Abstract
Adolescent obesity is a serious health problem associated with many comorbidities. Obesity-related alterations in circadian rhythm have been described for nocturnal blood pressure and for metabolic functions. We believe renal circadian rhythm is also disrupted in obesity, though this has not yet been investigated. This study aimed to examine renal circadian rhythm in obese adolescents before and after weight loss.
In 34 obese adolescents (median age 15.7 years) participating in a residential weight loss program, renal function profiles and blood samples were collected at baseline, after 7 months, and again after 12 months of therapy. The program consisted of dietary restriction, increased physical activity, and psychological support. The program led to a median weight loss of 24 kg and a reduction in blood pressure. Initially, lower diurnal free water clearance (− 1.08 (− 1.40–− 0.79) mL/min) was noticed compared with nocturnal values (0.75 (− 0.89–− 0.64) mL/min). After weight loss, normalization of this inverse rhythm was observed (day − 1.24 (− 1.44–1.05) mL/min and night − 0.98 (− 1.09–− 0.83) mL/min). A clear circadian rhythm in diuresis rate and in renal clearance of creatinine, solutes, sodium, and potassium was seen at all time points. Furthermore, we observed a significant increase in sodium clearance. Before weight loss, daytime sodium clearance was 0.72 mL/min (0.59–0.77) and nighttime clearance was 0.46 mL/min (0.41–0.51). After weight loss, daytime clearance increased to 0.99 mL/min (0.85–1.17) and nighttime clearance increased to 0.78 mL/min (0.64–0.93).
Conclusion: In obese adolescents, lower diurnal free water clearance was observed compared with nocturnal values. Weight loss led to a normalization of this inverse rhythm, suggesting a recovery of the anti-diuretic hormone activity. Both before and after weight loss, clear circadian rhythm of diuresis rate and renal clearance of creatinine, solutes, sodium, and potassium was observed.
What is Known: • Obesity-related alterations in circadian rhythm have been described for nocturnal blood pressure and for metabolic functions. We believe renal circadian rhythm is disrupted in obesity, though this has not been investigated yet. | |
What is New: • In obese adolescents, an inverse circadian rhythm of free water clearance was observed, with higher nighttime free water clearance compared with daytime values. Weight loss led to a normalization of this inverse rhythm, suggesting a recovery of the anti-diuretic hormone activity. • Circadian rhythm in diuresis rate and in the renal clearance of creatinine, solutes, sodium, and potassium was preserved in obese adolescents and did not change after weight loss. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adolescent obesity is a serious public health problem worldwide. During the last few decades, the prevalence of childhood and adolescent obesity has increased at an alarming rate, not only in the Western world but also in low- and middle-income countries (WHO) [35]. Up to 11–15% of Belgian children and adolescents are overweight and 3–5% are reportedly obese. Children with obesity have an increased risk of becoming obese adults [4, 11, 18, 20, 31] and, developing chronic conditions, such as cardiovascular disease, diabetes mellitus (DM), hypertension, and kidney disease [19, 28].
We hypothesized that adolescent obesity is associated with a disruption of renal circadian rhythm. Like many other biological processes, renal functions exhibit clear circadian variations. Urine production and voiding tend to predominate during the awakening phase, whereas increased urine storage and reduced micturition frequency predominate during the sleeping phase [25]. This circadian rhythm is believed to be driven by a self-sustained central system, located in the suprachiasmatic nucleus of the hypothalamus, communicating with peripheral systems in nearly every cell type [10, 25]. This system influences renal blood flow and circulating hormones affecting urine production and storage, sympathetic/parasympathetic tone, blood pressure (BP), and thus, glomerular filtration rate (GFR), and water and solute handling [9].
Disturbances in proper voiding rhythms are typically present in children with nocturnal enuresis and elderly individuals with nocturia [10]. Renal circadian rhythm in obesity has not yet been investigated. Previous studies, however, have observed obesity-related alterations in the circadian rhythm of other biological functions. Macumber et al. [21] demonstrated that obesity is associated with a relative decrease in nocturnal BP dipping in children. Pagano et al. [26] discussed the deranged circadian rhythm of metabolic functions and glucose homeostasis in obese adults.
