Abstract
Obstructive sleep apnea syndrome is a major cause of morbidity in the Down syndrome population and is commonly treated with adenoidectomy and/or tonsillectomy (AT). However, these children are at increased risk for perioperative respiratory adverse events (PRAEs). The objective of this study was to examine risk factors for major PRAEs requiring intervention in children with Down syndrome undergoing AT and to describe their postoperative monitoring environment. This retrospective study included all children with Down syndrome aged 0–18 years who underwent a preoperative polysomnogram followed by AT at a tertiary pediatric institution. Descriptive statistics were used to summarize baseline demographic and clinical characteristics. A multivariable model for prediction of PRAEs was constructed. A priori, it was decided that minimum oxygen saturation, apnea–hypopnea index, and average oxygen saturation asleep would be included, along with medical comorbidities associated with PRAEs at p < 0.2 in univariable analyses. Fifty-eight children were included in this study; twelve had a PRAE. Cardiac disease was associated with PRAEs on univariable analysis (p = 0.03). In multivariable analysis, average oxygen saturation asleep was associated with PRAEs (OR 1.50; 95% confidence interval 1.00, 2.41; p = 0.05). For all of the remaining variables, p > 0.15. Fifty-six children were admitted for monitoring overnight; four were admitted to the intensive care unit and fifty-two were admitted to the ward.
Conclusions: A multivariable model found evidence that lower average oxygen saturation while asleep was associated with PRAEs requiring intervention in children with Down syndrome. This study highlights the difficulty in predicting complications in this population.
What is known: |
• Obstructive sleep apnea syndrome is a major cause of morbidity in the Down syndrome population and is commonly treated with adenoidectomy and/or tonsillectomy. |
• However, children with Down syndrome are at increased risk for perioperative respiratory adverse events (PRAEs) following adenoidectomy and/or tonsillectomy. |
What is new: |
• We found that a lower average oxygen saturation asleep is associated with increased odds of PRAEs, adjusting for age, total apnea–hypopnea index, cardiac comorbidity, and minimum oxygen saturation. |
• This study highlights the difficulty in predicting complications in this population. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Down syndrome is a common genetic condition occurring in 13 in 10,000 live births [1]. Obstructive sleep apnea syndrome (OSAS) is a major cause of morbidity in the Down syndrome population and is diagnosed in up to 75% of children with Down syndrome [2] compared to 1–5% in the general population [3]. Children with Down syndrome are at increased risk of OSAS due to multiple anatomical and functional factors including midfacial hypoplasia, a narrow nasopharynx, relative macroglossia, adenotonsillar hypertrophy, hypotonia, obesity, and gastroesophageal reflux [4]. Treatment of OSAS is essential as it may be associated with neurodevelopment disorders [5], behavioral issues [5], and cardiovascular risk [6].
The first line treatment for OSAS with adenotonsillar hypertrophy is adenoidectomy and/or tonsillectomy (AT). AT improves OSAS severity but is curative in only 18–33% of children with Down syndrome [4, 7, 8]. Although relatively safe, AT is not without risks. Perioperative respiratory adverse events (PRAEs) range from major complications requiring intervention such as hypoxemia, upper airway obstruction, and pulmonary edema to minor complications such as transient, self-resolving desaturations. Children with Down syndrome are at increased risk of PRAEs, especially after AT, compared to the general population. Major PRAEs following AT occur in children with Down syndrome at a rate of 17–25% [9,10,11] as compared to a rate of 9.4% in the general population [12]. Severe OSAS (defined as an obstructive apnea–hypopnea index [AHI] > 10 events/h and minimum oxygen saturation < 80%), American Society of Anesthesiologists (ASA) physical status classification, preoperative pediatric intensive care unit (PICU) admission, and aerodigestive comorbidities have been described as risk factors for respiratory complications occurring overnight or on postoperative day one following AT [9]. However, predictive risk factors for PRAEs immediately after AT in children with Down syndrome are not well elucidated. The ability to accurately identify children with Down syndrome at increased risk for PRAEs immediately following AT would have significant benefits for healthcare resource utilization and perioperative planning.
We sought to determine the nocturnal gas exchange and comorbidity risk factors for PRAEs requiring intervention in children with Down syndrome undergoing AT or isolated adenoidectomy or tonsillectomy. Our secondary aim was to describe the postoperative monitoring environment for this population and to track hospital readmissions for respiratory complications within 30 days of AT.
