Abstract
“Developmental hemostasis” refers to the dynamic process of gradual hemostatic maturation. Conventional coagulation tests seem to fail to accurately depict the in vivo hemostasis, while viscoelastic tests, thromboelastography (TEG), and rotational thromboelastometry (ROTEM) appear very promising as they provide insight more rapidly and accurately into the hemostatic potential. We systematically reviewed the literature in PubMed to examine the use of TEG and ROTEM in neonates. Our search yielded 34 studies, of which 18 concerned healthy neonates and 16 sick neonates. These viscoelastic tests have shown accelerated initiation of coagulation, increased clot strength, and increased fibrinolysis in healthy neonates compared to children and adults. Cord blood leads to a hypercoagulable state as compared to whole blood when testing is performed with TEG. Pre-term neonates have a more hypocoagulable profile, but balanced hemostasis, related to term neonates, that evolves to a more procoagulant phenotype over the first month of life. Critically ill neonates exhibit a more hypocoagulable profile as compared to healthy neonates. TEG and ROTEM have shown predictive value for bleeding events in critically ill neonates and neonates undergoing cardiopulmonary bypass or therapeutic hypothermia.
Conclusion: TEG and ROTEM need to become part of the standard coagulation assessment in clinical settings in which hemostatic abnormalities are involved, as they seem to provide more rapid and accurate information regarding the hemostatic profile of the neonates. Their predictive value for bleeding events in critically ill neonates could lead to a more targeted therapy optimizing utilization of blood products.
What is Known: • Conventional coagulation tests seem to fail to accurately depict the in vivo hemostasis. • TEG and ROTEM delineate more rapidly and accurately the hemostatic potential. What is New: • TEG and ROTEM have shown predictive value for bleeding events. • TEG and ROTEM may lead to a more targeted transfusion therapy optimizing utilization of blood products. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemostasis is one of the most blurred fields of neonatal pathophysiology. “Developmental hemostasis” refers to the dynamic process of the gradual hemostatic maturation, that begins in utero and continues until adulthood [1, 2]. The standard coagulation tests, activated partial thromboplastin time (aPTT) and prothrombin time (PT), reflect the intrinsic and extrinsic coagulation pathways, respectively [3]. These tests are performed in plasma, where there are neither red blood cells nor platelets [4]. For that reason, they cannot accurately interpret the hemostatic mechanisms in vivo or reflect with precision the hemostatic profile and the bleeding diathesis of a neonate [2]. The cell-based model, which is based on the idea that hemostasis is controlled mainly by cellular components and not by the kinetics of the coagulation proteins, mirrors more accurately the hemostasis in vivo [3].
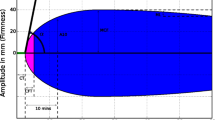
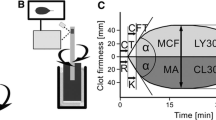
Viscoelastic tests such as thromboelastography/thromboelastometry (TEG/ROTEM) assess the interaction of the blood cells with coagulation proteins and, thus, reflect the cell-based model and consequently the hemostatic profile in vivo [2]. The provided information estimates the dynamics of clot development, stabilization, and dissolution (Table 1) [5]. Although viscoelastic tests have not been used in the diagnosis and treatment of bleeding disorders in the neonatal population, they seem promising. The published thresholds of ROTEM parameters for the diagnosis of coagulopathy are mainly derived from adult studies and there is no strong evidence in the current literature supporting diagnostic reference values [6]. Regarding neonates, available data are very limited and derived from studies of neonates undergoing cardiac surgery, suffering from neonatal complications such as sepsis, intraventricular hemorrhage (IVH), and hypoxemic ischemic encephalopathy undergoing therapeutic hypothermia [2, 7]. The main reason for the limited use of these tests in neonates is the lack of reference ranges in this population [2].
Considering that the transfusion of blood products is not benign, the transfusion rate must be balanced against the possible adverse events [8]. The use of these tests in transfusion algorithms has resulted in a reduction in blood product transfusions in pediatric and adult populations [9, 10]. For example, in patients undergoing extracorporeal membrane oxygenation (ECMO), changes in ROTEM variables seem more accurate in detecting changes in the anticoagulation effects of heparin compared to standard coagulation tests [11]. In patients undergoing cardiopulmonary bypass (CPB), the viscoelastic tests can also detect the protamine’s effects on coagulation as well as differentiate them from those of heparin [12]. The aforementioned studies enhance the idea that the viscoelastic tests can diagnose the specific coagulation impairment and guide a more targeted therapeutic approach.
We conducted a systematic review to examine the use of TEG and ROTEM in the neonatal population.
Methods
The methods and the results of this study were carried out according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement (PRISMA) [13].
Search strategy
We conducted a systematic search to PubMed database, until 28th January 2021, using the following specific keywords along with their combinations: “infant,” “newborn,” “neonate” and “thromboelastography,” “thromboelastometry,” “ROTEM,” “TEM,” “TEG,” “viscoelastic test.” We evaluated these keywords through the Medical Subject Headings (MeSH) and the final search string was (((infant[Title/Abstract]) OR (newborn[Title/Abstract])) OR (neonate[Title/Abstract])) AND ((((((thromboelastography[Title/Abstract]) OR (thromboelastometry[Title/Abstract])) OR (ROTEM[Title/Abstract])) OR (TEM[Title/Abstract])) OR (TEG[Title/Abstract])) OR (viscoelastic test[Title/Abstract])).
Eligibility criteria
We considered all study types referring to the use of the viscoelastic tests TEG/TEM in the neonatal population, except for review articles. Studies referring to other age groups or not published in English were excluded.
Study selection and data extraction
Two reviewers (GNK and RS) independently screened the literature search, reviewed the full text of all studies that were considered eligible according to the inclusion criteria, and extracted individually the studies’ data. For all studies, we recorded the name of the first author, year of publication, country of origin, sample size, type of blood sample, analyzing method (TEG/TEM), and findings.