Subsequently, obesity-related kidney dysfunction has been examined in adults. This dysfunction is believed to be indirectly mediated by comorbid conditions, such as DM and hypertension, and directly mediated by physical compression of the kidneys by visceral adiposity, inflammation, endothelial dysfunction [33], hyperinsulinemia [27], and a deranged endocrine activity of the visceral adipose tissue. Hyperinsulinemia leads to increased sodium and potassium reabsorption, independently of GFR and plasma aldosterone [22, 26]. Deranged endocrine activity may affect kidney function through three mechanisms: an overactive renin-angiotensin-aldosterone system (RAAS) [3, 6, 28, 30], a dysregulated natriuretic peptides system [29], or an overactive sympathetic nervous system [7]. These direct and indirect mechanisms contribute to impaired pressure natriuresis, increased renal tubular sodium reabsorption, and blood volume expansion [14, 19]. As a result, a compensatory increase in renal plasma flow (RPF), glomerular pressure, and hyperfiltration sets in to maintain sodium balance. This compensation spurs the development of glomerular hypertension, increased glomerular permeability, and proteinuria, and ultimately leads to glomerulomegaly and focal segmental glomerulosclerosis [5, 16, 29, 32]. Several studies showed that weight loss in obese patients can improve glomerular hemodynamics and, thus, counteract the development of obesity-related renal insufficiency [2, 5]. We propose that these aforementioned mechanisms of kidney disease contribute to a derangement of the renal circadian rhythm as well.
The objective of this prospective study was to examine renal circadian rhythm in obese adolescents before and after weight loss.
Materials and methods
Patient selection
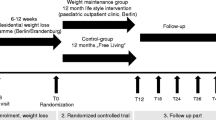
This prospective interventional study was performed at the medical pediatric rehabilitation center “Zeepreventorium”, De Haan, between July 2016 and June 2017. Obese adolescents aged 10 to 19-year-olds were recruited at the beginning of a 12-month residential obesity treatment program. Exclusion criteria were comorbidities (kidney failure, heart failure, liver failure, DM, and diabetes insipidus), pregnancy, and intake of medication with a potential effect on urine output (diuretics, desmopressin, ACE-inhibitors, AngII receptor antagonists, non-steroidal anti-inflammatory drugs, corticosteroids, lithium, and selective serotonin reuptake inhibitors). After data collection, 19 patients were excluded from analysis due to early discharge, incomplete urine sampling, incorrect urine sampling, impossibility of urine sampling due to a tibial fracture, or start of an ACE-inhibitor (Fig. 1).
Participants followed a multidisciplinary program, which consisted of moderate dietary restriction, psychological support, and regular physical activity. The diet consisted of 1600–1900 kcal/day, 200–250 g of carbohydrates, 25–35 g of fats, 75–90 g of proteins, and a sodium and potassium content of 2.5–4 g daily. Adolescents were encouraged to drinking at least 2 glasses of water (or milk during breakfast) with their meals and carry a water bottle at all times. This water replaced the soda or juice that the adolescents were used to drink before enrollment. Physical activity consisted of 2 h of supervised play and lifestyle activities daily, 100 min of physical education courses per week at school, and 150 min of supervised training sessions every week. Qualified physiotherapists supervised the participants during all training sessions [1].
Data collection
Data was collected at the following 3 time points: at the onset of the residential program in July 2016, mid-term after 7 months of treatment and at its completion after 12 months in June 2017. All patients completed a renal function profile (RFP) and a morning blood sample. BP, body weight, and height were measured. Standardized measurements were obtained at all time points: identical daily routines with standardized meals and fluid intake, similar sports activities, and comparable day-night rhythm.
Anthropometry and comorbidities
Anthropometric measurements were performed after breakfast, with the subjects wearing light school uniform clothing, without footwear. Bodyweight was measured on a digital scale with a precision of 0.1 kg and height was measured to the nearest 0.01 m using a wall-mounted stadiometer. Body mass index (BMI) was calculated as weight/heigth2 (kg/m2). BMI z-score was calculated as BMI adjusted to weight, height, and age. Overweight and obesity were defined as a BMI equal to or above the age- and gender-specific 85th and 97th percentile respectively according to regional charts in adolescents younger than 16 years. In adolescents older than 16 years, overweight and obesity were defined as BMI ≥ 30 and ≥ 35 respectively.
