Abstract
Purpose
To evaluate the efficacy and safety of a novel low dose chemotherapy as a remission induction regimen for elderly de novo AML patients ineligible for intensive chemotherapy.
Method
Fifty consecutive patients aged 60 to 85 with untreated de novo AML were enrolled. Patients with poor PS or defined non-hematological complications were given continuous drip infusion of low dose cytarabine (Ara-C), 20 mg/body and etoposide (VP-16), 50 mg/body for 10 days (AV group). Patients without those cormobidities were given intensive induction therapy (S group). After achieving complete remission (CR), S group patients and those with improved PS in AV group received consolidation chemotherapy with intensive regimen (S-S or AV-S group), and other patients received AV regimen repeatedly (AV-AV group).
Results
Eighteen (64%; 95% confidence interval (CI), 0.47–0.82) of 28 patients in AV group and 16 (73%; 95% CI, 0.54–0.91) of 22 patients in S group achieved CR, respectively. The 1-year OS rates of the patients in the AV-AV group (n = 9), AV-S group (n = 9), and S-S group (n = 16) were 22, 81, and 78%, respectively. Although the sample size was small, no significant difference was observed for the 1-year OS rate between the AV-S and S-S groups. Regimen related death were 4 patients in S group, while no patient in AV group.
Conclusion
Therapeutic strategy consisting of remission induction using AV regimen and consolidation using intensive regimen after improving PS is beneficial in the management of elderly AML patients who have difficulty in tolerating for intensive induction chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of acute myeloid leukemia (AML) increases with age and more than half of all patients are over 60 years of age at the time of diagnosis (Deschler and Lubbert 2006). Despite some reports of improved chemotherapeutic response and disease free survival in adults with AML (Berman et al. 1991; Wiernik et al. 1992; Yates et al. 1982), advanced age remains a significantly poor prognostic factor for both complete remission and survival. The optimal management strategy for those patients is still a controversial issue with opinions frequently diverse between intensive chemotherapy and conservative therapy. In fact, we cannot often help hesitating to select intensive chemotherapy for elderly patients due to prolonged myelosuppression, complex karyotypic abnormalities, preexisting myelodysplasia, underlying non-hematological complications, a poor performance status (PS) and organodysfunction with aging in comparison to younger patients.
We have tried to develop a therapeutic strategy for elderly patients with untreated de novo AML according to the presence of PS and non-hematological complications at the diagnosis. Accordingly, we treated those elderly patients with a new combination chemotherapy using continuous drip infusion of low dose cytarabine (Ara-C) and etoposide (VP-16) (AV therapy). We thus consider that administering Ara-C and etoposide by continuous intravenous infusion might possibly extend the contact time for drugs and leukemic cells, thereby enhancing the anti-leukemic effect (Koyama et al 1990). While, the patients without comorbidities received the intensive chemotherapy in a similar manner to younger patients with good PS. The present study aimed to evaluate the efficacy and safety of AV therapy as a remission induction regimen for elderly de novo AML patients ineligible standard intensive chemotherapy.
Patients and methods
Patients
The eligibility for enrollment included patients aged 60–85 with previously untreated de novo AML as defined by French–American–British (FAB) classification criteria (Bennett et al 1976). We only enrolled patients with a poor PS as defined by the WHO criteria 3 and 4, or non-hematological complications such as metabolic disease, an active infection without respiratory failure, a mild liver dysfunction due to a hepatic viral infection, and/or other malignant diseases. The exclusion criteria were as follows: (1) patients with acute promyelocytic leukemia (APL); (2) patients with antecedent myelodysplastic syndrome (MDS); (3) patients with therapy related AML; (4) patients with prior treatment for AML including chemotherapy, radiation, stem cell transplantation, and cytokines like as granulocyte colony-stimulating factor (G-CSF); and (5) obvious organ dysfunction (serum bilirubin level > 2 × upper normal limit, creatinine clearance < 50 ml/min and cardiac ventricular ejection fraction < 40%). The enrollment period was from July 1995 to December 2000. Written informed consent was obtained from all eligible patients before undergoing remission induction therapy. The study was approved by the institutional review board of our university.