Methods
Study population
This retrospective cohort study included all children with Down syndrome aged 0–18 years who underwent a preoperative polysomnogram (PSG) followed by palatine tonsillectomy, adenoidectomy, or adenoidectomy and tonsillectomy between March 2003 and January 2016 at the Children’s Hospital of Eastern Ontario, a tertiary academic referral center. All children with Down syndrome within the catchment area of the hospital are routinely followed at our Down syndrome clinic and referred for PSG evaluation if there is clinical suspicion of sleep-disordered breathing.
Study design
This study was approved by the Children’s Hospital of Eastern Ontario Research Ethics Board (14/113X). Due to the retrospective study design, individual informed consent was not required. The demographic and clinical features of children were assembled into a Research Electronic Data Capture database. Lifetime concurrent diagnoses were obtained from paper medical charts. Body mass index (BMI) z-scores were calculated according to Down Syndrome growth charts [13] and obesity was defined as a BMI greater than the 95th percentile.
Two parameters for congenital heart disease (CHD) were analyzed including a history of cardiac disease and a validated risk stratification CHD score based on residual lesion burden and functional status on the most recent echocardiogram prior to AT [14]. The CHD classification score was utilized in recognition that the full spectrum of heart lesions may be found on screening echocardiograms in children with Down syndrome, including many hemodynamically insignificant cardiac lesions that may have otherwise remained undetected. Minor CHD included cardiac conditions with or without medications as well as repaired CHD with normal cardiovascular function and no medications. Major CHD included repaired CHD with residual hemodynamic abnormality with or without medications. Severe CHD included children with uncorrected cyanotic heart disease, documented pulmonary hypertension, ventricular dysfunction requiring medications, or listed for heart transplant [14]. Children with spontaneous resolution of CHD and no residual cardiac lesion were categorized as not having CHD.
The data from each child’s most recent PSG prior to surgery was included in this study. Overnight, attended, in-laboratory diagnostic PSGs were conducted with standard PSG montage employed (Natus Xltek) and scored by sleep technologists according to the American Academy of Sleep Medicine recommendations. All PSGs were interpreted by one of two pediatric sleep medicine physicians according to American Academy of Sleep Medicine criteria [15]. PSG parameters examined included the total AHI (obstructive AHI [total number of obstructive apneas and hypopneas/hour] plus the central AHI [total number of central apneas and hypopneas/hour]), average oxygen saturation asleep (average oxygen saturation for the total sleep time, excluding oxygen saturation readings that are deemed to be artifact), minimum oxygen saturation during sleep (minimum oxygen saturation recorded during the total sleep time, excluding oxygen saturation readings that are deemed to be artifact), and sleep efficiency.
Medical records were reviewed for major PRAEs from the time of surgery for the duration of the child’s hospitalization. Major PRAEs included upper airway obstruction, hypoxemia, and pulmonary edema. Interventions that commonly occur for these PRAEs were selected based on the authors’ clinical experiences and included supplemental oxygen therapy, positive airway pressure therapy, intubation, jaw thrust, oral airway placement, frequent repositioning, and diuretics for pulmonary edema. Perioperative steroid administration was not considered an intervention as they were routinely given to reduce postoperative complications. The requirement for supplemental oxygen was defined as requiring oxygen for longer than one hour postoperatively or any requirement of supplemental oxygen thereafter during the admission. It is standard practice at our institution to provide supplemental oxygen for sustained desaturation below 92% and for frequent intermittent desaturations. Continuous oxygen saturation monitoring is utilized immediately postoperatively in the post-anesthetic care unit (PACU) and then individualized to the need of the child based on the risk of decompensation.
Postoperative locations of care included the PACU, daycare surgery, the ward, and the PICU. Children were observed in the PACU for 30–60 min postoperatively and then transferred to either daycare surgery or the ward once they were deemed stable by the anesthesiologist. In cases of same day discharge, children were observed another 1–2 h in daycare surgery prior to discharge home. Children with planned admissions to the PICU were directly transferred there from the operating room. The decision to admit children for postoperative monitoring was made on an individual basis rather than based on a protocol. Emergency department visits and admissions at the Children’s Hospital of Eastern Ontario within thirty days of AT were also tracked.