Data analysis
A systematic review of the studies that were regarded as eligible for inclusion was performed with a qualitative analysis and presentation of their data.
Results
Our search procedure yielded 199 studies. After duplicates were removed, we screened the title and abstract of 186 studies, and excluded 137 of them. Subsequently 49 studies were screened in full text for eligibility. Finally, 34 studies were included in our systematic review (Fig. 1) [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47].
Study characteristics
Eighteen [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] of the 34 studies referred to healthy neonates, while the remaining 16 [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] to sick neonates (Table 2). Fourteen studies [14,15,16,17,18,19, 21, 22, 24, 33, 36, 38, 42, 43] were case-control studies, 18 [20, 23, 25,26,27,28,29,30,31,32, 34, 35, 39,40,41, 45,46,47] were prospective cohort studies, and two [37, 44] were retrospective cohort studies. Eighteen studies [14,15,16, 18, 22,23,24,25, 27, 28, 32, 33, 35, 36, 41, 44, 45] used the TEG viscoelastic method and 16 [17,19-21, ,26,29,31,34,37-40,42,43,46,47] the ROTEM one. Most studies (18 out of 34) were conducted in Europe [16, 17, 20, 22, 23, 26, 28,29,30,31, 34, 36, 38, 41,42,43, 46, 47], 5 studies were conducted in Asia [15, 19, 21, 27, 37], 10 studies [14, 18, 24, 25, 32, 35, 39, 40, 44, 45] in the USA regarding the Americas, and one study [33] in South Africa.
Results from healthy neonates
Eight studies [14, 15, 17, 18, 21, 22, 25, 30] were conducted using cord blood samples. Suzuki et al. (1976) compared TEG results with and without urokinase, a plasminogen activator. The TEG patterns after the addition of urokinase were characteristic for consumption coagulopathy. Furthermore, their findings showed that an increased urokinase results in a remarkable lowering of plasminogen inhibitors a1-antithrypsin and antithrombin III compared to adults [15]. Three studies [14, 18, 21] showed accelerated initiation of coagulation and increased clot strength in neonates compared to children and adults, while one study [17] showed diminished clot strength compared to adults. One study [21] detected increased fibrinolysis in neonates compared to adults. One study [22] reported no differences in TEG values between neonates and adults, and one study [25] exhibited no differences in TEG values among neonates born vaginally with those delivered by cesarean section. Finally, Raffaeli et al. (2020) compared the TEG results in cord blood and whole blood samples of 60 neonates. Their results showed that cord blood leads to a procoagulant imbalance when testing is performed with TEG, while there are no differences in the conventional coagulation tests (PT and aPTT) [30].
Ten studies [16, 19, 20, 23, 24, 26,27,28,29, 31] were conducted using neonatal whole blood samples. One study [16] reported that healthy neonates have no coagulation defects despite prolonged conventional coagulation tests. One study [28] showed that pre-term neonates have a more hypocoagulable profile, but balanced hemostasis compared with term neonates, that evolves to a more procoagulant phenotype over the first month of life. One study [29] noted no differences in ROTEM variables between small-for-gestational-age (SGA) and appropriate-for-gestational-age (AGA) neonates. One study [27] exhibited acceleration of coagulation in female neonates and neonates delivered by cesarean section compared to male neonates and neonates delivered vaginally, respectively, while one study [31] showed that maternal problems during pregnancy as well as the delivery mode had no impact on the ROTEM variables of the neonates. Two studies [20, 24] showed accelerated coagulation in neonates compared to older children, and one study [19] exhibited accelerated coagulation in pre-term neonates compared to term neonates along with a strong correlation between gestational age (GA) with clotting time (CT) and clot formation time (CFT) of the ROTEM assays. Two studies [19, 27] showed positive correlation between GA and body weight (BW) with clot strength, while 4 studies [23, 24, 26, 27] showed increased fibrinolysis as the age decreases; one study [23] compared early pre-term neonates to moderate/late pre-term neonates, one study [26] compared pre-term to term neonates, one study [24] compared neonates to older children, and two of the studies [26, 27] found a negative correlation between GA and fibrinolysis. Finally, one study [31] found strong correlation between ROTEM variables and platelet count. In the same arena, regarding EXTEM parameters and hematocrit values, positive correlations were observed only for CT and CFT and a negative correlation was confirmed for alpha angle. Finally, hematocrit values were found to be positively correlated with FIBTEM CT, while negative correlations were observed between hematocrit values and FIBTEM A5 and α angle.
Ten studies [16, 18, 22,23,24,25, 27, 28, 30, 33] provided reference values for the TEG parameters (Table 3), and 7 studies [17, 19,20,21, 26, 29, 31] provided reference values for the ROTEM parameters (Table 4) in the neonatal population. The medians of TEG variables ranged for neonates <32 weeks GA: reaction time (R): 7.6–13.8 min, kinetics (K): 4.4–4.6 min, maximum amplitude (MA): 54.7–55.3 mm, lysis in 30′ (LY30): 0.1%; for neonates 32–37 weeks GA: R: 9.9–13.1 min, K: 4.7–6.7 min, MA: 51.9–55.8 mm, LY30: 0.0%; for neonates >37 weeks GA: R: 4.8–9 min; K: 1.40–7.2 min, MA: 54.5–65.01 mm, LY30: 0.1–1.92%. The medians of ROTEM variables ranged for pre-term neonates: EXTEM: CT: 44–185 s, CFT: 64–80 s, maximum clot firmness (MCF): 57–64 mm, lysis index in 60′ (LI60): 95%, while the medians of ROTEM variables ranged for term neonates: NATEM: CT: 355 s, CFT: 122.32 s, MCF: 55.40 mm; EXTEM: CT: 41–194 s, CFT: 70–98.4 s, MCF: 55.3–66 mm, LI30: 100%; INTEM: CT: 191 s, CFT: 76 s, MCF: 59 mm, LI30: 100%; FIBTEM: CT: 48–53.9 s, CFT: 39.0–74 s, MCF: 11.6–17 mm, LI30: 100%.