BP was measured three times in the seated position by an automated electronic sphygmomanometer (GE Healthcare, Carescape V100). Hypertension was defined as a mean systolic and/or diastolic BP equal to or above the age-, gender-, and height-specific 95th percentile [23]. Presence of insulin resistance was extracted from the medical files of the participants.
RFP and blood sampling
A RFP is a 24 h-urine collection in which urine samples are collected at fixed time points every 3 h, starting 3 h after the first-morning void. Daytime samples were taken at 9 am (1), 12 am (2), 15 pm (3), 18 pm (4), and 21 pm (5). The nighttime samples were taken at 0 am (6), 3 am (7), and 6 am (8). The volume of each sample, and of each micturition in between, was noted to calculate the 24 h, daytime and nighttime urine volume and diuresis rate. After an overnight fast, a sober serum sample was taken.
Biochemical analyses
Sodium, potassium, osmolality, and creatinine were measured in the blood sample and for each of the 8 urine samples. The renal clearance of creatinine, solutes, sodium, and potassium (Usubst × Uflow/Psubst), free water clearance (FWC) (urine flow-solute clearance), fractional excretion of sodium, and potassium (Usubst × Pcreat/Ucreat × Psubst) were calculated. eGFR was calculated and corrected for the body surface area (BSA) (creatinine clearance × 1.73/BSA).
Statistical analysis
Statistical analysis was performed using SPSS®, version 25. The median, interquartile range (IQR), and frequency were recorded as descriptive parameters. The Shapiro-Wilk test was used to identify the normal distribution of the variables. The McNemar test was used to compare two paired categorical variables. The continuous variables were analyzed using the non-parametric Wilcoxon matched-pairs signed-ranks test. Statistical significance was assessed using two-tailed tests and determined by p < 0.05. To identify independent predictors for altered circadian rhythm, a multivariate Cox regression analysis with calculation of the hazard ratio was performed.
The study was approved by the Ghent University Hospital review board (EC 2015/1438). The Declaration of Helsinki was followed and conducted in accordance with the legal regulations in Belgium. Written informed consent was obtained from both the parents and all individual participants included in the study.
Results
Patient characteristics and comorbidities
A total of 34 patients were eligible for analysis. Median age at baseline was 15.7 (14.1–16.7) years, and 73.5% of participants were female. One year of treatment led to a median weight loss of 24.0 (17.9–33.5) kg or 21.9 (18.7–28.3) %. The median BMI z-score at baseline was calculated at 2.42 (2.23–2.73) and decreased significantly (p < 0.001) after treatment (1.56 (1.28–1.92)). Each adolescent participating in the study showed a weight loss (minimum weight loss of 11.9 kg). Weight loss was more pronounced in the male group, leading to a median male weight loss of 41.9 kg compared with females (21.4 kg). After treatment, 6 adolescents had a normal BMI, 12 were overweight, and 16 remained obese. A significant decrease in BP was observed after both 7 and 12 months of weight loss. At the end of the treatment program, the systolic BP decreased significantly (p < 0.001) to a median of 114.5 (106.5–120.55) mmHg and the diastolic BP decreased (p = 0.043) to a median of 67.5 (62–72.25) mmHg. Initially, 27 (79.4%) patients were assessed as hypertensive. After 12 months of weight loss, only 4 (11.8%) patients were classified as hypertensive. Before weight loss, 28 (82.4%) patients had insulin resistance, which diminished significantly (p < 0.001) to 6 (17.6%) patients at the end of the program (Table 1 and Fig. 2).
Renal function profile
Diuresis rate
Before and after 12 months of weight loss, the diuresis rate was significantly lower during the nighttime compared with daytime measurements (p = 0.013 and p = 0.002, respectively). Nevertheless, this circadian rhythm appeared more pronounced after weight loss. A significant increase in diuresis rate was reported after 7 and 12 months of treatment (p < 0.001 and p < 0.001, respectively) (Table 2 and Fig. 3).
