Summary
Purpose
To analyze the effectiveness of decitabine combined with low-dose Ara‑C, aclarubicin, and rhG-CSF (DCAG) as a salvage therapy in patients with relapsed/refractory (R/R) acute myeloid leukemia (AML).
Patients and methods
This single-centered retrospective study included patients with relapsed/refractory AML between January 2013 and June 2020. The response rate and survival were calculated, and their correlations with clinical characteristics were estimated.
Results
Thirty-seven patients were enrolled. The overall response rate (ORR) was 56.8% with a complete remission (CR) rate of 51.4%. For patients with non-refractory and refractory AML, the CR rates were 87.5% and 30.4%, respectively (p < 0.001). No remission was found in four primary refractory AML. The incidence of infections was 73%, wherein 13.5% had severe infection, and 2.7% with treatment-related mortality. The median overall survival (OS) and relapse-free survival (RFS) were 10.6 (7.7–13.5) months and 5.0 (2.8–7.2) months, respectively. Patients in the poor risk category had a shorter OS (P = 0.004). The OS and RFS of patients with a recurrence time of < 6 months were shorter (P = 0.018 and P < 0.001, respectively).
Conclusions
The DCAG regimen was an effective and well-tolerated therapeutic alternative for R/R AML, particularly in non-refractory patients. Patients in the poor risk category and early relapse were adverse factors for remission rate and survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is a highly heterogeneous malignant disorder characterized by the aberrant clonal proliferation of hematopoietic stem or progenitor cells. Approximately 60–80% of adult patients with AML achieve complete remission (CR) after the induction chemotherapy. However, approximately 20% of patients have primary refractory AML and > 50% who achieve CR eventually relapse [1,2,3]. Relapsed/refractory (R/R) AML patients have a lower remission rate and various complications, leading to limited therapeutic alternatives and poor prognosis. The median overall survival (OS) of patients with R/R AML is approximately 6 months, with a 5-year OS rate of 10% [4]. Hypomethylating agents, such as decitabine, are specific inhibitors of DNA methyltransferase that can reactivate the expression of silenced tumor suppressor genes and restore aberrant cells to normal terminal differentiation and apoptosis [5, 6]. The effectiveness of decitabine monotherapy for R/R AML is poor, nevertheless, the combined regimens are considerable and well-tolerated [7, 8]. Moreover, the clinical factors that affect the remission rate and prognosis remain controversial. This study aimed to estimate the effectiveness, safety, and prognostic factors of the DCAG regimen for R/R AML and provide a clinical reference for the treatment of such patients.
Materials and methods
Patients
This study enrolled 37 relapsed/refractory AML patients who were treated in our center from January 2013 to June 2020. Relapsed AML was defined as the reappearance of leukemic blasts in the peripheral blood, more than 5% of blasts in the BM after CR, or extramedullary relapse. Refractory AML was diagnosed with the following criteria: (1) CR could not be achieved after two courses of chemotherapy; (2) patients with CR who received consolidation therapies, but relapsed within 12 months; (3) patients who relapsed for more than 12 months and could not achieve CR with conventional chemotherapy; (4) two or more relapses; or (5) persistence of extramedullary leukemia.
Treatment
All patients were treated with the DCAG regimen. Decitabine was administrated as a dose of 20 mg/m2·d intravenously for 5 consecutive days (d1-5), and chemotherapy regimens were administrated sequentially, as follows: cytarabine (10 mg/m2, q12 h, ih, d6-12), aclarubicin (14 mg/m2, qd, ivd, d6-9) and rhG-CSF (300 μg, qd, ih, d6-12).
Evaluation
Risk categories were assessed according to the ELN Guidelines (version 2017). The response criteria were defined according to the NCCN Guidelines (version 2020). Overall survival (OS) was defined as the time from the onset of induction to death or last contact. Relapse-free survival (RFS) was defined as the time from a CR to relapse, death, or last contact.
Adverse events
Toxicity was evaluated according to the National Cancer Institute Common Terminology.
Criteria for Adverse Events (CTCAE, version 4.0). All patients received prophylactic antimicrobials, supportive care, and blood transfusions, when necessary.