Results from sick neonates
Neonates undergoing extracorporeal membrane oxygenation
Three cohort studies [32, 44, 45] examined the TEG values in neonates undergoing ECMO. Stammers et al. (1995) evaluated the coagulation status of 17 neonates undergoing ECMO. TEG profiles detected hemostatic abnormalities in 46.5% of the cases, with platelet dysfunction being the most common etiology. TEG profiles were normal in 73.2% instances in the non-hemorrhagic group, and 40% in the hemorrhagic group. They concluded that TEG may help to guided transfusion therapy, because it provides useful information for the rapid diagnosis of hemorrhagic risk during coagulation assessment in neonates undergoing ECMO [32]. Phillips et al. (2020) retrospectively reviewed 46 neonates with congenital diaphragmatic hernia (CDH) supported by ECMO during 2008–2018. They assessed the blood product administration, TEG data, and hemorrhagic and thrombotic complications data. They concluded that institutional standardization of anticoagulation management of CDH neonates supported by ECMO including the use of TEG monitoring might lead to improvement of blood product treatment and decrease in bleeding complications in these neonates [44]. Snyder et al. (2020) evaluated the relationship of bivalirudin dose (anticoagulation therapy) to aPTT and TEG-R monitoring assays in 42 neonates with CDH undergoing ECMO. The results did not show dose-response relationships between bivalirudin and aPTT or TEG-R, although gradually increasing doses were needed to maintain therapeutic anticoagulation [45].
Neonates with congenital heart disease
Three cohort studies [34, 37, 41] tried to delineate as well as to examine the predictive value of the viscoelastic profile of neonates with congenital heart disease (CHD). Haizinger et al. (2006) compared rotational thromboelastography (ROTEG, former ROTEM) samples from 24 infants scheduled for minor surgical procedures with samples from 35 children with CHD scheduled for cardiopulmonary bypass (CPB). Their results showed that CT was prolonged and maximum clot firmness (MCF) reduced in CHD infants and varied widely, mainly in the neonatal group [34]. Kim et al. (2016) retrospectively examined the ROTEM data of 413 children with CHD and showed that the ROTEM pattern of these patients is similar to healthy children [37]. Ghirardello et al. (2020) conducted TEG to 151 very low birth weight (VLBW) neonates with patent ductus arteriosus (PDA). Their results showed that TEG does not predict spontaneous PDA closure in VLBW neonates. Moreover, they showed that fibrinolysis is enhanced in non-responders to PDA treatment [41].
Neonates undergoing cardiopulmonary bypass
Two prospective cohort studies [39, 40] were conducted on neonates undergoing CPB. Peterson et al. (2018) examined samples from 44 neonates before and after CPB in order to assess the heparine-protamine balance, using calibrated automated thrombography, thrombin-initiated fibrin clot kinetic assay (TFCK), aPTT, anti-FXa activity, and ROTEM. Sixteen neonates had excessive post-operative bleeding. The aPTT correlated significantly to TFCK, while anti-FXa and ROTEM assays were less correlative with aPTT. The aPTT had a stronger correlation with TFCK probably due to the similar measurement of fibrin formation as the endpoint for both assays. None of the coagulation tests could predict the post-operative bleeding [39]. Scott et al. (2018) investigated blood samples using ROTEM from 44 neonates before, during, and after CPB, as well as post-operatively in order to assess perioperative hemostatic profiles in neonates transfused with platelet and cryoprecipitate during CPB. Their results showed that platelet count and EXTEM A10 decreased significantly during CPB and increased significantly after CPB, while post-operative values were not significantly different from those before CPB. Moreover, EXTEM A10 > 46.5 mm and PLTEM A10 > 37.5 mm could outstandingly predict platelet count > 100 × 103/mL, and they were excellently associated with platelet count. Finally, fibrinogen concentration and FIBTEM A10 decreased significantly during CPB and normalized after cryoprecipitate transfusion. FIBTEM A10 > 9.5 mm was found to excellently predict fibrinogen >200 mg/dL, but it was less associated with fibrinogen concentration. Their findings supported that ROTEM during CPB is sensitive and specific for thrombocytopenia and hypofibrinogenemia [40].
Neonates undergoing therapeutic hypothermia
One study [35] examined the effects of cooling on the coagulation enzyme cascade using TEG. Forman et al. (2014) performed TEG in 24 encephalopathic neonates treated with systemic hypothermia. TEG parameters differed significantly between assays conducted at 37.0 versus 33.5 °C, indicating impaired coagulation at 33.5 °C. Their results also showed that K, α angle, MA, and coagulation index (CI) were significantly associated with clinical bleeding, pointing out that TEG is predictive of clinical bleeding in newborns undergoing hypothermia.
Neonates with intracranial hemorrhage
One study [36] outlined the TEG profiles of pre-term neonates with and without intracranial hemorrhage (ICH). Radicioni et al. (2015) conducted TEG and conventional coagulation tests in 49 pre-mature neonates, 19 of which developed ICH. Their results showed shorter R and K in the ICH group, pointing out a hypercoagulable state in those neonates. They also found a moderate positive correlation between clot lysis time (CLT) and GA.