Statistical analysis
Descriptive statistics were used to summarize baseline demographic and clinical characteristics. Frequency of PRAEs by type were tabulated by presence or absence of each type of comorbidity and Fisher’s exact test was used to test the association with PRAEs. Comorbidities with associations for which p < 0.20 were included in a multivariable logistic regression model for PRAEs requiring an intervention along with age at time of surgery, AHI, average oxygen saturation asleep, and minimum oxygen saturation. To accommodate the presence of a zero cell, Firth’s penalized logistic regression was used. Sensitivity analyses were completed to assess the robustness of these findings. First, the analysis was repeated by exclusively examining non-obese children. Second, the analysis was repeated by exclusively examining children who underwent combined adenoidectomy and tonsillectomy. All analyses were conducted using R version 3.6.2 [16].
Results
Baseline demographics
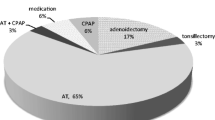
A total of 58 children were included (median [range] age 6.6 [1.3–16] years at the time of surgery, mean BMI z-score 0.2) (Table 1). The median (interquartile range [IQR]) AHI was 8.3 (5.3, 14.6) events/h. Five children underwent adenoidectomy, eleven underwent tonsillectomy, and forty-two underwent both adenoidectomy and tonsillectomy. The majority of children (54/58, 93.1%) received perioperative steroids.
There were 48/58 children (82.7%) with a history of CHD including patent ductus arteriosus, septal defects, coarctation of the aorta, and tetralogy of Fallot. Three children had pulmonary hypertension and were classified as having severe CHD. Other comorbidities included gastrointestinal disease (29/58, 50.0%), endocrine disease (19/58, 32.8%), and lower respiratory disease (17/58, 29.3%).
PRAEs
There were 12/58 children (21%) who had one or more major PRAEs including desaturation requiring supplemental oxygen (n = 11), upper airway obstruction requiring a combination of frequent repositioning, an oral airway, or jaw thrust (n = 5), and post-obstructive pulmonary edema requiring furosemide (n = 2). No children required noninvasive positive pressure ventilation or reintubation.
Risk factors for PRAEs
Major PRAEs were more frequent in children with a lifetime history of CHD on univariate testing (p = 0.03) (Table 2). The CHD risk stratification score was not predictive of PRAEs (p = 0.40). No other comorbidities were significantly associated with PRAEs.
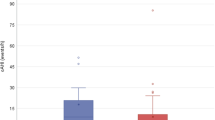
A multivariable logistic regression model was constructed for the a priori determined PSG variables and cardiac comorbidity (Table 3). A history of CHD was not associated with PRAEs on multivariable testing (p = 0.19). Average oxygen saturation asleep was associated with PRAEs (OR 1.50; 95% confidence interval 1.00, 2.41; p = 0.05). This estimated odds ratio corresponds to 50% increased odds of PRAE for every 1% decrease in average oxygen saturation asleep. Although there is significant overlap in oxygen saturations between children with and without PRAEs, it is notable that the two children with the lowest average oxygen saturations asleep (90–92%) suffered from PRAEs (Fig. 1). Both children had oxygen saturations of 95% or greater while awake and had a historical patent ductus arteriosus that either spontaneously resolved or was ligated. Neither subject was obese. Age, cardiac comorbidity, AHI, and minimum oxygen saturation were not associated with PRAEs on multivariable analysis. The same multivariable model was tested in a subgroup of non-obese children as a sensitivity analysis and performed similarly. Although we did not have sufficient numbers of obese children in our sample to determine the effect of obesity on PRAEs, it would be interesting to explore this in a future study. A sensitivity analysis exclusively examining children who underwent combined adenoidectomy and tonsillectomy also yielded similar results to the original model.
Asleep average oxygen saturation by PRAEs. Each dot indicates a patient’s average oxygen saturation during sleep obtained from preoperative polysomnography. The median (interquartile range) oxygen saturation among patients with PRAEs was 95.0 (94.0, 97.1) and among patients with no PRAEs was 96.4 (95.5, 97.2) (p = 0.13). PRAEs, perioperative respiratory adverse events; O2, oxygen
Postoperative monitoring environment
Figure 2 depicts the postoperative disposition of children included in our study. There were 56/58 children (97%) admitted postoperatively for monitoring for a length of 1 to 8 days. The majority of children did not have a PRAE (46/58, 79%). There were 2/58 children discharged home on the same day as their surgery, although both were planned to have an admission for monitoring. Conversely, 2/58 children were planned to have day surgery but had unexpected admissions to the ward due to PRAEs in the PACU.