Statistics
All statistical analyses were conducted with the Statistical Package for Social Science (SPSS, version 23.0). Chi-squared test or Fisher’s exact test was used to evaluate the effectiveness of induction therapy. Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test, stratified by baseline age, sex, risk category, gene mutations, disease status, and consolidation therapeutic regimens. A Cox proportional hazards model was used to further analyze the prognostic factors. Statistical significance was set at P < 0.05.
Results
Clinical characteristics
Thirty-seven relapse/refractory AML were analyzed retrospectively, including 18 males and 19 females, with a median age of 48 years (range, 12–68) years. At diagnosis, 13 of the 37 patients were in the favorable risk category, 9 were in the poor risk category, and the others were in the intermediate risk category. There were 14 cases of non-refractory AML and 23 cases of refractory AML (including four cases of primary refractory AML). When relapsed, the median WBC count was 2.46 × 109/L (range, 0.52–37.34 × 109/L), and the median BM blast was 31% (range, 7–93%) (Table 1).
Outcomes
The overall response rate (ORR) was 56.8% (21/37), with a complete remission (CR) rate of 51.4% (19/37). For patients with non-refractory and refractory AML, the CR rates were 87.5% (14/16) and 30.4% (7/23), respectively (p < 0.001). No remission was found in four primary refractory AML patients. The remission rate of patients in the poor risk category and the recurrence time less than 12 months were lower than others (P = 0.014 and P = 0.001, respectively); however, these were not influenced by age, BM blasts, or gene mutations (all P > 0.05) (Table 2).
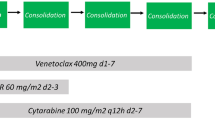
Considering the influence of subsequent chemotherapy on survival, we further analyzed consolidation therapeutic regimens (Fig. 1). The median OS and RFS were 10.6 (7.7–13.5) months and 5.0 (2.8–7.2) months, respectively (Fig. 2a, b). Univariate analysis showed that clinical characteristics, including age, sex, BM blasts, gene mutations, and non-refractory or refractory AML had no significant impact on survival (all P > 0.05) (Table 2). Patients who achieved a CR did not show a significant improvement in OS (p = 0.588) (Fig. 3). Patients in the poor-risk group had a shorter OS and no difference in RFS (P = 0.004 and P = 0.088, respectively) (Fig. 4a, b). Additionally, subgroup analysis showed that OS and RFS in patients with a recurrence time of < 6 months were shorter than those in other patients (P = 0.018 and P < 0.001, respectively) (Fig. 5a, b), which were not observed in patients with a recurrence time of < 12 months.
Seven patients, all of whom had refractory AML, underwent allo-HSCT. Among them, two patients underwent allo-HSCT after CR and both survived. Two patients, one with NR and one with PR, both received alternative chemotherapy regimens and were consolidated with allo-HSCT after CR was reattained, and one patient was alive. Another three patients underwent salvage transplantation and eventually died. Univariate analysis demonstrated that allo-HSCT might improve OS (P = 0.031) rather than RFS (P = 0.072) (Fig. 6a, b).
Considering the results of univariate analysis and previously reported data, we conducted a multivariate analysis incorporating age, sex, risk stratification, BM blasts, gene mutations, disease status, recurrence time, and consolidation therapies to clarify the prognostic factors. Nevertheless, multivariate analysis demonstrated that these factors did not affect survival (all P > 0.05) (Table 3).
Safety
During the induction therapy, all patients suffered from grade 4 hematological toxicity. The median times to neutropenia and platelet counts < 20 × 109/L were 17 days (range, 0–31 days) and 13 days (range, 0–30 days), respectively. Serious bleeding or organ dysfunction was not observed. The incidence of infections was 73% (27/37), including 12 cases of pneumonia, 3 cases of sepsis, 3 cases of cutaneous infection, 2 cases of oral mucositis, 1 case of intra-abdominal infection, and 6 cases of febrile neutropenia. According to CTCAE (version 4.0), 13.5% (5/37) of patients suffered from severe infections (grade 3 or 4). One patient died of severe pneumonia and respiratory failure, with a treatment-related mortality rate of 2.7% (1/37).
