 21]. It is interesting to note that, of the salivary carcinoma types evaluated to date by gene array, only two types, mucoepidermoid carcinoma (MEC) and adenoid cystic carcinoma, consistently showed distinct gene-expression profiles [18, 21]. MEC is the most common primary salivary gland malignancy in both adults and children [11, 32]. As with most salivary tumors, conventional pathologic parameters of MEC are not completely reliable, as even MEC assigned low to intermediate grade may behave poorly [3, 4, 25]. Furthermore, current treatment modalities for high-grade aggressive MEC are poor, making biomarkers for targeted therapy desirable.
Recently, the family of classic tumor suppressors and oncogenes was expanded to include microRNAs (miR), small 18- to 21-nt noncoding RNAs that regulate diverse cellular and molecular processes including cell death and proliferation [14]. Hundreds of human miR are predicted to regulate 10% to 30% of protein-encoding genes by interactions with their 3′-untranslated regions [19]. Little is known about the mechanisms underlying miR dysregulation in neoplastic processes. The final steps of miR maturation require Dicer, an RNase III-related enzyme [13, 20]. To date, this pathway has not been investigated in salivary gland neoplasms. We present our three-decade experience with 78 well-characterized MEC and correlate Dicer immunoreactivity with traditional clinicopathologic parameters.
Materials and methods
Case selection
This study was approved by the University of Pittsburgh Institutional Review Board (IRB#0601084). One hundred cases of MEC were retrieved from the Department of Pathology archives (1973–2004). Each case was reviewed by two of the authors (R.R.S. and S.C.) and graded using the point-based grading system proposed by Brandwein et al. [5] with one exception: bone invasion was excluded as a grading feature because of its function as a staging parameter. Clinical and demographic data were obtained from the electronic medical record and the institutional Head and Neck Tumor Registry. The tumor–node–metastases (TNM) stage and American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) stage grouping was assigned based on sixth-edition guidelines. The demographic and clinicopathologic features of the patients in this study are listed in Table 1.
Immunohistochemistry
Immunoperoxidase staining was performed as previously described [6]. Briefly, the slides were incubated at 4°C overnight with anti-Dicer antibodies (Clonegene, Hartford, CT, USA) at a 1:500 dilution. Several internal controls were used: the basal/parabasal layers of non-neoplastic squamous epithelium or skin, as well as both basal and luminal layers of excretory or intercalated ducts of salivary tissue. The intensity was graded on a three-tiered scale from 1 to 3. The following criteria were used to semi-quantitatively score the Dicer staining: score of “1” was defined by a staining intensity lower than that of the internal controls, a score of “2” was defined by a staining intensity equal to that of the internal controls, and a score of “3” was defined by a staining intensity greater than that of the controls (Fig. 1). Two pathologists (R.R.S. and S.C.) independently scored the stained slides.
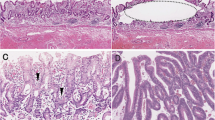
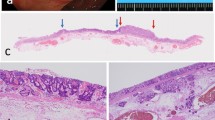
Scoring of Dicer expression in MEC. a, b Intermediate-grade MEC of the palate. Dicer immunoreactivity in MEC is lower than in basal squamous cells of adjacent normal mucosa (see inset), Dicer 1+. c, d Intermediate-grade MEC of the oral cavity. Dicer immunoreactivity in MEC is similar to that of basal squamous cells of adjacent normal mucosa, Dicer 2+. e, f High-grade MEC arising in parotid gland. Dicer immunoreactivity in MEC is higher than that of basal squamous cells of overlying normal epidermis (left one third of the image), Dicer 3+. Immunohistochemistry, original magnification ×100
Statistical analysis
All statistical analyses were performed with SPSS 14.0 software (SPSS, Chicago, IL, USA) and STATA V.9.0 (StataCorp LP, College Station, TX, USA). The relative frequencies of Dicer abnormalities by immunostaining with the subgroups of various parameters (grade, stage, and margin status) were compared using Fisher’s exact test (two-tail distribution). For these analyses, parameters were resolved into two-tiered groups as follows: grade—low/intermediate grade vs. high grade; stage—stage I/II vs. stage III/IV; and age—less than 55 years vs. greater than 55 years. Univariate disease-specific survival (DSS) was calculated using the Kaplan–Meier method with group-wise comparisons using the log-rank test. Multivariate analysis of DSS was performed using a Cox proportional hazards model, including all confounder variables that were significantly associated with DSS by univariate analysis and significantly associated with Dicer expression. The assumption of proportional hazards was evaluated for appropriateness by the assessment of scaled Schoenfeld residuals. A p value of less than 0.05 was considered statistically significant for all tests.