Of the children with planned admissions, 4/56 (7%) were initially admitted to PICU and 50/56 (89%) were admitted to the ward. Of the PICU admissions, 3/4 were planned admissions for the PICU. One admission was initially planned for the ward but was ultimately monitored in the PICU due to upper airway obstruction in the PACU.
There were 7/58 children (12%) who visited the emergency department within 30 days of AT. Overall, 4/58 children (7%) were readmitted for postoperative complications including pain, dehydration, and postoperative bleeding. None were readmitted for respiratory complications.
Discussion
Our study has confirmed that major PRAEs are common in children with Down syndrome following AT. In multivariable modeling, average oxygen saturation asleep was associated with PRAEs whereby a lower average oxygen saturation conferred a higher risk of PRAEs. There was no convincing evidence of an association between more traditionally used PSG metrics such as AHI and minimum oxygen saturation with PRAEs.
PRAEs and risk factors
Our study found a major PRAE rate of 21%, which is consistent with existing literature [9, 10]. No children within our cohort required reintubation or noninvasive positive pressure ventilation as compared to previous studies that reported these interventions in 0 to 3% of children with Down syndrome following AT [11, 17, 18].
Although recent pediatric literature reports PSG measures of abnormal gas exchange to be better at predicting PRAEs compared with the AHI [19], to our knowledge, average oxygen saturation asleep has not previously been studied as a predictor of PRAEs after AT. Children with lower average oxygen saturations are at higher risk for desaturation, resulting in higher rates of intervention. Physiologically, a low average oxygen saturation corresponds with a lower partial pressure of oxygen (PaO2) and is closer to the steep section of the oxyhemoglobin dissociation curve where even a small drop in PaO2 results in a large desaturation. We found that children with the lowest average oxygen saturations asleep had normal oxygen saturations awake; this highlights the importance of nocturnal oximetry for assessing PRAE risk following AT.
A low average oxygen saturation is characteristic of pulmonary disease whereas OSAS is characterized by a pattern of intermittent desaturations. The association between average oxygen saturation and PRAEs suggests that pulmonary disease severity, as opposed to OSAS severity, is a stronger predictor of PRAEs following AT. Although lower respiratory disease was not associated with PRAEs (Table 2), we believe that pulmonary disease may be underdiagnosed in children with Down syndrome. Children with Down syndrome are at increased risk of aspiration and recurrent pulmonary infections that can result in gas exchange abnormalities over time. Additionally, Down syndrome is characterized by a myriad of lung development abnormalities including pulmonary hypoplasia [20], alveolar simplification [21], and reduced airway number [22]. These conditions may not have been captured in our database. Furthermore, children with Down syndrome have an increased incidence of pulmonary hypertension which may contribute to hypoxemia. This is multifactorial and may be secondary to abnormal pulmonary vascular development [21], reduced endothelial production of nitric oxide [23], and/or an impairment in the regulation of vascular tone [24].
Cottrell et al. recently reported that severe OSAS, defined as an obstructive AHI > 10 events/hour and minimum oxygen saturation < 80%, is predictive of respiratory complications for children with Down syndrome undergoing AT [9]. Additional risk factors for respiratory issues following AT were ASA score, preoperative PICU admission, and aerodigestive comorbidities [9]. Their findings are similar to previous pediatric studies of general populations undergoing AT where traditional metrics of OSAS such as AHI and minimum oxygen saturation were found to be significant predictors of PRAEs [25, 26]. In contrast, we found that AHI and minimum oxygen saturation were not significant predictors of PRAEs. Our group had a lower AHI (median 8.3 events/hour; IQR 5.3, 16.6 events/h), higher minimum oxygen saturation (median 87.0%; IQR 80.2, 89.0%), and older average age at time of surgery (median 6.6 years; IQR 4.4, 10.1 years) comparatively [9]. Our finding that a low average oxygen saturation during sleep is associated with PRAEs suggests that nocturnal hypoxemia is a more important predictor of PRAEs in a population of children with Down syndrome and relatively mild to moderate OSAS. Another major difference between our study and the study completed by Cotrell et al. is the difference in timing of measured respiratory complications post AT. Cottrell et al. defined respiratory complications as supplemental oxygen therapy requirement on postoperative day one or a decrease in overnight saturation below preoperative baseline levels requiring an additional night of hospital monitoring [9]. Comparatively, our analysis captured PRAEs that occurred on the day of AT which better informs the safest postoperative monitoring environment for these children.