Critically ill neonates
Six studies [33, 38, 42, 43, 46, 47] were conducted in critically ill neonates. Grant et al. (1997) performed TEG to 12 neonatal surgical cases with sepsis, 15 neonatal surgical cases with early sepsis, and 16 neonatal non-septic surgical cases. TEG abnormalities were found in septic and early septic neonates (mainly hypocoagulability). The TEG was shown to have a 96% sensitivity and a 96% specificity for sepsis [33]. Sokou et al. (2017) performed EXTEM ROTEM assay in 35 neonates with confirmed sepsis and 56 neonates with suspected sepsis, while their control group consisted of 275 healthy neonates. Their results showed that septic neonates had significantly prolonged EXTEM CT and CFT, and reduced MCF, compared to neonates with suspected sepsis and healthy ones. EXTEM CT, CFT, and MCF revealed a more severe hypocoagulable profile in septic neonates with bleeding diathesis than the other two groups. Finally, EXTEM CFT was correlated with platelet count, Score for Neonatal Acute Physiology with Perinatal extension (SNAPPE), Tollner score, and day of full enteral feeding. Their findings supported that EXTEM parameters could predict sepsis in neonates, as well as its severity [38]. Lampridou M. et al. (2020) performed EXTEM and APTEM ROTEM assays in 44 neonates with confirmed sepsis and 22 neonates with suspected sepsis, while their control group consisted of 110 healthy neonates, in order to delineate the fibrinolytic profile in neonatal sepsis. Their findings, based on ROTEM fibrinolytic parameters [maximum lysis (ML) and lysis index at 60 min (LI60)], showed a more frequent fibrinolysis shutdown in septic neonates, but it could not effectively discriminate them from the other groups, or predict their clinical outcome [43]. Konstantinidi et al. (2020) performed EXTEM ROTEM assay in 16 neonates with perinatal asphyxia and 148 neonates with fetal distress, while their control group consisted of 273 healthy neonates. Their results showed that hypoxic neonates had prolonged CT and CFT and reduced A10, α angle, and MCF, indicating a hypocoagulable EXTEM profile, compared to healthy neonates [42]. Parastatidou S. et al. (2021) performed EXTEM and FIBTEM ROTEM assays, including maximum clot elasticity (MCE), platelet-specific TEM (PLTEM) MCE, and PLTEM MCF, in 110 thrombocytopenic neonates with sepsis, suspected sepsis, or hypoxia, in order to investigate their predictive power for bleeding events in thrombocytopenic critically ill neonates. Their results showed that thrombocytopenic critically ill neonates with bleeding events had significantly lower PLTEM MCE and PLTEM MCF values compared to those without bleeding events. Platelet count was found to be strongly positively correlated with EXTEM A5 and A10, while EXTEM A10 was found to be the more predictive parameter for bleeding events, with a value < 37 mm as the optimal cut-off point [46]. Sokou et al. (2021) confirmed the predictive value of EXTEM A10 in critically ill neonates for bleeding events in the first 24 h of life and managed to show that EXTEM LI60 is also a strong predictor. They performed EXTEM ROTEM assay in 332 full-term and pre-term critically ill neonates—16 neonates with perinatal asphyxia, 151 neonates with fetal distress, 121 neonates with sepsis, and 46 neonates with suspected sepsis. Apart from the above correlations, they managed to develop a multivariable prediction model for the first 24 h bleeding risk (NeoBRis) in critically ill neonates [47].
Discussion
Neonates have a hemostatic deficit negatively correlated with GA, BW, and the maturity of the liver function. Despite this “immaturity,” the neonatal hemostatic system is perfectly functionally balanced, as healthy neonates do not have any bleeding or thrombotic diathesis [48]. Studies that used TEG and ROTEM showed that the GA is positively correlated with accelerated coagulation, clot formation time, and clot strength, and negatively correlated with fibrinolysis [19, 23, 24, 26,27,28].
Four studies [17,18,19, 21] showed that neonates have accelerated coagulation and clot formation time, but poorer clot strength and higher fibrinolytic activity compared to older children and adults. The shorter coagulation time in neonates might be attributed to the increased levels of procoagulant factors, such as fibrinogen, factor V, factor VIII, and von Willebrand factor (vWF) as compared to adults [1, 49]. The diminished clot strength in neonates is probably on account of the impaired polymerization properties of the neonatal fibrin, partially because of the elevated levels of fibrinogen-bound sialic acid [50]. The increased fibrinolytic activity in neonates might be due to the increased tissue-plasminogen activator (t-PA) levels and the reduced levels of the fibrinolysis inhibitors plasminogen activator inhibitor (PAI) and α2-antiplasmin, compared to adults [51].
Two studies [16, 22] showed no differences in TEG variables between neonates and adults. Kettner et al. (2008) used heparinase-modified TEG in neonates previously treated with vitamin K and blood products, making the results biased [16]. Mirabella et al. (2017) used kaolin-activated TEG, pointing out that the activator of the TEG assay should be considered when interpreting the TEG results, and that there are no differences between neonates and adults as far as kaolin-activated TEG variables is concerned [22].
Despite the fact that 17 studies [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31, 33] have provided reference ranges for TEG/ ROTEM variables, we must not neglect that viscoelastic tests, and coagulation testing in general, may be affected by pre-analytical factors, including sampling and sample handling, as well as operator-to-operator variability. The Perinatal and Pediatric Hemostasis Subcommittee of the International Society on Thrombosis and Hemostasis (ISTH) published consensus recommendations for hemostasis tests in children, suggesting that all laboratories and units should establish age-, analyzer-, and reagent-appropriate reference ranges [52]. Because of the difficulty in obtaining “universal” reference ranges, it is very important to obtain institutional reference ranges for every intensive care unit using viscoelastic tests.
It seems that viscoelastic tests could be used for a more rapid estimation of the bleeding diathesis in sick neonates. Four studies [35, 40, 46, 47] tried to point out the predictive ability of TEG and ROTEM regarding bleeding events in sick neonates.
Neonates undergoing CPB are at increased risk for bleeding events due to their immature coagulation system, the significant hemodilution from the CPB prime, the long operative times at low temperatures, and the extensive suture lines [53]. Scott et al. (2018) showed that EXTEM A10 can predict thrombocytopenia, and FIBTEM A10 can predict hypofibrinogenemia in neonates undergoing CPB [40].
