Abstract
The effect of genetic polymorphisms for glutathione S-transferase ( GST) M1, GSTT1, GSTP1-1 ( GSTP1), cytochrome P450 2E1 ( CYP2E1) and aldehyde dehydrogenase 2 ( ALDH2) on the risk of hepatocellular carcinoma (HCC) was observed in 78 Japanese patients with HCC and 138 non-cancer hospital controls. We found a positive association between cumulative amounts of alcohol consumption (≧600,000 ml in a lifetime) and the risk of HCC (OR=4.52, 95% CI 2.39–8.55). However, cigarette smoking was not significantly related to the risk of HCC (OR=1.23, 95% CI 0.57–2.68). The allelic frequencies of GSTM1, GSTT1, GSTP1, CYP2E1and ALDH2of HCC patients were not significantly different from those of controls when odds ratios were only adjusted for age and gender except for any 2 alleles of ALDH2in drinkers (OR=2.53, 95% CI 1.21–5.31). However, the frequency of any C2alleles of CYP2E1 and any 2 alleles of ALDH2were significantly higher than those of controls (OR=5.77, 95% CI 1.24–27.39, OR=9.77, 95% CI 1.63–58.60) when covariates including viremia were selected by using stepwise logistic regression analysis. We conclude that habitual alcohol drinking is likely to lead to an increased risk of HCC, and any C2alleles of CYP2E1as well as any two alleles of ALDH2were also associated with an increased risk of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary liver cancer usually complicates several chronic liver diseases, mainly those induced by hepatitis B virus (HBV) and hepatitis C virus (HCV) (Ruiz et al. 1992; Tsai et al. 1994). Especially, HBV and HCV prevalence was found to be associated with 95% of hepatocellular carcinoma (HCC) patients in Japan (The Liver Cancer Study Group of Japan 2000). The proportions of HBV and HCV were 16.5% and 74.8%, respectively. Not only is HCC an inevitable consequence of chronic HBV or HCV infection, but also other HCC risk factors, such as tobacco smoking, alcohol drinking and aflatoxin exposure (Ross et al. 1992), are related to susceptibility to HCC. Epidemiological studies have shown a possible correlation between ethanol abuse and the development of HCC (Chen et al. 1991; Mohamed et al. 1992). Otherwise, the possibilities of a relationship between tobacco smoking and the occurrence of HCC are controversial (Trichopoulos et al. 1987; Tsukuma et al. 1993; Kuper et al. 2000; Tanaka et al. 1992; Hadziyannis et al. 1995).
Many chemical carcinogens are also metabolically converted into active forms that have harmful effects on the liver. The metabolizing enzymes, including glutathione S-transferases (GSTs), cytochrome P-450s (CYPs) and aldehyde dehydrogenase 2 (ALDH2), play an important role in the detoxification or activation of carcinogens. This metabolic activation depends on genetic variations, which may be responsible for individual differences. GSTM1, GSTT1and GSTP1may play a part in the activation and detoxification of procarcinogens in tobacco smoke (Guengerich 1991; Mannervik and Danielson 1988). Individual variations in enzyme activities have been demonstrated for several GSTs. Some of these variations are genetically linked and may affect individual cancer risk.
When drinking alcohol, some of the proposed mechanisms for ethanol-related carcinogenesis are closely linked to the action of acetoaldehyde. Approximately half of the Japanese population lacks ALDH2 activity because of a structural point mutation in the ALDH2 gene. This genetic polymorphism, which is seen in Asians, including Japanese, but not in Caucasians, results in catalytic deficiency of aldehyde metabolism (Harada and Zhang 1993). Besides ALDH2, the ethanol inducible CYP2E1 catalyses the oxidation of ethanol itself. In addition, CYP2E1 is of critical importance in the metabolic activation of many carcinogens, including N-nitrosamines, benzene and aniline, that are present in tobacco smoke. Therefore, previous reports have shown that CYP2E1 might modulate the risk of HCC (Ladero et al. 1996).
In this study, we have made the hypothesis that alcohol abuse and/or tobacco smoking is a risk factor for the development of HCC, and we have examined the effects of the GSTs ( M1, T1, P1-1), CYP2E1and ALDH2polymorphism on the susceptibility of HCC among Japanese people in relation to their smoking or alcohol-drinking status.