Cardiac comorbidity has previously been reported as a predictor for PRAEs in general populations [25]. We may not have reproduced this in our multivariable analysis secondary to the heterogeneity of cardiac disease within our population and a relatively small sample size, although strong associations were not seen with PRAEs even when the most severe cardiac disease was considered based on the validated functional CHD risk stratification score. Faraoni et al. reported that major and severe CHD are risk factors for mortality and reintubation compared with children with minor or no CHD [27]. The risk stratification score has not been validated for less severe postoperative complications and this may have affected the ability of the stratification score to predict PRAEs in our study population.
Disposition
The vast majority of children in our cohort had pre-planned postoperative hospital admission (56/58, 97%), as this is a known population at high risk of postoperative complications. However, most children did not suffer PRAEs (46/58, 79%) and theoretically many of these children may not have required postoperative overnight monitoring.
The reported postoperative PICU admission rate in children with Down syndrome is highly variable from 3.3 to 25% [11, 17] and is heavily influenced by differences in monitoring practices. Some institutions admit children electively to PICU for monitoring whereas others admit following PRAE occurrence. At our institution, the decision for admission to PICU is reserved for children deemed to be at high risk or those who have suffered perioperative complications. This is reflected in our PICU admission rate of 4/58 (7%).
We report a readmission rate of 7% (4/58), which is in keeping with previously reported readmission rates of 6.5–10% [9, 17]. None of the readmissions in our study were due to respiratory complications.
Generalizability and limitations
Our institution serves as a large referral center and follows all children with Down syndrome within the catchment area of the hospital. As such, our study sample is representative of all children with Down syndrome in our region. The main limitation is the size of our cohort, which is similar in size to many previous studies on AT in children with Down syndrome. The size of our cohort resulted in wide confidence intervals in our multivariable analysis. Another limitation to the generalizability of our study is that studied children required formal PSG completion prior to AT and this is not routinely performed on all children prior to AT. Given the scarcity of access to PSGs in our region [28], our population likely includes a selection bias, whereby children who are more complex or deemed to be at higher risk for OSAS undergo PSG. Despite this, the median AHI and PRAE rate was in the lower range of those reported for a tertiary care pediatric hospital population. We also chose to analyze PRAEs following a group of procedures including adenoidectomy and tonsillectomy, isolated adenoidectomy, and isolated tonsillectomy. Despite the heterogeneity of procedures, the results of a sensitivity analysis whereby the multivariable model was repeated exclusively examining children who underwent combined adenoidectomy and tonsillectomy yielded similar results to the original model. Future larger studies should consider the specific surgical procedure (adenoidectomy and tonsillectomy, adenoidectomy, tonsillectomy) in addition to comorbidities, in the prediction of PRAEs. Also, we are unable to account for the level of provider expertise involved in the care of this surgical population which may have influenced our rate of PRAEs [29]. Finally, our study was limited in our ability to predict unplanned hospital admissions, as the vast majority of children had pre-planned postoperative hospital admissions.
In summary, PRAEs requiring intervention following AT are relatively common in children with Down syndrome, the majority requiring supplemental oxygen and/or simple management of transient airway obstruction. We found that a lower average oxygen saturation asleep is associated with increased odds of PRAEs, adjusting for age, total AHI, cardiac comorbidity, and minimum oxygen saturation. There are 50% increased odds of PRAE for every 1% decrease in average oxygen saturation asleep. Future studies should consider alternative measures of nocturnal hypoxemic burden that incorporate frequency, duration, and depth of desaturation, to predict PRAEs in these children. This may prove particularly useful to direct disposition planning in limited PSG access settings.
Data availability
Data available on request from the authors.
Code availability
Code available on request from the authors.