Hypothermia increases coagulation disturbances mainly due to the induced decrease of the enzymatic activity involved in the coagulation process. Below 33 °C, both enzyme activity and platelet function are significantly reduced, while at 33 °C platelet aggregation and adhesion, but not platelet function, are significantly reduced compared with 37 °C [54]. Forman et al. (2014) supported that TEG variables (K, α angle, MA, CI) can predict bleeding events in neonates undergoing therapeutic hypothermia. However, the authors found that although lower temperature leads to hypocoagulability, more TEG parameters were associated with bleeding in a higher temperature [35].
Sepsis is a systematic inflammatory response to infection. This response has been associated with activation of the coagulation cascade, consumption of anticoagulation factors, and inhibited fibrinolysis [55]. Perinatal hypoxia, interruption of fetal blood flow and impairment of gas exchange, is a condition comprising liver and bone marrow oxygenation leading to disturbances in neonatal hemostasis and platelet function [56]. Parastatidou et al. (2020) showed that EXTEM A10 is a strong predictor for bleeding events in thrombocytopenic neonates with sepsis or hypoxia. More precisely, adjusted analysis by logistic regression revealed that EXTEM A10 (OR = 0.88, 95% CI: 0.81–0.95, p = 0.003), PLTEM ΜCE (OR = 0.90, 95% CI: 0.82–0.99, p = 0.043), and PLTEM MCF (OR = 0.96, 95% CI: 0.92–0.99, p = 0.039) were significantly correlated with clinical bleeding. All these ORs were close to 1, indicating that results should be interpreted with caution [46]. Sokou et al. (2021) showed that both EXTEM A10 and LI60 are strong predictors (β coefficient: − 0.013, 95% CI: − 0.143, − 0.061, p < 0.001 and β coefficient: +0.064, 95% CI: +0.020, +0.108, p = 0.005, respectively) for bleeding events in critically ill neonates and have included them into a prediction model of hemorrhage in this clinical setting [47].
Although the existing data are still limited, the value of viscoelastic tests in predicting bleeding risk seems promising. What is clear is that the viscoelastic tests can better identify a condition of altered hemostasis than the standard coagulation tests do, possibly contributing in optimizing transfusion treatment in ill neonates [2, 10].
Conclusion
It is imperative that larger multicenter studies are performed to establish reference ranges, as well as diagnostic and therapeutic values of all TEG and ROTEM assays. The viscoelastic tests are probably clinically useful as part of the standard coagulation tests, because of their more rapid and accurate estimation of hemostasis. Considering that transfusions in neonates are applied mostly prophylactic, and that they have been proved cost ineffective and associated with complications, TEG/ROTEM needs to be evaluated in the context of transfusion algorithms as they seem to have the potential to optimize blood utilization.
Data Availability
Available upon request.
Abbreviations
- A5:
-
Clot strength at 5 min
- A10:
-
Clot strength at 10 min
- A15:
-
Clot strength at 15 min
- A20:
-
Clot strength at 20 min
- A25:
-
Clot strength at 25 min
- A30:
-
Clot strength at 30 min
- AGA:
-
Appropriate-for-gestational-age
- aPTT:
-
Activated partial thromboplastin time
- BW:
-
Body weight
- CCHD:
-
Complex congenital heart disease
- CDH:
-
Congenital diaphragmatic hernia
- CFT:
-
Clot formation time
- CHD:
-
Congenital heart disease
- CI:
-
Coagulation index
- CL:
-
Clot lysis
- CPB:
-
Cardiopulmonary bypass
- CT:
-
Clotting time
- ECMO:
-
Extracorporeal membrane oxygenation
- EXTEM:
-
Extrinsically activated thromboelastometry
- FIBTEM:
-
Fibrin-based extrinsically activated thromboelastometry
- GA:
-
Gestational age
- Hct:
-
Hematocrit
- ICH:
-
Intracranial hemorrhage
- INTEM:
-
Intrinsically activated thromboelastometry
- IVH:
-
Intraventricular hemorrhage
- ISTH:
-
International Society on Thrombosis and Hemostasis
- K:
-
Kinetics
- LI30:
-
Lysis index at 30 min
- LI45:
-
Lysis index at 45 min
- LI60:
-
Lysis index at 60 min
- LY30:
-
Lysis in 30 min
- LY60:
-
Lysis in 60 min
- MA:
-
Maximum amplitude
- MCE:
-
Maximum clot elasticity
- MCF:
-
Maximum clot firmness
- ML:
-
Maximum lysis
- NATEM:
-
Non-activated TEM
- NeoBRis:
-
Neonatal bleeding risk index
- PAI:
-
Plasminogen activator inhibitor
- PLTEM:
-
Platelet-specific thromboelastometry
- PDA:
-
Patent ductus arteriosus
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses statement
- PT:
-
Prothrombin time
- R:
-
Reaction time
- ROTEG:
-
Rotational thromboelastography
- ROTEM:
-
Rotational thromboelastometry
- SGA:
-
Small-for-gestational-age
- SNAPPE:
-
Score for Neonatal Acute Physiology with Perinatal extension
- TEG:
-
Thromboelastography
- TFCK:
-
Thrombin-initiated fibrin clot kinetic
- t-PA:
-
Tissue-plasminogen activator
- VLBW:
-
Very low birth weight
- vWF:
-
Von Willebrand factor
References
Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Powers P (1987) Development of the human coagulation system in the full-term infant. Blood. 70(1):165–172