Results
Clinicopathologic features
Twenty-two of 100 cases diagnosed as MEC from 1973–2004 were reclassified as adenosquamous carcinoma (AsqCA) based on the presence of surface involvement by dysplasia, carcinoma in situ, a discrete adenocarcinoma component, significant nuclear pleomorphism, and/or presence of abundant keratinization [2].
Of the remaining 78 cases, the median age was 51.5 years (range 7–81) with a slight female predilection (F/M = 1.2:1). The parotid and minor salivary glands of the oral cavity/oropharynx were the two most common sites involved. In this series, the most common MEC grade was high, followed by intermediate and low. Stage I was the most common stage for tumors, followed by stage IV. Margins were positive in 26.7% of cases, while lymph nodes were positive in 27.7% of cases. The recurrence rate was 28.7%.
Low/intermediate-grade MEC were more frequently low stage (stage I/II, 35/38, 92.1%) as compared to high grade MEC (stage I/II, 8/25, 32%; p < 0.001). Of the three stage-III/IV tumors in the low/intermediate-grade category, all were intermediate grade. Only 4 of 45 (8.9%) low/intermediate-grade MEC had positive lymph nodes as compared to 14 of 33 (42.4%) in high-grade MEC (p < 0.001). All cases with positive lymph nodes in the low/intermediate category were intermediate grade. Similarly, only 5 of 45 (12.2%) low/intermediate-grade MEC had recurrences, while 15 of 29 (51.7%) high-grade MEC recurred (p < 0.001). In the low/intermediate grade-MEC with recurrences, four were intermediate grade, and only one was low-grade MEC.
Overall, the 5- and 10-year DSS were 73.6% and 69.9%, respectively. Median follow-up for surviving patients was 4.42 years (range, <1 week–20.93 years). Univariate predictors of DSS are summarized in Table 2 and Fig. 2. Age >55 years, grade, stage, and positive margin status were all univariate predictors of poor outcome, while gender and site were not.
Dicer IHC in normal salivary glands, normal squamous mucosa
In normal salivary tissue, Dicer intensity was highest in the basal/myoepithelial and luminal epithelium of intercalated, striated, and excretory ducts (Fig. 3). Serous acini showed weaker reactivity, while mucous acini were negative. The distal myoepithelial cells surrounding the acini showed variable reactivity. In squamous mucosa, Dicer intensity was highest in the basal/parabasal layers. Generally, the stromal elements of salivary glands (fibrous tissue, adipose tissue, and nerves) were non-reactive for Dicer. The vascular endothelium was occasionally positive for Dicer.
Dicer IHC in mucoepidermoid carcinoma
Dicer was expressed predominantly in the epidermoid and intermediate cell types, both within the solid and cystic areas of MEC. Overall, 47 of 78 (60.3%) cases showed abnormalities of Dicer expression, with 22 of 78 (28.2%) showing increased expression and 25 of 78 (32.1%) showing decreased expression. Dicer over-expression was significantly more common in high-grade, stage-III/IV, margin-positive MEC. Decreased expression was significantly more common in the tumors of patients over 55 years of age. Table 3 summarizes Dicer expression levels with respect to grade, stage, and margin status. Overall, abnormal Dicer expression (both over- and under-expression combined) was significantly more frequent in high grade, stage III/IV, and tumors of patients over 55 years. By univariate analysis, both over- and under-expression of Dicer correlated with poorer survival (Fig. 4). Age and stage were independent predictors of poor DSS on multivariate analysis.