Discussion
Relapsed/refractory AML patients always have a lower remission rate and various complications, leading to limited therapeutic alternatives and poor prognosis. No standard salvage treatment is available for these patients. A review revealed that several intensive regimens achieved relatively high CR rates, ranging from 44 to 59.4% in adult patients with R/R AML. However, most of these regimens do not result in substantial CR duration or overall survival [9]. Novel non-cytotoxic approaches have reshaped the therapeutic landscape of chemoresistant AML [10, 11]. However, for economic or other reasons, some patients do not receive these novel drugs. Therefore, alternative therapies are recommended to control disease progression and minimize treatment-related mortality. Aberrant DNA methylation is an essential component of the pathogenesis of leukemia [5]. Decitabine can inhibit DNA methyltransferases (DNMTs) and reactivate silenced tumor suppressor genes, and is a promising therapeutic approach. Decitabine can increase sensitivity to cytotoxic drugs [7, 12].
In an international multicenter retrospective database, the CR/CRi rate of hypomethylating agents (HMAs) in patients with relapsed/refractory AML was 16%, the median OS was 6.5 months, and the OS for patients achieving CR/CRi was 21 months [13]. Another study reported a CR rate of 15.7%, with a median OS of 177 days in relapsed/refractory AML patients [14]. These data underscore the fact that HMAs monotherapy did not result in long-term remission as a front-line treatment in patients with R/R AML. For these patients, the reported CR rate for intensive chemotherapy ranges from 38 to 67% [15,16,17,18,19,20,21,22]. Several studies have demonstrated that the remission rate of decitabine-based chemotherapy is similar to that of intensive chemotherapy, and the former has superior tolerance. A retrospective study reported that low-dose decitabine in combination with aclacinomycin and cytarabine achieved a better outcome than FLAG in patients with R/R AML and illustrated that decitabine may further increase sensitivity to cytotoxic drugs further [7, 12, 23, 24].
In our study, all the patients received the DCAG regimen as salvage therapy. Our results revealed the CR rate of 51.4%. Compared with intensive chemotherapy, the remission rate was relatively high. The CR rate for non-refractory patients was 87.5%, whereas that for refractory AML was 30.4%. Subgroup analyses showed that the CR rate of non-refractory AML was significantly higher than that of refractory AML, which is inconsistent with a large international patient cohort [13]. However, this regimen is unsuitable for the treatment of primary refractory AML. It also revealed that the CR rate of patients with a recurrence time of < 12 months was inferior to that of others, suggesting that the regimen could not overcome early relapse.
In addition, our results showed that the remission rate in the poor-risk group was inferior to that in the other groups, similar to the results of other salvage regimens. It has been reported that the CR rate of intensive chemotherapy in patients with epigenetic mutations, such as ASXL1 and DNMT3A, is lower. In our study, the remission rate was not affected by these genes, suggesting that the DCAG regimen might overcome some unfavorable mutations. TP53 mutation, which results in impaired function of the p53 protein leading to impaired apoptosis and cellular immortality, provides malignant cells with innate resistance to intensive chemotherapy, with a CR rate in the range of 20–40% [25, 26]. Several studies have suggested that hypomethylating agents might be an alternative therapeutic regimen for TP53-mutated AML; however, our data are limited because only one patient was enrolled and attained CR. Therefore, we should be prudent in choosing indications.
In survival analysis, decitabine-based chemotherapy showed a median OS and RFS of 10.6 months and 5 months, respectively, which seemed to be no worse than or even better than that of intensive chemotherapy. In our study, the OS of poor-risk AML patients was shorter than that of others, but the RFS was similar, which indicated that even though this regimen might postpone recurrence, it still could not improve survival. In addition, a recurrence time less than 6 months was an adverse factor for prognosis, which further suggests that the DCAG regimen might not overcome early relapse. Moreover, there was no significant difference in survival between patients with CR and non-CR, which was related to differences in follow-up treatment. Among the seven patients who underwent allo-HSCT, only two achieved CR after DCAG regimens, suggesting that allo-HSCT was essential for prolonging survival. Subgroup analysis also showed that allo-HSCT might improve OS, further confirming the above results.