Konstantinidi A, Sokou R, Parastatidou S, Lampropoulou K, Katsaras G, Boutsikou T, Gounaris AK, Tsantes AE, Iacovidou N (2019) Clinical application of thromboelastography/thromboelastometry (TEG/TEM) in the neonatal population: a narrative review. Semin Thromb Hemost 45(05):449–457. https://doi.org/10.1055/s-0039-1692210
Hoffman M, Monroe D (2001) A cell-based model of hemostasis. Thromb Haemost 85(06):958–965. https://doi.org/10.1055/s-0037-1615947
Haas T, Fries D, Tanaka KA, Asmis L, Curry NS, Schöchl H (2015) Usefulness of standard plasma coagulation tests in the management of perioperative coagulopathic bleeding: is there any evidence? Br J Anaesth 114(2):217–224. https://doi.org/10.1093/bja/aeu303
Schmidt AE, Israel AK, Refaai MA (2019) The utility of thromboelastography to guide blood product transfusion. Am J Clin Pathol 152(4):407–422. https://doi.org/10.1093/ajcp/aqz074
Veigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT (2016) A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med 24(1):114. https://doi.org/10.1186/s13049-016-0308-2
Oswald E, Stalzer B, Heitz E, Weiss M, Schmugge M, Strasak A, Innerhofer P, Haas T (2010) Thromboelastometry (ROTEM ® ) in children: age-related reference ranges and correlations with standard coagulation tests. Br J Anaesth 105(6):827–835. https://doi.org/10.1093/bja/aeq258
Del Vecchio A, Franco C, Petrillo F, D’Amato G (2016) Neonatal transfusion practice: when do neonates need red blood cells or platelets? Am J Perinatol 33(11):1079–1084. https://doi.org/10.1055/s-0036-1586106
Serraino GF, Murphy GJ (2017) Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: updated systematic review and meta-analysis. Br J Anaesth 118(6):823–833. https://doi.org/10.1093/bja/aex100
Wikkelsø A, Wetterslev J, Møller AM, Afshari A (2017) Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: a systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 72(4):519–531. https://doi.org/10.1111/anae.13765
Najafi A, Nikeish M, Etezadi F, Pourfakhr P, Imani F, Khajavi MR, Shariat Moharari R (2015) Comparison of aPTT and CT parameter of the ROTEM test to monitor heparin anti-coagulation effect in ICU patients: an observational study. Acta Med Iran 53(10):643–646
Mittermayr M, Velik-Salchner C, Stalzer B, Margreiter J, Klingler A, Streif W, Fries D, Innerhofer P (2009) Detection of protamine and heparin after termination of cardiopulmonary bypass by thrombelastometry (ROTEM®): results of a pilot study. Anesth Analg 108(3):743–750. https://doi.org/10.1213/ane.0b013e31818657a3
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Mahasandana C, Hathaway WE (1973) Circulating anticoagulants in the newborn: relation to hypercoagulability and the idiopathic respiratory distress syndrome. Pediatr Res 7(7):670–672. https://doi.org/10.1203/00006450-197307000-00010
Suzuki S, Wake N, Yoshiaki K (1976) New neonatal problems of blood coagulation and fibrinolysis. J Perinat Med 4:213–220. https://doi.org/10.1515/jpme.1976.4.4.213
Kettner SC, Pollak A, Zimpfer M, Seybold T, Prusa AR, Herkner K, Kuhle S (2004) Heparinase-modified thrombelastography in term and preterm neonates. Anesth Analg 98(6):1650–1652. https://doi.org/10.1213/01.ANE.0000115149.25496.DD
Cvirn G, Gallistl S, Kutschera J, Wagner T, Ferstl U, Jurgens G, Koestenberger M (2008) Clot strength: a comparison between cord and adult blood by means of thrombelastometry. J Pediatr Hematol Oncol 30(3):210–213. https://doi.org/10.1097/MPH.0b013e318162bd2c
Edwards RM, Naik-Mathuria BJ, Gay AN, Olutoye OO, Teruya J (2008) Parameters of thromboelastography in healthy newborns. Am J Clin Pathol 130(1):99–102. https://doi.org/10.1309/LABNMY41RUD099J2
Strauss T, Levy-Shraga Y, Ravid B, Schushan-Eisen I, Maayan-Metzger A, Kuint J, Kenet G (2010) Clot formation of neonates tested by thromboelastography correlates with gestational age. Thromb Haemost 103(02):344–350. https://doi.org/10.1160/TH09-05-0282
Ravn HB, Andreasen JB, Hvas A-M (2017) Does whole blood coagulation analysis reflect developmental haemostasis? Blood Coagul Fibrinolysis 28(3):218–223. https://doi.org/10.1097/MBC.0000000000000585
Sidlik R, Strauss T, Morag I, Shenkman B, Tamarin I, Lubetsky A, Livnat T, Kenet G (2016) Assessment of functional fibrinolysis in cord blood using modified thromboelastography. Pediatr Blood Cancer 63(5):839–843. https://doi.org/10.1002/pbc.25865
Mirabella L, Cotoia A, Colacicco G, Tullo L, Salatto P, Mollica G et al (2017) Reference values for coagulation assessment in full-term newborns. Minerva Anestesiol 83(4):369–374. https://doi.org/10.23736/S0375-9393.16.11375-6
Motta M, Guaragni B, Pezzotti E, Rodriguez-Perez C, Chirico G (2017) Reference intervals of citrated-native whole blood thromboelastography in premature neonates. Early Hum Dev 115(September):60–63. https://doi.org/10.1016/j.earlhumdev.2017.09.014
Sewell EK, Forman KR, Wong ECC, Gallagher M, Luban NLC, Massaro AN (2017) Thromboelastography in term neonates: an alternative approach to evaluating coagulopathy. Arch Dis Child Fetal Neonatal Ed 102(1):F79–F84. https://doi.org/10.1136/archdischild-2016-310545
Schott NJ, Emery SP, Garbee C, Waters J (2018) Thromboelastography in term neonates. J Matern Neonatal Med 31(19):2599–2604. https://doi.org/10.1080/14767058.2017.1349747