Dicer was also frequently abnormally expressed in adenosquamous carcinoma in 17 of 22 (77.3%) cases, with 8 of 22 (36.4%) showing increased immunoreactivity and 9 of 22 (40.9%) with decreased immunoreactivity.
Discussion
The current MEC cohort is among the largest single-institution experiences in the past decade and validates stage, Brandwein grade, margin status, and age as prognostic factors. Prior large series are characterized by limited application of modern grading and staging systems [25], large referral population (hence not truly a single-institution experience) [11] or a lack of adequate assessment of stage altogether [8]. The Brandwein grade is the most modern grading system and shows improved correlation with outcome as compared to the Armed Forces Institute of Pathology grading system [5]. We did not compare these grading systems directly, but we were able to provide supportive evidence for the utility of the Brandwein system. Ultimately, in our cohort, only age and stage are independent predictors of poor DSS. Grade and stage may have been too closely linked to show an independent predictive value for grade alone.
An ancillary but significant finding in our study was the high prevalence of AsqCA (22%) that were inappropriately classified as MEC. AsqCA is essentially a variant of squamous cell carcinoma with divergent glandular differentiation. Morphologic criteria for its separation from high-grade MEC include surface dysplasia/carcinoma in situ, prominent keratinization, and discrete gland formation, often present in the deeper portions of the tumor [2]. Unfortunately, separation of these entities may still be challenging, particularly on small biopsies or when surface mucosa is ulcerated. Historically, at our institution, until the mid 1980s, the distinction between high-grade MEC and AsqCA was not made, which was another contributing factor to the discrepancy rate. Both MEC and AsqCA have poor outcome, with a 5-year survival of 13–20% for AsqCA and 0–43% for high-grade MEC, and mandate aggressive management [17].
The molecular pathogenesis of MEC is not fully elucidated. Perhaps the best characterized MEC-specific genetic alteration is the t(11;19)(q21;p13) initially identified by traditional karyotyping [24]. This translocation was shown to generate a mucoepidermoid carcinoma translocated 1 (MECT1)–mastermind-like 2 (MAML2) gene fusion consisting of exon 1 of MECT1 fused to exons 2–5 of MAML2 [29]. Fusion-positive patients are generally younger, have smaller, well-differentiated tumors, and a significantly lower risk of local recurrence, metastasis or tumor-related death. However, a few translocation-positive aggressive high-grade MEC have been recently described [28]. The participation of the miR pathway in these molecular alterations, if any, has yet to be characterized.
To the best of our knowledge, this is the first report demonstrating correlation between Dicer protein immunoreactivity and survival in human carcinomas. Previously, another group showed correlation between the level of Dicer mRNA and post-operative survival of patients with non-small cell lung carcinoma [16]. We have recently presented additional details on Dicer expression in non-small cell lung carcinoma and showed the role of Dicer in prostate adenocarcinoma [6, 7]. Dicer expression varies in squamous cell carcinoma of the esophagus and does not show significant association with patient survival [26]. Combined with Dicer changes in MEC described here, alteration of Dicer levels may represent a previously unrecognized common theme in carcinogenesis. Differential miR expression is the most likely explanation for worse prognosis in patients with both up- or down-regulation of Dicer. miR expression in MEC has yet to be characterized. However, in cell lines of head and neck squamous cell carcinoma, 55 miR were differentially expressed [30].
We also describe the immunoexpression profile of Dicer in normal salivary/mucoserous glands. Dicer appears to be distributed at higher levels in intercalated and striated ducts, both in basal and luminal layers. The functional importance of this expression pattern might be explained by the anti-apoptotic function of Dicer [23]. It is possible that Dicer expression in normal salivary gland mirrors the cellular constituents of salivary gland with proliferative or regenerative capacity.