Treatment design
Low dose Ara-C and VP16 regimen (AV therapy); patients either with a poor PS or defined non-hematological complications were given continuous drip infusion of low dose Ara-C, 20 mg/body/day and VP-16, 50 mg/body/day for 10 days. If marrow hypoplasia or a reduction of leukemic blasts of less than 5% could not been obtained on day 10, both Ara-C and VP-16 were added for another 4 days. If a complete remission (CR) could not been achieved, then the patients received the re-induction therapy which was the same as the first regimen.
Intensive regimen; the patients without a poor PS or any other complications were given the intensive regimen consisting of daunorubicin (DNR), 30 mg/m2, by bolus infusion on days 1, 2, and 3, behenoyl Ara-C (BHAC), 150 mg/m2, by drip infusion for 10 days and 6-mercaptopurine (6—MP), 70 mg/m2, orally for 10 days. If marrow hypoplasia or a reduction of leukemic blasts of less than 5 % could not been obtained on day 7, then DNR was added on days 8 and 9. If CR could not been achieved, then the patients received the re-induction therapy which was the same as the first regimen.
After achieving CR, patients received three courses of intensive post-remission chemotherapy. The first course consisted of Ara-C, 150 mg/m2, by continuous infusion for 7 days and mitoxantrone, 6 mg/m2, by bolus infusion on days 1, 2, and 3. The second course consisted of DNR, 30 mg/m2 for 3 days, VP-16 70 mg/m2, by drip infusion for 5 days, and BHAC, 150 mg/m2, for 7 days. The third course consisted of aclarubicin, 14 mg/m2, by bolus infusion and BHAC, 150 mg/m2 for 7 days. These courses were applied for patients who achieved CR by intensive induction regimen, and for those who obtained CR by AV regimen and recovered PS. Patients, who achieved CR by AV therapy but still had a poor PS, again received three courses of AV therapy.
Response definitions
The response criteria were defined as follows: a CR included normalization of previous cytologic abnormalities in the bone marrow (blasts < 5%, normal proportion of erythropoiesis) and normalization of peripheral blood counts (disappearance of blasts, neutrophil > 1.5 × 109/l and platlets > 100 × 109/l). It was essential to maintain the condition of the peripheral blood for 4 weeks except for beginning the next chemotherapy in order to confirm CR. A relapse was determined in the event of an increase of blasts in the marrow (>10%) or the appearance of them in the peripheral blood. For CR patients, the duration of remission [disease free survival (DFS)] was defined from the date of bone marrow examination associated with CR until either relapse, death, or the final data acquisition (August 31, 2005). The overall survival (OS) was defined from the date of diagnosis until death or the final data acquisition.
Statistical analysis
A comparison of the variables between the two treatment groups at baseline was performed either by Student’s t test or Fisher’s exact test. The effects of age, sex, PS, FAB classification, chromosomal abnormality, hemoglobin, white blood cell (WBC) count, lactate dehydrogenase (LDH) level, nucleated cell count of bone marrow, CD7 and CD34 expression of blasts and regimen of induction chemotherapy were analyzed for the CR rates using the chi-square test. Survival analyses for OS and DFS were performed using the log-rank test based on the Kaplan-Meier method. A multivariate analysis was performed using the Cox regression technique to define the prognostic significance of selected variables. A P value of less than 0.05 was considered to be significant.
Results
Patients
Fifty-four patients were registered, but four patients were not considered eligible for the study because their clinical status was too poor to receive chemotherapy. Therefore, 50 patients were finally enrolled. The median age of the patients was 69-year-old, and the median follow-up period in the surviving patients was 53 months. Twenty-eight patients were treated with AV therapy as induction remission (AV group), and 22 patients were treated with the intensive regimen (S group). Cytogenetic abnormalities were grouped according to published criteria adopted by the Southwest Oncology Group (SWOG) (Slovak et al 2000). The clinical characteristics including the karyotype of the two group patients were well balanced except for the PS and the defined non-hematological complications as shown in Table 1. The non-hematological complications in AV group were 12 patients with pneumonia, 4 patients with cardiac dysfunction, and 3 patients with mild renal dysfunction. The FAB classifications was M0; 7, M1; 3, M2; 8, M4; 4, M5; 2, M6; 3, and M7; 1 in AV group patients, and M0; 2, M1; 5, M2; 8, M4; 4, and M5; 3 in the S group patients.