Materials and methods
Subjects
A total of 78 HCC patients seen in the University of Occupational and Environmental Health (UOEH) Hospital in Japan from June 1997 to April 1998 were enrolled in the present study. Acid-citrate-dextrose-anti-coagulated blood was drawn from 78 patients with HCC and from 138 hospital controls with no evidence of cancer in any organ. Cases and controls were unmatched. The demographic data of both case and control groups are shown in Table 1. All study subjects completed a questionnaire administrated by a trained interviewer, covering medical, residential, occupational and smoking and drinking history. The lifetime amount of cigarette smoking was quantified by the Brinkman-Coates index, which is the product of the daily number of cigarettes smoked and years of smoking. The cumulative amount of ethanol consumption was quantified by drink-years, which was calculated by multiplying the volume of ethanol a year by the number of drinking-years. None of the subjects had had any exposure to carcinogens, heavy metals or radiation in their occupational history.
This study was approved by the ethics committee of medical care and research of the University of Occupational and Environmental Health (UOEH) under the guidelines of the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Genotyping
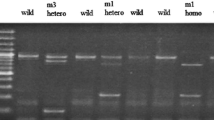
Genomic DNA was isolated from peripheral leukocytes by proteinase K digestion and phenol/chloroform extraction and ethanol precipitation. A multiplex polymerase chain reaction (PCR) method was used to detect the presence or absence of GSTM1and GSTT1 (Katoh et al. 1996). The genotype of GSTP1 (A to G substitution at nucleotide 313) was determined by PCR/RFLP according to Watson et al. (1998). The genetic polymorphism in the 5′-flanking region of CYP2E1was determined by PCR amplification followed by digestion with Rsa1, using the method described previously (Adami et al. 1992). The dominant allele ( c1) was sensitive to Rsa1digestion and the c2allele was resistant to Rsa1digestion. The genotypes of ALDH2were identified as the homozygous genotype of a normal ALDH2 (1/1), the homozygous genotype of an inactive ALDH2 (2/2) and the heterozygous genotype of normal and inactive ALDH2 (1/2) by the method of Harada and Zhang (1993).
Statistical analysis
Statistical analysis was performed by comparing each gene polymorphism of five metabolic enzymes in HCC patients with the hospital controls. Odds ratios and 95% confidence intervals were adjusted for age and gender by multiple logistic regression analysis with the SPSS for Windows Medical Pack (SPSS Inc., Chicago). Needing to combine heterozygous genotypes ( GSTM1/T1/P1, CYP2E1ALDH2) to examine the interaction between environmental and genetic factors as well as smoking or drinking status, we carried out stratification analysis of HCC risk associated with genotypes.
Results
Table 2 demonstrates the risk of HCC by drinking, smoking habits and viremias. The age- and gender-adjusted OR of heavy drinkers, who consumed alcohol above a threshold of 600,000 ml during their lifetime, was significantly higher (OR=5.19, 95% CI 2.53–10.64) than in non-drinkers and light drinkers who consumed alcohol under 600,000 ml during their lifetime. On the other hand, there was no tendency of increased risk in the smoker strata (OR=1.23, 95% CI 0.57–2.68). We also confirmed a strong association between viremia and HCC (OR=805.17, 95% CI 134.37–4,824.52).
The age- and gender-adjusted frequencies of GSTM1, GSTT1, GSTP1, CYP2E1 and ALDH2genotypes associated with HCC are shown in Table 3. There was no significant difference between controls and HCC in terms of frequency distribution of their genes. To evaluate the interaction between the genotypes, we analyzed the combination of the genes. No significant association was observed for any interaction of genes (data not shown).
Furthermore, we calculated the OR for data that was classified by smoking or drinking to evaluate the effect of the gene in combination with smoking or drinking. The summarized data and the ORs are shown in Table 4, together with the 95% confidence interval. The frequency of any 2 allele of ALDH2had a significant correlation with increased risk of HCC among alcohol drinkers (OR=2.53, 95% CI 1.21–5.31). However, other genotype distributions of HCC were not significantly different from those of the controls (data not shown).