The implications of Dicer expression abnormalities may extend beyond prognosis. Since the disease-specific survival for high-grade/high-stage MEC is poor, additional therapeutic modalities are desirable. In recent years, understanding of RNA interference (RNAi)-based gene silencing has offered yet another therapeutic approach to cancer. Importantly, Dicer level correlates with the efficiency of RNAi. For instance, over-expression in HeLa cells leads to a 30% enhancement of a shRNA-mediated gene silencing [22].
There is increasing evidence that potential RNAi-based therapeutics will induce significant side effects, including off-target silencing and activation of the interferon system [1]. Furthermore, some adverse effects appear to be caused by competition of exogenous siRNAs with the endogenous miR for proteins of miR machinery (e.g., Exportin-5) [12]. While this remains largely speculative, the widespread changes of Dicer expression in MEC, particularly in high-grade MEC, may ultimately help to predict the efficacy of potential RNAi-based drugs.
In summary, this study represents the first comprehensive analysis of Dicer expression in a clinicopathologically well-characterized cohort of MEC patients. In addition, we herein characterize Dicer expression in normal salivary tissue. We show a higher frequency of abnormal Dicer expression in high-grade, high-stage MEC when compared to low/intermediate-grade, low stage-MEC. Almost two thirds of MEC show abnormal Dicer expression. Abnormal Dicer immunoreactivity (over- and under-expression combined or separately) correlated with poorer survival.
References
Aagaard L, Rossi JJ (2007) RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev 59:75–86
Alos L, Castillo M, Nadal A, Caballero M, Mallofre C, Palacin A, Cardesa A (2004) Adenosquamous carcinoma of the head and neck: criteria for diagnosis in a study of 12 cases. Histopathology 44:570–579
Auclair PL, Goode RK, Ellis GL (1992) Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer 69:2021–2030
Batsakis JG, Luna MA (1990) Histopathologic grading of salivary gland neoplasms: I. Mucoepidermoid carcinomas. Ann Otol Rhinol Laryngol 99:835–838
Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, Bodian C, Urken ML, Gnepp DR, Huvos A, Lumerman H, Mills SE (2001) Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 25:835–845
Chiosea S, Jelezcova E, Chandran U, Acquafondata M, McHale T, Sobol RW, Dhir R (2006) Up-regulation of dicer, a component of the MicroRNA machinery, in prostate adenocarcinoma. Am J Pathol 169:1812–1820
Chiosea S, Jelezcova E, Chandran U, Luo J, Mantha G, Sobol RW, Dacic S (2007) Overexpression of Dicer in precursor lesions of lung adenocarcinoma. Cancer Res 67:2345–2350
Evans HL (1984) Mucoepidermoid carcinoma of salivary glands: a study of 69 cases with special attention to histologic grading. Am J Clin Pathol 81:696–701
Francioso F, Carinci F, Tosi L, Scapoli L, Pezzetti F, Passerella E, Evangelisti R, Pastore A, Pelucchi S, Piattelli A, Rubini C, Fioroni M, Carinci P, Volinia S (2002) Identification of differentially expressed genes in human salivary gland tumors by DNA microarrays. Mol Cancer Ther 1:533–538
Frierson HF Jr., El-Naggar AK, Welsh JB, Sapinoso LM, Su AI, Cheng J, Saku T, Moskaluk CA, Hampton GM (2002) Large scale molecular analysis identifies genes with altered expression in salivary adenoid cystic carcinoma. Am J Pathol 161:1315–1323
Goode RK, Auclair PL, Ellis GL (1998) Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 82:1217–1224
Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA (2006) Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441:537–541
Hutvagner G, Zamore PD (2002) A microRNA in a multiple-turnover RNAi enzyme complex. Science 297:2056–2060
Hwang HW, Mendell JT (2006) MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer 94:776–780
Karja VJ, Syrjanen KJ, Kurvinen AK, Syrjanen SM (1997) Expression and mutations of p53 in salivary gland tumours. J Oral Pathol Med 26:217–223