Response to treatment and survival
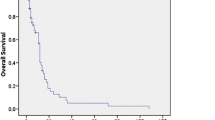
Eighteen [64%; 95% confidence interval (CI), 0.47–0.82] of the 28 patients in the AV group and 16 (73%; 95% CI, 0.54–0.91) of 22 patients in the S group achieved CR, respectively. Eleven of 18 patients in the AV group and 12 of 16 patients in the S group achieved CR after one course of induction chemotherapy. The 2-year OS rates were 28 and 46% and the 5-year OS rates were 12 and 23% in the AV group and S group, respectively (Fig. 1a). There was no significant difference in OS rate between AV group and S group (P = 0.086). In patients who achieved CR, the 2-year DFS rates were 17 and 34% and the 5-year DFS rates were 13 and 33% in the AV group and the S group, respectively (Fig. 1b). In addition, regarding the DFS rate, there was no significant difference between AV group and S group (P = 0.059).
The overall survival (OS) rates and disease free survival (DFS) rates of patients treated with the AV regimen or standard intensive regimens by the Kaplan–Meier method. Graph A OS rates according to induction regimens. A solid line indicates the OS rate of patients treated with AV regimen (AV group: n = 28) and a dotted line indicates that of the patients treated with the standard intensive regimen (S group: n = 22). The 2-year/5-year OS rates of the AV group and the S group were 28%/12% and 46%/22%, respectively. There was no significant difference in the OS rate between the AV group and the S group (P = 0.086). Graph B DFS rates according to induction regimens. A solid line indicates the DFS rate of the AV group (n = 18) and a dotted line indicates that of the S group (n = 16). The 2-year/5-year DFS rates of the AV group and the S group were 17%/13% and 34%/33%, respectively. No significant difference was observed in the DFS rate between the AV group and the S group (P = 0.059). Tic marks indicate censored observations
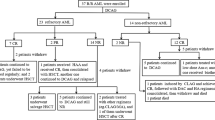
The clinical outcomes of patients according to the induction and consolidation regimens are summarized in Fig. 2. In the AV group, nine patients received AV therapy repeatedly as consolidation (AV-AV group), while nine patients received intensive chemotherapy as consolidation (AV-S group) since an improvement in the PS was obtained after CR. In the S group, all CR patients received consolidation chemotherapy with the intensive regimen (S-S group). The 1-year OS rates/1-year DFS rates of the patients in the AV-AV group, the AV-S group, and S-S group were 22%/11%, 81%/67%, and 78%/74%, respectively (Fig. 3). When only mentioned in AV-S group and S-S group, the 5-year OS rates/DFS rates were 33%/35% and 31%/34%, respectively. Although the sample size was small, no significant differences were observed for the OS and DFS between AV-S group and S-S group. The survival curves both for OS and DFS in the AV-AV group were significantly lower than in the AV-S and S-S group (P < 0.05).