Change in different parameters at 3 h fixed time points before and after 12 months of weight loss therapy. a Change in diuresis rate (mL/min), b change in creatinine clearance (mL/min). Differences between baseline and after treatment measurement of every time point were calculated with the Wilcoxon matched-pairs signed-ranks test. p values < 0.05 were considered statistically significant and are mentioned with an asterisk (*). The end of the whiskers represents the 5th and 95th percentile of the measurements
Creatinine clearance
A significant decrease in nighttime creatinine clearance compared with daytime clearance was demonstrated before (p < 0.001) and after (p = 0.043) weight loss. Creatinine clearance was not statistically different between baseline measurement and follow-up time points (p = 0.4 and p = 0.071, respectively) (Table 2 and Fig. 3). We calculated the eGFR as creatinine clearance per 1.73 m2 and noted a significant increase (p = 0.007) to a median eGFR after weight loss of 137.57 (123.50–155.73) mL/min.
Free water clearance
Before and after 7 months of treatment, nocturnal FWC was significantly higher than daytime values (p < 0.001 and p < 0.001, respectively). After 12 months of weight loss, nighttime FWC did not differ from daytime levels (p = 0.091). After 7 and 12 months of treatment, no significant change in FWC was observed (p = 0.082 and p = 0.321, respectively). However, we noted a trend toward lower FWC after weight loss (Table 2 and Fig. 4). When calculating the FWC per 1.73 m2, we noted no significant change (p = 0.062) from baseline (− 0.73 (− 1.05–− 0.61) to after 12 months of treatment (− 1.04 (− 1.30–− 0.70). A multivariate logistic regression analysis was done to evaluate gender, obesity, and hypertension after treatment as potential risk factors for a disrupted circadian rhythm of FWC. However, none of these factors was determined to be potential risk factors (gender (p = 0.41), hypertension (p = 0.95), obesity after treatment (p = 0.84)).
Change in water handling and solutes clearance at 3 h fixed time points before and after 12 months of weight loss therapy. a Solute clearance (mL/min); b sodium clearance (mL/min); c potassium clearance (mL/min); d change in free water clearance (mL/min). Differences between baseline and after treatment measurement of every time point were calculated with the Wilcoxon matched-pairs signed-ranks test. p values < 0.05 were considered statistically significant and are mentioned with an asterisk (*). The end of the whiskers represents the 5th and 95th percentile of the measurements
Solute clearance
Compared with daytime values, a significant decrease in solute clearance during the night was reported at all time points (p < 0.001, p < 0.001, and p < 0.001, respectively). A significant increase in solute clearance was seen after 7 (p < 0.001) and 12 months of weight loss (p < 0.001) (Table 2 and Fig. 4).
Sodium clearance
A significant circadian rhythm with lower nighttime sodium clearance values than daytime measurements was observed at all time points (p < 0.001, p = 0.013, p = 0.029, respectively). U4 shows a clear dip in sodium clearance before and after treatment. For sodium clearance, a significant increase was noted after 7 and 12 months of weight loss (p = 0.03 and p < 0.001, respectively) (Table 2 and Fig. 4). Fractional excretion of sodium exhibited a similar pattern with a significant increase from 0.44 (0.34–0.51) % to 0.62 (0.48–0.73) % (p < 0.001) after treatment.
Potassium clearance
Nocturnal potassium clearance was significantly lower than daytime measurement at all points (p < 0.001, p < 0.001, and p < 0.001, respectively). A significant increase in potassium clearance was reported after 7 (p < 0.001) and 12 months (p < 0.001) (Table 2 and Fig. 4). A significant increase in baseline fractional excretion of potassium (5.79 (4.96–6.55) %) was observed after 12 months (7.69 (6.43–9.82) %) (p < 0.001).
Discussion
The objective of this prospective study was to examine renal circadian rhythm in obese adolescents before and after weight loss.