Karube Y, Tanaka H, Osada H, Tomida S, Tatematsu Y, Yanagisawa K, Yatabe Y, Takamizawa J, Miyoshi S, Mitsudomi T, Takahashi T (2005) Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci 96:111–115
Keelawat S, Liu CZ, Roehm PC, Barnes L (2002) Adenosquamous carcinoma of the upper aerodigestive tract: a clinicopathologic study of 12 cases and review of the literature. Am J Otolaryngol 23:160–168
Leivo I, Jee KJ, Heikinheimo K, Laine M, Ollila J, Nagy B, Knuutila S (2005) Characterization of gene expression in major types of salivary gland carcinomas with epithelial differentiation. Cancer Genet Cytogenet 156:104–113
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20
Maniataki E, Mourelatos Z (2005) A human, ATP-independent, RISC assembly machine fueled by pre-miRNA. Genes Dev 19:2979–2990
Maruya S, Kim HW, Weber RS, Lee JJ, Kies M, Luna MA, Batsakis JG, El-Naggar AK (2004) Gene expression screening of salivary gland neoplasms: molecular markers of potential histogenetic and clinical significance. J Mol Diagn 6:180–190
Mikuma T, Kawasaki H, Yamamoto Y, Taira K (2004) Overexpression of Dicer enhances RNAi-mediated gene silencing by short-hairpin RNAs (shRNAs) in human cells. Nucleic Acids Symp Ser (Oxf) 0:191–192
Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K (2005) Aberrant T cell differentiation in the absence of Dicer. J Exp Med 202:261–269
Nordkvist A, Gustafsson H, Juberg-Ode M, Stenman G (1994) Recurrent rearrangements of 11q14–22 in mucoepidermoid carcinoma. Cancer Genet Cytogenet 74:77–83
Spiro RH (1986) Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg 8:177–184
Sugito N, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Kurehara H, Ando T, Mori R, Takashima N, Ogawa R, Fujii Y (2006) RNASEN regulates cell proliferation and affects survival in esophageal cancer patients. Clin Cancer Res 12(24):7322–7328
Suzzi MV, Alessi A, Bertarelli C, Cancellieri A, Procaccio L, Dall’olio D, Laudadio P (2005) Prognostic relevance of cell proliferation in major salivary gland carcinomas. Acta Otorhinolaryngol Ital 25:161–168
Tirado Y, Williams MD, Hanna EY, Kaye FJ, Batsakis JG, El-Naggar AK (2007) CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: implications for histogenesis and biologic behavior. Genes Chromosomes Cancer 46:708–715
Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, O’Neil K, Stover K, El-Naggar A, Griffin JD, Kirsch IR, Kaye FJ (2003) t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet 33:208–213
Tran N, McLean T, Zhang X, Zhao CJ, Thomson JM, O’Brien C, Rose B (2007) MicroRNA expression profiles in head and neck cancer cell lines. Biochem Biophys Res Commun 358:12–17
Van Heerden WF, Raubenheimer EJ, Dreyer L (2005) The role of DNA ploidy and Ki-67 in the grading of mucoepidermoid carcinomas. Anticancer Res 25:2589–2592
Waldron CA, el-Mofty SK, Gnepp DR (1988) Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol 66:323–333
Acknowledgments
This study was supported by a grant from the Postdoctoral Pathology Research Training Program (PPRTP, RT = 00147, CC = 99FUND) from the Department of Pathology, University of Pittsburgh Medical Center to SC. The study was also supported by the Stout Family Fund for Head and Neck Cancer Research at the Eye & Ear Foundation of Pittsburgh and the Head and Neck Oncology Registry. S.Y. Lai was supported by an NIH Mentored Career Development Award (K08 DE018061-01). Authors would like to thank Kim Marie Adams and Jennifer Ridge Hetrick for their administrative support. All experiments comply with the current laws of the USA. Authors would like to thank Marie Acquafondata for excellent technical support.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiosea, S.I., Barnes, E.L., Lai, S.Y. et al. Mucoepidermoid carcinoma of upper aerodigestive tract: clinicopathologic study of 78 cases with immunohistochemical analysis of dicer expression. Virchows Arch 452, 629–635 (2008). https://doi.org/10.1007/s00428-007-0574-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0574-5