Clinical outcomes of the patients according to the induction and consolidation regimens. Twenty-eight patients were treated with AV therapy as induction remission, and 22 patients were treated with the standard intensive regimen. Eighteen (64%) of 28 patients in the AV group and 16 (73%) of 22 patients in the S group achieved CR, respectively. In patients who achieved CR by AV therapy, nine patients received AV therapy repeatedly as consolidation (AV-AV group), and nine patients received standard intensive chemotherapy as consolidation (AV-S group) since an improvement of the PS was obtained after CR. All patients, who achieved CR with the standard intensive induction-regimen, received consolidation chemotherapy with the standard intensive regimen (S-S group). pts number of patients
The overall survival (OS) rates and disease free survival (DFS) rates of the patients according to the induction and consolidation regimens by the Kaplan–Meier method. Graph A The solid line indicates the OS rate of the patients who achieved CR by AV regimen and treated with standard intensive chemotherapy as consolidation (AV-S group: n = 9). A dotted line indicates the OS rate of patients treated with a standard intensive regimen for both induction and consolidation (S-S group: n = 16). A broken line indicates OS rate of the patients treated with an AV regimen through all chemotherapeutic courses (AV-AV group: n = 9). Graph B A solid line, a dotted line, and a broken line indicate the DFS rates of AV-S group (n = 9), S-S group (n = 16), and AV-AV group (n = 9), respectively. The 1-year OS rates/1-year DFS rates of the patients in the AV-AV group, AV-S group, and S-S group were 22%/11%, 81%/67%, and 78%/74%, respectively. When only mentioned in AV-S group and S-S group, the 5-year OS rates/DFS rates were 33%/35% and 31%/34%, respectively. Although the sample size was small, no significant differences were observed for the OS and the DFS rates between the AV-S group and S-S group. Those rates in the AV-AV group were significantly lower than for the AV-S and S-S groups (P < 0.05). Tic marks indicate censored observations
Prognostic factors
Only a poor PS was identified as an adverse prognostic factor associated with CR based on univariate analysis. Regarding OS, a univariate analysis identified the following factors have an adverse prognostic effect: poor PS, leukocytosis (>30 × 109/l), elevated LDH (>1,000 IU/l), hypoalbuminemia (<3.5 g/dl), and no achievement of CR. A multivariate analysis of prognostic factors associated with OS identified the following factors to demonstrate independent adverse significance: poor PS (RR 0.27, 95% CI 0.44–0.91, P < 0.001) and no achievement of CR (RR 0.63, 95% CI 0.16–0.45, P < 0.05). A multivariate analysis for DFS identified no prognostic factors. In this study, age, chromosomal abnormality, surface markers, and the regimen of induction were not identified as prognostic factors for either the CR rates or OS.
Safety
The regimen related mortality during remission induction therapy was observed in four patients in the S group, while no such patients were seen in the AV group. The causes of death in the S group were pneumonia in one patient and sepsis in three. In AV group, no adverse effect that needed withdrawal and/or postponement of chemotherapy was observed. The non-hematological adverse effects by AV regimen are summarized in Table 2. Almost all gastro-duodenal adverse effects were tolerable by the administration of antiemetic and/or antiacid drugs. As for the hematological adverse effects, myelosuppression was the major toxicity occurring during induction chemotherapy with AV regimen. The median number of days for the neutrophiles to recover to 0.5 × 109/l was 28 days after the initiation of chemotherapy. Similarly, the median period for platelets to recover to 5.0 × 1010/l without transfusion was 29 days. Febrile neutropenia with grade 3 was observed in 16 patients (57%) with the highest frequency, but all of them improved after the administration of antibiotics. On the other hands, in the S group, the median number of days for the neutrophiles to recover to 0.5 × 109/l was 29 days after the initiation of chemotherapy, which was almost the same degree as that of the AV group. However, the degree of myelosuppression was more severe in S-group. Concretely, the median number of days for WBC counts under 0.1 × 109/l was 7 (range 5–12) days in S group, while 3 (0–6) days in AV group (P < 0.05). Thereby, as described in the beginning of this section, life-threatening events were only observed in the S group.
Discussion
How to treat elderly patients with AML still remains an important topic of debate regardless more than half of the all AML patients are older than 60 years of age (Estey 2000). Although the beneficial effects of intensive chemotherapy for elderly patients were reported (Lowenberg et al. 1989; Rees et al. 1996) and thereby recommended (Chen et al 2005; Hiddemann et al. 1999), many oncologists feel uncomfortable of offering intensive chemotherapy to elderly patients with a poor PS or comorbidity because of the high risk of induction mortality. For those patients, various regimens to reduce the toxicity associated with intensive chemotherapy have been proposed such as dose attenuation and/or use of cytokines like as G-CSF. A low dose Ara-C regimen has been extensively studied and it is currently regarded as one of the representative therapies for advanced MDS and AML patients in the elderly (Tilly et al. 1990; Visani et al. 2004), because of the assumption that it is less toxic than conventional intensive treatments. These studies have shown CR rates about 30–50% and a lower number of early deaths. Combination therapy of low dose Ara-C with other cytotoxic drugs therefore seems to be more promising to improve CR rates. Based on this point of view, we therefore employed AV therapy for elderly AML patients.