In the present study, an inverse circadian rhythm of FWC was observed, both before and after 7 months of treatment, with lower daytime compared with nighttime values. This finding is inconsistent with the theory that in healthy children and adults, increased secretion of ADH is expected during the night. The elevated excretion of ADH comes along with a reduction in FWC during nighttime compared with daytime. This mechanism is essential, as it leads to the production of more concentrated urine during the night and thus, a reduction in micturition frequency [17]. The disrupted day-night rhythm of FWC in our population may be provoked by a reversed ADH activity with lower ADH levels during the night, leading to a higher nocturnal FWC during the night. Another possible explanation is the nocturnal mobilization of obesity-related peripheral edema as obesity may be a cause of lower extremity lymphedema and venous insufficiency. As a result, interstitial fluid accumulates in the lower extremities while standing during daytime and is reabsorbed into the circulation when laying down during sleep. This shift represents a surplus of free water that the kidneys need to remove trough a stimulated natriuretic peptides system and suppressed ADH [8, 12, 13]. After 12 months of weight loss, no significant difference in day and nighttime FWC was reported, suggesting a normalization of the circadian rhythm.
Clear circadian rhythms of diuresis rate and of the renal clearance of creatinine, solutes, sodium, and potassium were observed at baseline and after completion of the weight loss program. The dip of sodium clearance in sample U4 can be explained by the daily 2 h of play and lifestyle activities before U4 sampling. Blunted renal circadian rhythms in accordance with disrupted daily rhythms of BP and other metabolic functions in obese patients were expected, as described in previous studies [21, 26]. This largely preserved renal circadian rhythm may be explained by the short duration of obesity in our adolescent study population, perhaps being too short to affect the rhythm of renal function. Another possibility is that our study sample is a variant of the obese population with preserved renal circadian rhythm. Further research to investigate these circadian rhythms in the obese population is necessary.
An overall median weight loss of 24 kg did not affect creatinine clearance and even led to a significant increase in eGFR. A decrease was expected since Chagnac et al. [5] and Navarro-Díaz et al. [24] reported a substantial decrease in renal hyperfiltration after bariatric surgery in obese adults. The eGFR was calculated as milliliter per minute per 1.73 m2 and showed a significant increase after 12 months of weight loss. This discrepancy is likely an artifact attributable to the decrease in BSA and not due to a change in renal size or function. Several possible explanations for the lack of change in creatinine clearance in this study are plausible. Firstly, the follow-up period of this study was short and, 47% of adolescents were still obese after 12 months of treatment. As a result, weight loss may not have been sufficient or prolonged enough to affect changes in renal filtration. Second, it is possible that the hyperfiltration theory is not correct or not applicable for obese adolescents.
A decrease in the obesity-related hyperactive RAAS established by weight loss has been described in previous studies [6, 15, 34] and was confirmed by our findings. A significant increase in sodium clearance was observed after 12 months of weight loss, which can be explained by lower aldosterone concentrations. This increase in sodium clearance only becomes evident at the end of the treatment program, suggesting that adequate weight loss is necessary. The observed elevation in potassium clearance after weight loss cannot necessarily be attributed to diminished activity of the RAAS. This elevation may be explained by an increased intake of potassium since meals were not identical at each test moment. The significant decrease in both systolic and diastolic BP after weight loss can be explained by a normalization of the RAAS, as well.
Limitations of this study include the small number of participants, the short follow-up period and the fact that plasma renin and aldosterone measurements were not included in the study protocol. Moreover, this study is only generalizable to adolescents without obesity-related comorbidities, as these patients were excluded from the analysis. Another limitation is the inability to isolate weight loss from a multidisciplinary program including many lifestyle modifications. Furthermore, it was not possible to use values that corrected for BSA, because of its large variability within and between the obese patients. In addition, the exact water intake by the adolescents was not measured. This could be a confounder, as increased water consumption after initiation of the program can influence the renal function profiles. The GFR was calculated and not measured through inulin clearance in the 24-h urine sample. In addition, 24-h blood pressure measurement was not performed. Lastly, due to practical and ethical limitations of the study, we could not include a control group of healthy non-obese adolescents.
Conclusion
In the present study, 34 obese adolescents were monitored during a 12-month weight loss program. This program led to a median weight loss of 24 kg, a reduction in both systolic and diastolic BP, and improvement of insulin resistance.
Initially, an inverse circadian rhythm of FWC was observed, with lower daytime compared with nighttime values. This may be provoked by a deranged ADH activity and the nocturnal mobilization of obesity-related peripheral edema. After 12 months of weight loss, normalization of this inverse rhythm was noticed.