Abbreviations
- AHI:
-
Apnea-hypopnea index
- ASA:
-
American Society of Anesthesiologists
- AT:
-
Adenoidectomy and/or tonsillectomy
- BMI:
-
Body mass index
- CHD:
-
Congenital heart disease
- IQR:
-
Interquartile range
- PACU:
-
Post-anesthetic care unit
- PaO2:
-
Partial pressure of oxygen
- PICU:
-
Pediatric intensive care unit
- PRAE:
-
Perioperative respiratory adverse event
- PSG:
-
Polysomnogram
- OSAS:
-
Obstructive sleep apnea syndrome
References
Mai CT, Isenburg J, Langlois PH, Alverson C, Gilboa SM, Rickard R et al (2015) Population-based birth defects data in the United States, 2008 to 2012: presentation of state-specific data and descriptive brief on variability of prevalence. Birth Defects Res Clin Mol Teratol 103:972–993. https://doi.org/10.1002/bdra.23461
Lee C-F, Lee C-H, Hsueh W-Y, Lin M-T, Kang K-T (2018) Prevalence of obstructive sleep apnea in children with down syndrome: a meta-analysis. J Clin Sleep Med 14:867–875. https://doi.org/10.5664/jcsm.7126
Lumeng JC, Chervin RD (2008) Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 5:242–252. https://doi.org/10.1513/pats.200708-135MG
Farhood Z, Isley JW, Ong AA, Nguyen SA, Camilon TJ, LaRosa AC et al (2017) Adenotonsillectomy outcomes in patients with Down syndrome and obstructive sleep apnea: Down syndrome tonsillectomy systematic review. Laryngoscope 127:1465–1470. https://doi.org/10.1002/lary.26398
Chervin RD (2006) Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics 117:e769–e778. https://doi.org/10.1542/peds.2005-1837
Smith DF, Amin RS (2019) OSA and Cardiovascular Risk in Pediatrics. Chest 156:402–413. https://doi.org/10.1016/j.chest.2019.02.011
Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A (2017) Outcome of adenotonsillectomy in children with Down syndrome and obstructive sleep apnoea. Arch Child 102:331–336. https://doi.org/10.1136/archdischild-2015-310351
Merrell JA, Shott SR (2007) OSAS in Down syndrome: T&A versus T&A plus lateral pharyngoplasty. Int J Pediatr Otorhinolaryngol 71:1197–1203. https://doi.org/10.1016/j.ijporl.2007.04.009
Cottrell J, Zahr SK, Propst EJ, Narang I, Amin R, Chiang J et al (2020) Morbidity and mortality from adenotonsillectomy in children with trisomy 21. Int J Pediatr Otorhinolaryngol 138:110377. https://doi.org/10.1016/j.ijporl.2020.110377
Abdel-Aziz M, Azooz K, Naguib N, Reda R, Kamel A (2017) The effect of adenotonsillectomy on obstructive sleep apnea in children with Down syndrome. Acta Otolaryngol (Stockh) 137:981–985. https://doi.org/10.1080/00016489.2017.1312016
Goldstein NA, Armfield DR, Kingsley LA, Borland LM, Allen GC, Post JC (1998) Postoperative complications after tonsillectomy and adenoidectomy in children with Down syndrome. Arch Otolaryngol Head Neck Surg 124:171–176
De Luca CG, Pacheco-Pereira C, Aydinoz S, Bhattacharjee R, Tan H-L, Kheirandish-Gozal L et al (2015) Adenotonsillectomy complications: a meta-analysis. Pediatrics 136:702–718. https://doi.org/10.1542/peds.2015-1283
Zemel BS, Pipan M, Stallings VA, Hall W, Schadt K, Freedman DS et al (2015) Growth charts for children with Down syndrome in the United States. Pediatrics 136:e1204–e1211. https://doi.org/10.1542/peds.2015-1652
Faraoni D, Vo D, Nasr VG, DiNardo JA (2016) Development and validation of a risk stratification score for children with congenital heart disease undergoing noncardiac surgery. Anesth Analg 123:824–830. https://doi.org/10.1213/ANE.0000000000001500
Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV (2020) For the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: rules, terminology and technical specifications, Version 2.6. www.aasmnet.org. Darien, Illinois: American Academy of Sleep Medicine, 2020. n.d.
R Core Team (2018) R: A language and environment for statistical computing. Austria: R Foundation for Statistical Computing. Available from: https://www.R-project.org/. n.d.