Finally, we had a multivariate analysis including viremias; variables available for selection include age, gender, drinking status, smoking status, viremia and each genotype of five enzymes (Table 5). The frequencies of C2alleles of CYP2E1 (OR=5.77, 95% CI 1.24–27.39) and 2 alleles of ALDH2 (OR=9.77, 95% CI 1.63–58.60) were significantly higher than those of controls (Table 5)
Discussion
We have observed the correlation between habitual alcohol drinking and the risk of HCC for many years. Our results showed that there was a significant association between heavy alcohol drinking, which is over 600,000 ml in a lifetime, and an increase in the risk of HCC: the OR for alcohol drinkers was 4.52 (95% CI 2.39–8.55) in our HCC patients (Table 2). This relationship is in agreement with most of the many previous reports on this topic (Mohamed et al. 1992; Kuper et al. 2000). This seems to be a valid finding because alcohol has been assumed to be a promoter or growth enhancer of HCC (Adami et al. 1992).
We also examined the association between tobacco smoking and the risk of HCC. Some risk excess was observed among tobacco smokers (OR=1.23, 95% CI 0.57–2.68) compared with non-smokers, but it was not significant.
Otherwise, the data on smoking and risk of HCC are contradictory (Trichopoulos et al. 1987; Tsukuma et al. 1993; Kuper et al. 2000; Tanaka et al. 1992; Hadziyannis et al. 1995). Our data revealed that there was likely to be no positive relationship between tobacco smoking and HCC. If tobacco smoking is one of the causes of HCC, this discrepancy could be due to some biases. The first one was that the smoking histories were excessively error prone. The second was that it was impossible to distinguish between two kinds of non-smoker. One of them had never smoked in their life, and another had quit smoking, but had a past history of smoking. The last was that the alcohol habit confounded it in the present study. We need a further examination without biases such as smoking history, alcohol and viremia.
We present data on the frequency of the ALDH2genotype in HCC. A significant relationship between the occurrence of certain cancers and the ALDH2polymorphism has been reported, particularly in alcoholics (Hori et al. 1997). Other reports also indicated that the differences of ALDH2genotypes has no association with HCC development (Takeshita et al. 2000). However, in a multivariate analysis including the viral factor, the frequency of any 2 allele of ALDH2was significantly different from controls (OR=9.77, 95%CI 1.63–58.60). Moreover, we found evidence of a significant effect of drinking depending on the difference of the genetic polymorphism of ALDH2. Statistically, there was an association between any 2 allele of ALDH2and HCC patients in habitual drinkers (OR=2.53, 95% CI 1.12–5.31). It is likely that alcoholic liver diseases with the ALDH2heterozygote (1/2) are more severe than those with the ALDH2homozygote (1/1) (Enomoto et al. 1991), since those with the ALDH2heterozygote (1/2) would have higher internal exposure to acetaldehyde after drinking alcohol (Takeshita et al. 1997). Ohhira et al. (1996) studied primary hepatocellular carcinoma associated with alcoholic liver disease without hepatitis virus infection. In the analysis of genetic polymorphism of ALDH2, all of the subjects had the ALDH2homozygote (1/1or2/2). Otherwise, Shibata et al. (1998) showed that ORs resulting from the ALDH2homozygote and some accumulated amount of alcohol intake by age 40 based on community controls were statistically significant in HCC. Although it is inconsistent which is a risk factor, the homozygote or heterozygote gene, these results might imply that individual differences of ALDH2genotypes change the risk of HCC by alcohol consumption.
A multivariate analysis showed that an increase of risk for HCC also was found to a significant degree in the difference of CYP2E1genotypes (OR=5.77, 95% CI 1.24–27.39). The rate of CYP2E1 activity increases in the liver after alcohol induction. This means that the c2 CYP2E1 gene increases in habitual drinkers, especially those with chronic liver disease (Ladero et al. 1996; Tsutsumi et al. 1994a, 1994b). As a result, the activation of carcinogens increases in the liver. It is possible that the CYP2E1 activity in the human liver is associated with the susceptibility of HCC. There are two different mechanisms that influence its rate of activity. One of them is the genetic functional difference between c1and c2alleles. The other depends on environmental factors, mainly ethanol or other inducers, which also frequently show a carcinogenic potential in the liver. Earlier reports have suggested the CYP2E1polymorphisms may play an important role in smoking-related HCC. Homozygosity for the c1/c1genotype significantly increased the risk of developing HCC in cigarette smokers (Yu et al. 1995). In contrast, there was no significant association between HCC risk and genotype c1/c2or c2/c2in all HCC patients (Lee et al. 1997).