Furthermore, this study showed clear circadian rhythms in diuresis rate and the renal clearance of creatinine, solutes, sodium, and potassium at the beginning and end of the weight loss program. Possible explanations for this largely preserved renal circadian rhythm include a short duration of obesity in our adolescent study population.
A decrease in the obesity-related hyperactive RAAS was suggested as a result of the significant increase in sodium clearance that was observed. Future studies with a larger study population, healthy controls, and a longer follow-up period must be performed to confirm our findings.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ADH:
-
Anti-diuretic hormone
- AGT:
-
Angiotensinogen
- AngII:
-
Angiotensin II
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- BSA:
-
Body surface area
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- FWC:
-
Free water clearance
- GFR:
-
Glomerular filtration rate
- RAAS:
-
Renin-angiotensin-aldosterone system
- RFP:
-
Renal function profile
- RPF:
-
Renal plasma flow
References
Braet C, Tanghe A, De Bode P, Franckx HVWM (2003) Inpatient treatment of obese children: a multicomponent programme without stringent calorie restriction. https://doi.org/10.1007/s00431-003-1155-5
Bruyndonckx L, Hoymans VY, De Guchtenaere A, Van Helvoirt M, Van Craenenbroeck EM, Frederix G, Lemmens K, Vissers DK, Vrints CJ, Ramet J, Conraads VM (2015) Diet, exercise, and endothelial function in obese adolescents. Pediatrics 135(3):e653–e661. https://doi.org/10.1542/peds.2014-1577
Cabandugama PK, Gardner MJ, Sowers JR (2017) The renin angiotensin aldosterone system in obesity and hypertension. Med Clin North Am 101(1):129–137. https://doi.org/10.1016/j.mcna.2016.08.009
Caprio S, Pierpont B, Kursawe R (2018) The “adipose tissue expandability” hypothesis: a potential mechanism for insulin resistance in obese youth. Horm Mol Biol Clin Investig 33(2). https://doi.org/10.1515/hmbci-2018-0005
Chagnac A (2003) The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14(6):1480–1486. https://doi.org/10.1097/01.ASN.0000068462.38661.89
Cooper R, McFarlane-Anderson N, Bennett F, Wilks R, Puras A, Tewksbury D, Ward R, Forrester T (1997) ACE, angiotensinogen and obesity: a potential pathway leading to hypertension. J Hum Hypertens 11(2):107–111. https://doi.org/10.1038/sj.jhh.1000391
da Silva AA, do Carmo J, Dubinion J, Hall JE (2009) The role of the sympathetic nervous system in obesity-related hypertension. Curr Hypertens Rep 11(3):206–211
Davies HO, Popplewell M, Singhal R, Smith N, Bradbury AW (2017) Obesity and lower limb venous disease - the epidemic of phlebesity. Phlebology 32(4):227–233. https://doi.org/10.1177/0268355516649333
De Guchtenaere A, Vande Walle C, Van Sintjan P, Raes A, Donckerwolcke R, Van Laecke E, Hoebeke P, Vande Walle J (2007) Nocturnal polyuria is related to absent circadian rhythm of glomerular filtration rate. J Urol 178(6):2626–2629. https://doi.org/10.1016/j.juro.2007.08.028
Dossche L, Walle JV, Van Herzeele C (2016) The pathophysiology of monosymptomatic nocturnal enuresis with special emphasis on the circadian rhythm of renal physiology. Eur J Pediatr 175(6):747–754. https://doi.org/10.1007/s00431-016-2729-3
Gómez SF, Casas Esteve R, Subirana I, Serra-Majem L, Fletas Torrent M, Homs C, Bawaked RA, Estrada L, Fíto M, Schröder H (2018) Effect of a community-based childhood obesity intervention program on changes in anthropometric variables, incidence of obesity, and lifestyle choices in Spanish children aged 8 to 10 years. Eur J Pediatr 177(10):1531–1539. https://doi.org/10.1007/s00431-018-3207-x