Sokou R, Foudoulaki-Paparizos L, Lytras T, Konstantinidi A, Theodoraki M, Lambadaridis I, Gounaris A, Valsami S, Politou M, Gialeraki A, Nikolopoulos GK, Iacovidou N, Bonovas S, Tsantes AE (2017) Reference ranges of thromboelastometry in healthy full-term and pre-term neonates. Clin Chem Lab Med 55(10):1592–1597. https://doi.org/10.1515/cclm-2016-0931
Liu Q, Xu C, Chen X, Wang J, Ke Z, Hu H (2019) Establishing a reference range for thromboelastograph parameters in the neonatal period. Int J Lab Hematol 41(4):530–535. https://doi.org/10.1111/ijlh.13043
Raffaeli G, Tripodi A, Cavallaro G, Cortesi V, Scalambrino E, Pesenti N, Artoni A, Mosca F, Ghirardello S (2020) Thromboelastographic profiles of healthy very low birthweight infants serially during their first month. Arch Dis Child Fetal Neonatal Ed 105(4):412–418. https://doi.org/10.1136/archdischild-2019-317860
Sokou R, Konstantinidi A, Stefanaki C, Tsantes AG, Parastatidou S, Lampropoulou K, Katsaras G, Tavoulari E, Iacovidou N, Kyriakou E, Gounaris A, Bonovas S, Tsantes AE (2019) Thromboelastometry: studying hemostatic profile in small for gestational age neonates—a pilot observational study. Eur J Pediatr 178(4):551–557. https://doi.org/10.1007/s00431-019-03331-w
Raffaeli G, Tripodi A, Manzoni F, Scalambrino E, Pesenti N, Amodeo I, Cavallaro G, Villamor E, Peyvandi F, Mosca F, Ghirardello S (2020) Is placental blood a reliable source for the evaluation of neonatal hemostasis at birth? Transfusion. 60(5):1069–1077. https://doi.org/10.1111/trf.15785
Theodoraki M, Sokou R, Valsami S, Iliodromiti Z, Pouliakis A, Parastatidou S, Karavana G, Ioakeimidis G, Georgiadou P, Iacovidou N, Boutsikou T (2020) Reference values of thrombolastometry parameters in healthy term neonates. Children. 7(12):259. https://doi.org/10.3390/children7120259
Stammers AH, Willett L, Fristoe L, Merrill J, Stover T, Hunt A, Morrow J, Newberry J (1995) Coagulation monitoring during extracorporeal membrane oxygenation: the role of thrombelastography. J Extra Corpor Technol 27(3):137–145
Grant HW, Hadley GP (1997) Prediction of neonatal sepsis by thromboelastography. Pediatr Surg Int 12(4):289–292. https://doi.org/10.1007/BF01372152
Haizinger B, Gombotz H, Rehak P, Geiselseder G, Mair R (2006) Activated thrombelastogram in neonates and infants with complex congenital heart disease in comparison with healthy children. Br J Anaesth 97(4):545–552. https://doi.org/10.1093/bja/ael206
Forman KR, Wong E, Gallagher M, McCarter R, Luban NLC, Massaro AN (2014) Effect of temperature on thromboelastography and implications for clinical use in newborns undergoing therapeutic hypothermia. Pediatr Res 75(5):663–669. https://doi.org/10.1038/pr.2014.19
Radicioni M, Bruni A, Bini V, Villa A, Ferri C (2015) Thromboelastographic profiles of the premature infants with and without intracranial hemorrhage at birth: a pilot study. J Matern Neonatal Med 28(15):1779–1783. https://doi.org/10.3109/14767058.2014.968773
Kim JY, Shin YR, Kil HK, Park MR, Lee JW (2016) Reference intervals of thromboelastometric evaluation of coagulation in pediatric patients with congenital heart diseases: a retrospective investigation. Med Sci Monit 22:3576–3587. https://doi.org/10.12659/MSM.901256
Sokou R, Giallouros G, Konstantinidi A, Pantavou K, Nikolopoulos G, Bonovas S, Lytras T, Kyriakou E, Lambadaridis I, Gounaris A, Douramani P, Valsami S, Kapsimali V, Iacovidou N, Tsantes AE (2018) Thromboelastometry for diagnosis of neonatal sepsis-associated coagulopathy: an observational study. Eur J Pediatr 177(3):355–362. https://doi.org/10.1007/s00431-017-3072-z
Peterson JA, Maroney SA, Zwifelhofer W, Wood JP, Yan K, Bercovitz RS, Woods RK, Mast AE (2018) Heparin-protamine balance after neonatal cardiopulmonary bypass surgery. J Thromb Haemost 16(10):1973–1983. https://doi.org/10.1111/jth.14245
Scott JP, Niebler RA, Stuth EAE, Newman DK, Tweddell JS, Bercovitz RS, Benson DW, Cole R, Simpson PM, Yan K, Woods RK (2018) Rotational thromboelastometry rapidly predicts thrombocytopenia and hypofibrinogenemia during neonatal cardiopulmonary bypass. World J Pediatr Congenit Hear Surg 9(4):424–433. https://doi.org/10.1177/2150135118771318
Ghirardello S, Raffaeli G, Crippa BL, Gulden S, Amodeo I, Consonni D, Cavallaro G, Schena F, Mosca F (2020) The thromboelastographic profile at birth in very preterm newborns with patent ductus arteriosus. Neonatology. 117(3):316–323. https://doi.org/10.1159/000507553
Konstantinidi A, Sokou R, Tsantes AG, Parastatidou S, Bonovas S, Kouskouni E, Gounaris AK, Tsantes AE, Iacovidou N (2020) Thromboelastometry variables in neonates with perinatal hypoxia. Semin Thromb Hemost 46(04):428–434. https://doi.org/10.1055/s-0040-1709473
Lampridou M, Sokou R, Tsantes AG, Theodoraki M, Konstantinidi A, Ioakeimidis G, Bonovas S, Politou M, Valsami S, Iliodromiti Z, Boutsikou T, Iacovidou N, Nikolopoulos G, Tsantes AE (2020) ROTEM diagnostic capacity for measuring fibrinolysis in neonatal sepsis. Thromb Res 192:103–108. https://doi.org/10.1016/j.thromres.2020.05.028