In this study, the CR rates were not different between the AV group and S group, in spite of an unfavorable selection of patients for AV group. The CR rate in the AV group (64%) was higher than those in other studies on older patients with AML (Lowenberg et al. 1989; Tilly et al. 1990; Goldstone et al. 2001). Moreover, the DFS and OS rates in the AV-S group who had achieved CR by AV therapy and received intensive consolidation were in a similar range to those reported for younger adults (Mayer et al 1994).
In addition, a clear observation in this study is the importance of post-remission chemotherapy. Usually, PS consists of two factors: namely, host-related factors such as comorbidity, and leukemia-related factors such as leukocytosis or infection as associated complications. Leukemia-related factors can be improved by achieving CR regardless of the induction regimen, and as a result, patient’s PS after CR must also improved. In this study, half of all patients who achieved CR in the AV group were able to receive standard intensive consolidation chemotherapy after an improvement of PS. This fact suggested that leukemia-related factors were important elements of the PS, and moreover, that standard intensive chemotherapy was possible for those with a poor PS if they once achieved CR. The findings that there was no difference in the OS and DFS rates between the AV-S group and the S-S group suggests that intensive consolidation chemotherapy contributes to an improvement of survival. In addition, the compliance of the intensive approach was generally acceptable probably due to the good PS. In contrast, if the PS of patients is poor after the achievement of CR, low dose post-remission chemotherapy could thus be one option. However, the optimal intensity of consolidation regimen is still open to debate (Stone et al. 2001; Anderson et al. 2002) and thus requires further study.
Recently, large retrospective studies on the treatment outcome of elderly patients with AML and MDS have been reported (Chen et al. 2005; Kantarjian et al. 2006). Kanterjian et al. proposed prognostic models, based on standard readily available characteristics such as age, PS, leukocytosis and an unfavorable karyotype, which may assist in therapeutic and investigational decisions (Kantarjian et al. 2006). However, in our study, some parameters such as age, chromosomal abnormality, leukocytosis, and regimen of induction were not identified as prognostic factors for CR rates and/or OS. These results probably reflect the lower statistical power of our sample size. In our study, a few patients survived longer than 5 years in AV-S group. Those patients had lower blasts percentages in the marrow at first in comparison to other patients. The disease characteristics of the patients tend to mostly MDS rather than AML. As a result, the AV regimen was thus speculated to be effective for patients with such diseases as overt leukemia with slowly growing blasts. In the future, the selection of adequate induction chemotherapy based on such prognostic models should be considered in elderly AML patients. In poor-risk group, the mortality during induction chemotherapy with standard intensive regimens was still high, and thereby it seems adequate to recommend AV therapy as an option for AML patients who have difficulty in tolerating intensive chemotherapy.
Febrile neutropenia (FN) occurred in 57% of the patients in the AV group as a major toxicity in the first induction therapy. In addition, myelosuppression was an important adverse effect during the induction therapy, but no life-threatening sepsis or hemorrhaging was observed. As a result, no patients required withdrawal, and all safely concluded the AV regimen.
In conclusion, AV therapy was found to be effective, at least as a remission induction therapy and well tolerated by elderly patients with a poor PS or some non-hematological complications. In addition, therapeutic strategy consisting of remission induction using low dose regimens such as AV therapy and consolidation therapy using an intensive regimen should therefore be taken into consideration in the management of such AML patients.