Gene mutations significantly influence prognosis. Notably, the widely accepted prognostic impact of gene mutations mostly results from intensive chemotherapy. Nevertheless, the influence of these mutations, such as TP53 and DNA methylation mutations (including DNMT3A, TET2, IDH1/2, and ASXL1), on patients treated with the DCAG regimen has been relatively less reported, and the results remain controversial. TP53 mutation tend to be observed in elderly patients, whereas it is usually associated with poor prognosis and low response to chemotherapy [25,26,27,28]. Welch et al. reported that patients with TP53 mutation responded to decitabine; however, the improved response rate did not translate into prolonged survival [13, 29]. In our study, only one patient with TP53 mutation who achieved CR failed to receive regular treatment and relapsed. Our results also demonstrated that the DCAG regimen could be applied to patients with methylation mutations and the survival were similar. We anticipate the expansion of samples and design of prospective studies for further verification.
Grade 4 hematologic toxicity commonly occurred during treatment, and the incidence of infection was 73%. The median time for neutropenia and a platelet count of < 20 × 109/L was similar to that for intensive chemotherapy. In our study, all patients have relapsed or refractory AML with a poor performance status, which accounted for the higher incidence of infection; nevertheless, the rate of severe infection was relatively lower. The treatment-related mortality was 2.7%, suggesting that this regimen was well-tolerated for these patients.
In conclusion, the DCAG regimen was an effective and well-tolerated therapeutic alternative for R/R AML, particularly in non-refractory patients. Furthermore, this regimen may overcome some certain unfavorable factors. Our results also revealed that poor-risk AML and early relapse were adverse factors for survival. Considering the inherent bias in retrospective studies and the limited number of enrolled patients, these results should be interpreted with caution, and further clinical trials are essential.
References
Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults:2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.
Walter RB, Othus M, Brunett AK, et al. Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI,HOVON/SAKK,SWOG and MD Anderson Cancer Center. Leukemia. 2015;29(2):312–20.
Cui L, Liu Y, Pang Y, et al. Emerging agents and regimens for treatment of relapsed and refractory acute myeloid leukemia. Cancer Gene Ther. 2020;27(1-2):1–14.
Ganzel C, Sun Z, Cripe LD, et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience. Am J Hematol. 2018;93(8):1074–81.
Kim TK, Gore SD, Zeidan AM. Epigenetic therapy in acute myeloid leukemia: current and future directions. Semin Hematol. 2015;52(3):172–83.
Negrotto S, Ng KP, Jankowska AM, et al. CpG methylation patterns and decitabine treatment response in acute myeloid leukemia cells and normal hematopoietic precursors. Leukemia. 2012;26(2):244–54.
Jiang X, Wang Z, Ding B, et al. The hypomethylating agent decitabine prior to chemotherapy improves the therapy efficacy in refractory/relapsed acute myeloid leukemia patients. Oncotarget. 2015;6(32):33612–22.
Song LX, Xu L, Li X, et al. Clinical outcome of treatment with a combined regimen of decitabine and aclacinomycin/cytarabine for patients with refractory acute myeloid leukemia. Ann Hematol. 2012;91(12):1879–86.
Megías-Vericat JE, Martínez-Cuadrón D, Sanz MÁ, et al. Salvage regimens using conventional chemotherapy agents for relapsed/refractory adult AML patients: a systematic literature review. Ann Hematol. 2018;97(7):1115–53.
Tiong IS, Wei AH. New drugs creating challenges in acute myeloid leukemia. Genes Chromosomes. Cancer. 2019;58(12):903–14.
Thol F. What to use to treat AML: the role of emerging therapies. Hematology Am Soc Hematol Educ Program. 2021;2021(1):16–23.
Tawfik B, Sliesoraitis S, Lyerly S, et al. Efficacy of the hypomethylating agents as frontline, salvage, or consolidation therapy in adults with acute myeloid leukemia (AML). Ann Hematol. 2014;93(1):47–55.
Stahl M, DeVeaux M, Montesinos P, et al. Hypomethylating agents in relapsed and refractory AML:outcomes and their predictors in a large international patient cohort. Blood Adv. 2018;2(8):923–32.
Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma. 2013;54(9):2003–7.
Lee SR, Yang DH, Ahn JS, et al. The clinical outcome of FLAG chemotherapy without idarubicin in patients with relapsed or refractory acute myeloid leukemia. J Korean Med Sci.2009;24(3):498–503.
Wang LJ, Ding J, Zhu CY, et al. Clinic outcome of FLAG regimen treating patients with refractory and relapse acute myeloid leukemia. J Exp Hematol. 2016;24(1):19–24.
Martin MG, Welch JS, Augustin K, et al. Cladribine in the treatment of acute myeloid leukemia: a single-institution experience. Clin Lymphoma Myeloma. 2009;9(4):298–301.
Price SL, Lancet JE, George TJ, et al. Salvage chemotherapy regimens for acute myeloid leukemia: is one better efficacy comparison between CLAG and MEC regimens. Leuk Res. 2011;35(3):301–4.
Trifilio SM, Rademaker AW, Newman D, et al. Mitoxantrone and etoposide with or without intermediate dose cytarabine for the treatment of primary induction failure or relapsed acute myeloid leukemia. Leuk Res. 2012;36(4):394–6.
Kohrt HE, Patel S, Ho M, et al. Second-line mitoxantrone, etoposide, and cytarabine for acute myeloid leukemia: a single-center experience. Am J Hematol. 2010;85(11):877–81.
Fan CH, Yu MJ, Mai MY, et al. Efficacy of HAA regimen in the treatment of 64 patients with refractory/relapsed acute myeloid leukemia. Chin J Hematol. 2016;37(2):100–4.
Li L, Zhang X, Yu H, et al. Low-dose hypomethylating agent decitabine in combination with aclacinomycin and cytarabine achieves a better outcome than standard FLAG chemotherapy in refractory/relapsed acute myeloid leukemia patients with poor-risk cytogenetics and mutations. Onco Targets Ther. 2018;11:6863–70.
Chen Y, Dai M, Liu Q. Efficacy and safety of DAC combined with CAG regimen (decitabine in combined with aclacinomycin, cytarabine and G‑CSF) as a second induction regimen compared with Non-DAC regimen for acute myeloid leukemia who failed the first course of standard induction IA chemotherapy. Blood. 2019;134((sup1):5104–5104.
Wang L, Luo J, Chen G, et al. Chidamide, decitabine, cytarabine, aclarubicin, and granulocyte colony-stimulating factor (CDCAG) in patients with relapsed/refractory acute myeloid leukemia: a single-arm, phase 1/2 study. Clin Epigenetics. 2020;12(1):132.
Kadia TM, Jain P, Ravandi F, et al. TP53 mutations in newly diagnosed acute myeloid leukemia: Clinicomolecular characteristics, response to therapy, and outcomes. Cancer. 2016;122(22):3484–91.
Hunter AM, Sallman DA. Current status and new treatment approaches in TP53 mutated AML. Best Pract Res Clin Haematol. 2019;32(2):134–44.
Rücker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119(9):2114–21.
Hou HA, Chou WC, Kuo YY, et al. TP53 mutations in de novo acute myeloid leukemia patients: longitudinal follow-ups show the mutation is stable during disease evolution. Blood Cancer J. 2015;5(7):e331.
Welch JS, Petti AA, Miller CA, et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N Engl J Med. 2016;375(21):2023–36.
Acknowledgements
We thank the staff at the Department of Hematology, The First Hospital of Jilin University, for their assistance with this work.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Fu, L. Su, Y. Li and Y. Tan declare that they have no competing interests.
Ethical standards
Ethics approval: This study was approved by the Ethics Committee of The First Hospital of Jilin University (approval number: 2023–515) and conducted in accordance with the Declaration of Helsinki. Consent to participate: Informed consent was obtained from all individual participants included in the study. Consent to publish: All the participants had consented to the submission to the journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, Y., Su, L., Li, Y. et al. Decitabine combined with low-dose cytarabine, aclarubicin and rhG-CSF regimen may be a potential alternative for relapsed/refractory acute myeloid leukemia: A single-center study. memo (2023). https://doi.org/10.1007/s12254-023-00933-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12254-023-00933-x