In this study, the possible effects of GSTs metabolic enzymes in modulating the development of HCC were not confirmed among alcohol drinkers or tobacco smokers. Members of the GST family are important candidates for involvement in susceptibility to commonly occurring forms of cancer, because they may regulate an individual's ability to metabolize environmental carcinogens. Normal or increased GST enzyme activity or levels may protect susceptible tissues from somatic mutations in DNA by facilitating the conjugation and subsequent elimination of electrophilic carcinogens. Absent or deficient GST enzyme activity may result in poorer elimination of electrophilic carcinogens, particularly in the presence of very active electrophilic activation by phaseI enzymes. If an individual's inherited genotype at a GST locus does not permit the efficient metabolism of compounds involved in carcinogens, then that individual may be at increased cancer risk.
For example, the GSTM1/ GSTT1 is polymorphic in humans. GSTM1has been shown to be polymorphic and is absent in 35–60% of individuals (Bell et al. 1993; Katoh et al. 1995). Similarly, GSTT1is also polymorphic and is absent in 10–65% of human populations (Chenevix-Trench et al. 1995). The lack of GSTM1 activity is due to the inherited homozygous deletion of the genes, and GSTM1deficiency has been linked with risk for various cancers (Bell et al. 1993; Brockmoller et al. 1996; Rebbeck 1997). Less is known about the association between GSTT1and cancer risk, but persons with the GSTT1null type show reduced ability to detoxify metabolites of 1,3-butadiene (Pemble et al. 1994) and ethylene oxide (Wiencke et al. 1995). A report suggested that the GSTT1null type might be a risk modifier in the occurrence of colorectal cancer (Deakin et al. 1996). Also, the difference of GSTM1/T1polymorphisms may be subject to increased risk of urothelial cancer in tobacco smokers (Katoh et al. 1998).
The GSTP1is also widely expressed in normal epithelial tissue and is particularly abundant in the urinary, respiratory and digestive tracts, suggesting a possible role for GSTP1in the detoxification and elimination of toxic products in these tissues. GSTP1 is a major enzyme involved in the inactivation of carcinogens in cigarette smoke, such as benzo(a)pyrene diol epoxide and acrolein, as well as other cigarette smoke toxins. The gene is also suggested to be involved in the development of acquired resistance towards anti-cancer drugs. The GG genotype of GSTP1was significantly more frequent among patients with oral squamous cell carcinoma and lung cancer (Katoh et al. 1999; Ryberg et al. 1997).
Overall, the differences of genetic polymorphisms on GST enzymes have no association with the development of HCC, although alcohol drinking showed a significant association with it. The discrepancy between our results and previous reports could be explained by the following suggestions: first, there was a racial difference in the frequencies of each genotype (Kato et al. 1992); for another reason, the risk of genetic polymorphism to HCC could be overshadowed by the great etiologic role of HBV and HCV viremia in the development of HCC (Tsukuma et al. 1990; Yu et al. 1994; Donato et al. 1998). However, HBV positive patients had significantly lower GST activity than those who were HBV negative (Zhou et al. 1997). These results suggest that the risk of HCC is not only associated with GST polymorphism, but also GST activity.
In conclusion, we found that there was a significant association between CYP2E1and ALDH2polymorphisms with the interaction of alcohol and the risk of HCC in Japanese people.