Phillips RC, Shahi N, Leopold D, Levek C, Shirek G, Hilton S, Hyslop R, Gien J, Kinsella JP, Buckvold S, Liechty KW, Kim JS, Marwan AI (2020) Thromboelastography-guided management of coagulopathy in neonates with congenital diaphragmatic hernia supported by extracorporeal membrane oxygenation. Pediatr Surg Int 36(9):1027–1033. https://doi.org/10.1007/s00383-020-04694-0
Snyder CW, Goldenberg NA, Nguyen ATH, Smithers CJ, Kays DW (2020) A perioperative bivalirudin anticoagulation protocol for neonates with congenital diaphragmatic hernia on extracorporeal membrane oxygenation. Thromb Res 193(April):198–203. https://doi.org/10.1016/j.thromres.2020.07.043
Parastatidou S, Sokou R, Tsantes AG, Konstantinidi A, Lampridou M, Ioakeimidis G, Panagiotounakou P, Kyriakou E, Kokoris S, Gialeraki A, Douramani P, Iacovidou N, Piovani D, Bonovas S, Nikolopoulos G, Tsantes AE (2021) The role of ROTEM variables based on clot elasticity and platelet component in predicting bleeding risk in thrombocytopenic critically ill neonates. Eur J Haematol 106(2):175–183. https://doi.org/10.1111/ejh.13534
Sokou R, Piovani D, Konstantinidi A, Tsantes AG, Parastatidou S, Lampridou M, Ioakeimidis G, Gounaris A, Iacovidou N, Kriebardis AG, Politou M, Kopterides P, Bonovas S, Tsantes AE (2021) A risk score for predicting the incidence of hemorrhage in critically ill neonates: development and validation study. Thromb Haemost 121(02):131–139. https://doi.org/10.1055/s-0040-1715832
Revel-vilk S (2012) The conundrum of neonatal coagulopathy. Am Soc Hematol Educ Progr 2012:450–454
Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Castle V, Powers P (1988) Development of the human coagulation system in the healthy premature infant. Blood. 72(5):1651–1657
Francis JL, Armstrong DJ (1982) Sialic acid and enzymatic desialation of cord blood fibrinogen. Pathophysiol Haemost Thromb 11(4):223–228. https://doi.org/10.1159/000214667
Pinacho A, Páramo JA, Ezcurdia M, Rocha E (1995) Evaluation of the fibrinolytic system in full-term neonates. Int J Clin Lab Res 25(3):149–152. https://doi.org/10.1007/BF02592557
Ignjatovic V, Kenet G, Monagle P (2012) Perinatal and Paediatric Haemostasis Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Developmental hemostasis: recommendations for laboratories reporting pediatric samples. J Thromb Haemost 10(2):298–300. https://doi.org/10.1111/j.1538-7836.2011.04584.x
Guzzetta NA, Allen NN, Wilson EC, Foster GS, Ehrlich AC, Miller BE (2015 Feb) Excessive postoperative bleeding and outcomes in neonates undergoing cardiopulmonary bypass. Anesth Analg 120(2):405–410. https://doi.org/10.1213/ANE.0000000000000531
Wolberg AS, Meng ZH, Monroe DM, Hoffman M (2004) A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma Inj Infect Crit Care 56(6):1221–1228. https://doi.org/10.1097/01.TA.0000064328.97941.FC
Peker E, Akbayram S, Geylani H, Dogan M, Kirimi E (2011) Global fibrinolytic capacity in neonatal sepsis. Clin Appl Thromb 17(6):E64–E69. https://doi.org/10.1177/1076029610384113
Bauman ME, Cheung P-Y, Massicotte MP (2011) Hemostasis and platelet dysfunction in asphyxiated neonates. J Pediatr 158(2):e35–e39. https://doi.org/10.1016/j.jpeds.2010.11.011
Code availability
N/A.
Author information
Authors and Affiliations
Contributions
GNK conceived the study, participated in its design, collection, and interpretation of the data, and also performed the data collection and extraction, as well as the statistical analyses, and helped to draft the manuscript.
RS contributed to the data collection and extraction, in the analysis of the results and in drafting the manuscript.
AGT contributed to the data collection and extraction, in the analysis of the results and in drafting the manuscript.
DP contributed to the data collection and extraction, in the analysis of the results and in drafting the manuscript.
SB contributed to the data collection and extraction, in the analysis of the results and in drafting the manuscript.
AK contributed to the data collection and extraction, in the analysis of the results and in drafting the manuscript.
GI contributed to the data collection and extraction, as well as in the analysis of the results.
SP contributed to the data collection and extraction, as well as in the analysis of the results.
DG contributed to the data collection and extraction, as well as in the analysis of the results.
AM contributed to the data collection and extraction, as well as in the analysis of the results.
GM conceived the study and participated in the manuscript’s design and coordination.
AET conceived the study and participated in the manuscript’s design and coordination.
All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All analyses were based on previous published studies; thus, no ethical approval was required.
Consent to participate
N/A.
Consent for publication
N/A.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Katsaras, G.Ν., Sokou, R., Tsantes, A.G. et al. The use of thromboelastography (TEG) and rotational thromboelastometry (ROTEM) in neonates: a systematic review. Eur J Pediatr 180, 3455–3470 (2021). https://doi.org/10.1007/s00431-021-04154-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04154-4