Yumusakhuylu AC, Binnetoglu A, Demir B, Baglam T, Sari M (2016) Is it safe to perform adenotonsillectomy in children with Down syndrome? Eur Arch Otorhinolaryngol 273:2819–2823. https://doi.org/10.1007/s00405-016-4012-7
McColley SA, April MM, Carroll JL, Naclerio RM, Loughlin GM (1992) Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 118:940–943
Molero-Ramirez H, Kakazu MT, Baroody F, Bhattacharjee R (2019) Polysomnography parameters assessing gas exchange best predict postoperative respiratory complications following adenotonsillectomy in children with severe OSA. J Clin Sleep Med 15:1251–1259. https://doi.org/10.5664/jcsm.7914
Cooney TP, Thurlbeck WM (1982) Pulmonary hypoplasia in Down’s syndrome. N Eng J Med 307:1170–1173
Bush D, Abman SH, Galambos C (2017) Prominent Intrapulmonary bronchopulmonary anastomoses and abnormal lung development in infants and children with Down syndrome. Pediatrics 180:156-162.e1. https://doi.org/10.1016/j.jpeds.2016.08.063
Schloo BL, Vawter GF, Reid LM (1991) Down syndrome: Patterns of disturbed lung growth. Hum Pathol 22:919–923. https://doi.org/10.1016/0046-8177(91)90183-P
Cua CL, Rogers LK, Chicoine LG, Augustine M, Jin Y, Nash PL et al (2011) Down syndrome patients with pulmonary hypertension have elevated plasma levels of asymmetric dimethylarginine. Eur J Pediatr 170:859–863. https://doi.org/10.1007/s00431-010-1361-x
Cappelli-Bigazzi M, Santoro G, Battaglia C, Palladino MT, Carrozza M, Russo MG et al (2004) Endothelial cell function in patients with Down’s syndrome. Am J Cardiol 94:392–395. https://doi.org/10.1016/j.amjcard.2004.04.047
Katz SL, Monsour A, Barrowman N, Hoey L, Bromwich M, Momoli F et al (2020) Predictors of postoperative respiratory complications in children undergoing adenotonsillectomy. J Clin Sleep Med 16:41–48. https://doi.org/10.5664/jcsm.8118
Keamy DG, Chhabra KR, Hartnick CJ (2015) Predictors of complications following adenotonsillectomy in children with severe obstructive sleep apnea. Int J Pediatr Otorhinolaryngol 79:1838–1841. https://doi.org/10.1016/j.ijporl.2015.08.021
Faraoni D, Zurakowski D, Vo D, Goobie SM, Yuki K, Brown ML et al (2016) Post-operative outcomes in children with and without congenital heart disease undergoing noncardiac surgery. J Am Coll Cardiol 67:793–801. https://doi.org/10.1016/j.jacc.2015.11.057
Katz SL, Witmans M, Barrowman N, Hoey L, Su S, Reddy D et al (2014) Paediatric sleep resources in Canada: the scope of the problem. Pediatr Child Health 19:367–372. https://doi.org/10.1093/pch/19.7.367
Taenzer AH, Spence BC (2018) The afferent limb of rapid response systems: continuous monitoring on general care units. Crit Care Clin 34:189–198
Acknowledgements
We would like to thank Dr. Indra Narang for reviewing this manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. Dr. Katz has received a speaker honorarium from Biogen, unrelated to this work.
Author information
Authors and Affiliations
Contributions
All authors have read and approved of this article’s submission. Dr. Katz was the principal investigator for the study, conceived and designed the study. Drs. Barrowman, Bromwich, Momoli, and Murto contributed to the design of the study and revised the manuscript. Dr. Barrowman was involved in the statistical analysis of data. Dr. Xiao contributed to the design of the study, completed data collection, and wrote the first draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Research Ethics Board of the Children’s Hospital of Eastern Ontario approved this study.
Consent to participate
This type of study does not require informed consent.
Consent to publish
This type of study does not require individual consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Work for this study was performed at the Children’s Hospital of Eastern Ontario, 401 Smyth Road, Ottawa, Ontario, Canada, K1H 8L1.
Rights and permissions
About this article
Cite this article
Xiao, L., Barrowman, N., Momoli, F. et al. Risk factors for respiratory adverse events after adenoidectomy and tonsillectomy in children with down syndrome: a retrospective cohort study. Eur J Pediatr 181, 2399–2408 (2022). https://doi.org/10.1007/s00431-022-04438-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04438-3