References
Adami HO, Hsing AW, McLaughlin JK, Trichopoulos D, Hacker D, Ekbom A, Persson I(1992) Alcoholism and liver cirrhosis in the etiology of primary liver cancer. Int J Cancer 51:898–902
Bell DA, Taylor JA, Paulson DF, Robertson CN, Mohler JL, Lucier GW(1993) Genetic risk and carcinogen exposure: a common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst 85:1159–1164
Brockmoller J, Cascrbi I, Kerb R, Roots I(1996) Combined analysis of inherited polymorphisms in arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1; microsomal epoxide hydolase, and cytochrome P450 enzymes as modulators of bladder cancer risk. Cancer Res 56:3915–3925
Chen CJ, Liang KY, Chang AS, Chang YC, Lu SN, Liaw YF,Chang WY, Sheen MC, Lin TM(1991) Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familiar tendency on hepatocellular carcinoma. Hepatology 13:398–406
Chenevix-Trench G, Young J, Coggan M, Board P(1995) Glutathione S-transferase M1 and T1 polymorphisms: susceptibility to colon cancer and age onset. Carcinogenesis 16:1655–1657
Deakin M, Elder J, Hendrickse C (1996) Glutathione S-transferase GSTT1 genotypes and susceptibility to cancer: Studies of interactions with GSTM1 in lung, oral, gastric and colorectal cancers. Carcinogenesis 17:881–884
Donato F, Boffetta P, Puoti M(1998) A meta-analysis of epidemiological studies on the combined effect of Hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer 75:347–354
Enomoto N, Takase S, Takada N, Takada A (1991) Alcohol liver disease in heterozygotes of mutant and normal aldehyde dehydrogenase-2 genes. Hepatology 13:1071–1075
Guengerich FP (1991) Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzyme. Chem Res Toxicol 4:391–407
Hadziyannis S, Tabor E, Kaklamani E, Tzonou A, Stuver S, Tassopoulos N, Mueller N, Trichopoulos D (1995) A case-control study of hepatitis B and C virus infections in the etiology of hepatocellular carcinoma. Int J Cancer 60:627–631
Harada S, Zhang S (1993) New strategy for detection of ALDH2 mutant. Alchol Alchohol [Suppl] 1A:11–13
Hori H, Kawano T, Endo M, Yuasa Y (1997) Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and human esophageal squamous cell carcinoma susceptibility. J Clin Gastroenterol 25:568–575
Kato S, Shields PG, Caporaso NE, Hoover RN, Trump BF, Sugimura H, Weston A, Harris CC (1992) Cytochrome P450IIE1 genetic polymorphisms, racial variation, and lung cancer risk. Cancer Res 52:6712–6715
Katoh T, Inatomi H, Nagaoka A, Sugita A (1995) Cytochrome P450 1A1 gene polymorphism and homozygous deletion of the glutathione S-transferase M1 gene in urothelial cancer patients. Carcinogenesis 16:655–657
Katoh T, Nagata N, Kuroda Y, Itoh H, Kawahara A, Kuroki N, Ookuma R, Bell DA (1996) Glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) genetic polymorphism and susceptibility to gastric and colorectal adenocarcinoma. Carcinogenesis 17:1855–1859
Katoh T, Inatomi H, Kim H, Yang M, Matsumoto T, Kawamoto T (1998) Effect of glutathione S-transferase(GST)M1 and GSTT1 genotypes on urothelial cancer risk. Cancer Letters 132:147–152
Katoh T, Kaneko S, Takasawa S, Nagata N, Inatomi H, Ikemura K, Itoh H, Matsumoto T, Kawamoto T, Bell DA (1999) Human glutathione S-transferase P1 polymorphism and susceptibility to smoking related epithelial cancer; oral, lung, gastric, colorectal and urothelial cancer. Pharmacogenetics 9:165–169
Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, Trichopolous D, Stuver SO (2000) Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer 85:498–502
Ladero JM, Agundez JAG, Rodriguez-Lescure A,Diaz-Rubio M, Benitez J (1996) RsaIpolymorphism at the cytochrome P450 2E1 locus and risk of hepatocellular carcinoma. Gut 39:330–333
Lee HS, Yoon JH, Kamimura S, Iwata K,Watanabe H, Kim CY (1997) Lack of association of cytochrome P450 2E1 genetic polymorphisms with the risk of human hepatocellular carcinoma. Int J Cancer 71:737–740
Mannervik B, Danielson UH (1988) Glutathione transferases structure and catalytic activity. CRC Crit Rev Biochem 23:283–337
Mohamed AE, Kew MC, Groeneveld HT (1992) Alcohol consumption as a risk factor for hepatocellular carcinoma in urban southern Africa Blacks. Int J Cancer 51:537–541
Ohhira M, Fujimoto Y, Matsumoto A, Ohtake T, Ono M, Kohgo Y(1996) Hepatocellular carcinoma associated with alcoholic liver disease: a clinicopathological study and genetic polymorphism of aldehyde dehydrogenase 2. Alcohol Clin Exp Res 20 [Suppl 9):378A–382A
Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB (1994) Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J 300 (Pt 1):271–276
Rebbeck TR (1997) Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomarkers Prevent 6:733–743
Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE (1992) Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet 339:943–946
Ruiz J, Sangro B, Cuende JI, Beloqui O, Riezu-Boj JI, Herrero JI, Prieto J (1992) Hepatitis B and C viral infections in patients with hepatocellular carcinoma. Hepatology 16:637–641
Ryberg D, Skaug V, Hewer A, Phillips DH, Harries LW, Wolf CR, Øgreid D, Ulvik A, Vu P, Haugen A (1997) Genotypes of glutathione transferase M1 and P1 and their significance for lung DNA adduct levels and cancer risk. Carcinogenesis 18: 1285–1289
Shibata A, Fukuda K, Nishiyori A, Ogimoto I, Sakata R, Tanikawa K (1998) A case-control study on male hepatocellular carcinoma based on hospital and community controls. J. Epidemiology 8:1–5
Takeshita T, Kawai T, Morimoto K (1997) Elevated levels of hemoglobin-associated acetaldehyde related to alcohol drinking in the atypical genotype of low Km aldehyde dehydrogenase. Cancer Res 57:1241–1243
Takeshita T, Yang X, Inoue Y, Sato S, Morimoto K (2000) Relationship between alcohol drinking, ADH2 and ALDH2 genotype, and risk for hepatocellular carcinoma in Japanese. Cancer Letters 149:69–76
Tanaka K, Hirohata T, Takeshita S, Hirohata I, Koga S, Sugimachi K, Kanematsu T, Ohryohji F, Ishibashi H (1992) Hepatitis B virus, cigarette smoking and alcohol consumption in the development of hepatocellular carcinoma.: a case control study in Fukuoka, Japan. Int J Cancer 51:509–514
The Liver Cancer Study Group of Japan(2000) Survey and follow-up study of primary liver cancer in Japan Report 14 (in japanese). Acta Hepatol Japonica 41:799–811
Trichopoulos D, Day NE, Kaklamani E, Tzonou A, Munoz N, Zavitsanos X, Koumantaki Y, Trichopoulou A (1987) Hepatitis B virus, tobacco smoking and ethanol consumption in the etiology of hepatocellular carcinoma. Int J Cancer 39: 45–49
Tsai JF, Chang WY, Jeng JE, Ho MS,Lin ZY, Tsai JH (1994) Hepatitis B and C virus infection as risk factors for liver cirrhosis and cirrhotic hepatocellular carcinoma. A case-control study. Liver 14:98–102
Tsukuma H, Hiyama T, Oshima A, Sobue T, Fujimoto I, Kasugai H, Kojima J, Sasaki Y, Imaoka S, Horiuchi N, Okuda S (1990) A case-control study of hepatocellular carcinoma in Osaka, Japan. Int J Cancer 45:231–236
Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H, Kawashima T (1993) Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 328:1797–1801
Tsutsumi M, Takada A, Wang JS(1994a) Genetic polymorphisms of cytochrome P450 2E1 related to the development of alcoholic liver disease. Gastroenterology 107:1430–1435
Tsutsumi M, Wang JS, Takase S, Takada A (1994b) Hepatic messenger RNA contents of cytochrome 450 2E1 in patients with different P450 2E1 genotypes. Alchol Alcohol 29 [Suppl 1]:29–32
Watson MA, Stewart RK, Smith GBJ, Massey TE,BellDA (1998) Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis 19:275–280
Wiencke JK, Pemble S, Ketterer B, Kelsey KT (1995) Gene deletion of glutathione transferase theta1: correlation with induced genetic damage and potential role in endogenous mutagenesis. Cancer Epidemiol Biomarkers Prev 4:253–259
Yu MW, Chen CJ, Luo JC, Brandt-Rauf PW, Carney WP, Santella RM (1994) Correlations of chronic hepatitis B virus infection and cigarette smoking with elevated expression of neu oncoprotein in the development of hepatocellular carcinoma. Cancer Res 54:5106–5110
Yu MW, Gladek-Yarborough A, Chiamprasert S, Santella RM, Liaw YF, Chen CJ (1995) Cytochrome P450 2E1 and glutathione S-transferase M1 polymorphisms and susceptibility to hepatocellular carcinoma. Gastroenterology 109:1266–1273
Zhou T, Evans AA, London WT, Xia X, Zou H, Shen F, Clapper ML (1997) Glutathione S-transferase expression in hepatitis B virus-associated human hepatocellular carcinogenesis. Cancer Res 57:2749–2753
Acknowledgements
This work was supported in part by a grant-in-aid from the Ministry of Education, Science and Culture of Japan and a grant-in-aid from the Ministry of Welfare and Labor of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munaka, M., Kohshi, K., Kawamoto, T. et al. Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and the risk of hepatocellular carcinoma. J Cancer Res Clin Oncol 129, 355–360 (2003). https://doi.org/10.1007/s00432-003-0439-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-003-0439-5