References
Anderson JE, Kopecky KJ, Willman CL, Head D, O’Donnell MR, Luthardt FW, Norwood TH, Chen IM, Balcerzak SP, Johnson DB, Appelbaum FR (2002) Outcome after induction chemotherapy for older patients with acute myeloid leukemia is not improved with mitoxantrone and etoposide compared to cytarabine and daunorubicin: a Southwest Oncology Group study. Blood 100:3869–3876
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C (1976) Proposals for the classification of the acute leukaemias. French–American–British (FAB) co-operative group. Br J Haematol 33:451–458
Berman E, Heller G, Santorsa J, McKenzie S, Gee T, Kempin S, Gulati S, Andreeff M, Kolitz J, Gabrilove J et al (1991) Results of a randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood 77:1666–1674
Chen CC, Yang CF, Yang MH, Lee KD, Kwang WK, You JY, Yu YB, Ho CH, Tzeng CH, Chau WK, Hsu HC, Gau JP (2005) Pretreatment prognostic factors and treatment outcome in elderly patients with de novo acute myeloid leukemia. Ann Oncol 16:1366–1373
Deschler B, Lubbert M (2006) Acute myeloid leukemia: epidemiology and etiology. Cancer 107:2099–2107
Estey EH (2000) How I treat older patients with AML. Blood 96:1670–1673
Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE (2001) Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood 98:1302–1311
Hiddemann W, Kern W, Schoch C, Fonatsch C, Heinecke A, Wormann B, Buchner T (1999) Management of acute myeloid leukemia in elderly patients. J Clin Oncol 17:3569–3576
Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E, Garcia-Manero G, Wierda W, Pierce S, Shan J, Estey E (2006) Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer 106:1090–1098
Koyama S, Itou S, Shibata A (1990) [Low dose continuous infusion therapy with etoposide (VP-16) and cytosine arabinoside (Ara-C) for a patient with refractory acute myelogenous leukemia]. Rinsho Ketsueki 31:1891–1892
Lowenberg B, Zittoun R, Kerkhofs H, Jehn U, Abels J, Debusscher L, Cauchie C, Peetermans M, Solbu G, Suciu S et al (1989) On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol 7:1268–1274
Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, Omura GA, Moore JO, McIntyre OR, Frei E 3rd (1994) Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med 331:896–903
Rees JK, Gray RG, Wheatley K (1996) Dose intensification in acute myeloid leukaemia: greater effectiveness at lower cost. Principal report of the Medical Research Council’s AML9 study. MRC Leukaemia in Adults Working Party. Br J Haematol 94:89–98
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR (2000) Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 96:4075–4083
Stone RM, Berg DT, George SL, Dodge RK, Paciucci PA, Schulman PP, Lee EJ, Moore JO, Powell BL, Baer MR, Bloomfield CD, Schiffer CA (2001) Postremission therapy in older patients with de novo acute myeloid leukemia: a randomized trial comparing mitoxantrone and intermediate-dose cytarabine with standard-dose cytarabine. Blood 98:548–553
Tilly H, Castaigne S, Bordessoule D, Casassus P, Le Prise PY, Tertian G, Desablens B, Henry-Amar M, Degos L (1990) Low-dose cytarabine versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J Clin Oncol 8:272–279
Visani G, Malagola M, Piccaluga PP, Isidori A (2004) Low dose Ara-C for myelodysplastic syndromes: is it still a current therapy? Leuk Lymphoma 45:1531–1538
Wiernik PH, Banks PL, Case DC Jr, Arlin ZA, Periman PO, Todd MB, Ritch PS, Enck RE, Weitberg AB (1992) Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood 79:313–319
Yates J, Glidewell O, Wiernik P, Cooper MR, Steinberg D, Dosik H, Levy R, Hoagland C, Henry P, Gottlieb A, Cornell C, Berenberg J, Hutchison JL, Raich P, Nissen N, Ellison RR, Frelick R, James GW, Falkson G, Silver RT, Haurani F, Green M, Henderson E, Leone L, Holland JF (1982) Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood 60:454–462
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsurumi, H., Kanemura, N., Hara, T. et al. Therapeutic strategy of untreated de novo acute myeloid leukemia in the elderly: the efficacy of continuous drip infusion with low dose cytarabine and etoposide. J Cancer Res Clin Oncol 133, 547–553 (2007). https://doi.org/10.1007/s00432-007-0203-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-007-0203-3