Greene AK, Grant FD, Slavin SA (2012) Lower-extremity lymphedema and elevated body-mass index. N Engl J Med 366(22):2136–2137. https://doi.org/10.1056/NEJMc1201684
Gulur DM, Mevcha AM, Drake MJ (2011) Nocturia as a manifestation of systemic disease. BJU Int 107(5):702–713. https://doi.org/10.1111/j.1464-410X.2010.09763.x
Hall JE, Henegar JR, Dwyer TM, Liu J, da Silva AA, Kuo JJ, Tallam L (2004) Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther 11(1):41–54. https://doi.org/10.1053/j.arrt.2003.10.007
Harp JB, Henry SA, DiGirolamo M (2002) Dietary weight loss decreases serum angiotensin-converting enzyme activity in obese adults. Obes Res 10(10):985–990. https://doi.org/10.1038/oby.2002.134
Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE (2001) Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 12(6):1211–1217
Kamperis K, Hansen M, Hagstroem S, Hvistendahl G, Djurhuus J, Rittig S (2004) The circadian rhythm of urine production, and urinary vasopressin and prostaglandin E2 exretion in healthy children. J Urol 171(6 Part 2):2571–2575. https://doi.org/10.1097/01.ju.0000110421.71910.c0
Kiba K, Hirayama A, Yishikawa M, Yutaka Y, Torimoto K, Nobutaka S, Tanaka N, Fujimoto K, Uemura H (2018) Increased urine production due to leg fluid displacement reduces hours of undisturbed sleep. LUTS Low Urin Tract Symptoms 10(3):253–258. https://doi.org/10.1111/luts.12176
Kovesdy C, Furth S, Zoccali C, World Kidney Day (2017) Obesity and kidney disease: hidden consequences of the epidemic. Indian J Nephrol 27(2):85–92. https://doi.org/10.4103/ijn.IJN_61_17
Lurbe E, Alvarez V, Liao Y, Torro I, Cremades B, Redón J, Cooper R Obesity modifies the relationship between ambulatory blood pressure and natriuresis in children. Blood Press Monit 5(5-6):275–280
Macumber IR, Weiss NS, Halbach SM, Hanevold CD, Flynn JT (2016) The association of pediatric obesity with nocturnal non-dipping on 24-hour ambulatory blood pressure monitoring. Am J Hypertens 29(5):647–652. https://doi.org/10.1093/ajh/hpv147
Marcus Y, Shefer G, Stern N (2013) Adipose tissue renin–angiotensin–aldosterone system (RAAS) and progression of insulin resistance. Mol Cell Endocrinol 378(1–2):1–14. https://doi.org/10.1016/j.mce.2012.06.021
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114(2 Suppl 4th Report):555–576
Navarro-Díaz M, Serra A, Romero R, Bonet J, Bayés B, Homs M, Pérez N, Bonal J (2006) Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol 17(12 suppl 3):S213–S217. https://doi.org/10.1681/ASN.2006080917
Noh J-Y, Han D-H, Yoon J-A, Kim M-H, Kim S-E, Ko I-G, Kim K-H, Kim C-J, Cho S (2011) Circadian rhythms in urinary functions: possible roles of circadian clocks? Int Neurourol J 15(2):64–73. https://doi.org/10.5213/inj.2011.15.2.64
Pagano ES, Spinedi E, Gagliardino JJ (2017) White adipose tissue and circadian rhythm dysfunctions in obesity: pathogenesis and available therapies. Neuroendocrinology 104(4):347–363. https://doi.org/10.1159/000453317
Rocchini AP, Katch V, Kveselis D, Moorehead C, Martin M, Lampman R, Gregory M (1989) Insulin and renal sodium retention in obese adolescents. Hypertension 14(4):367–374. https://doi.org/10.1161/01.HYP.14.4.367
Rüster C, Wolf G (2013) The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin Nephrol 33(1):44–53. https://doi.org/10.1016/j.semnephrol.2012.12.002
Sarzani R, Salvi F, Dessì-Fulgheri P, Rappelli A (2008) Renin–angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens 26(5):831–843. https://doi.org/10.1097/HJH.0b013e3282f624a0
Shatat IF, Flynn JT (2011) Relationships between renin, aldosterone, and 24-hour ambulatory blood pressure in obese adolescents. Pediatr Res 69(4):336–340. https://doi.org/10.1203/PDR.0b013e31820bd148
Simental-Mendía LE, Hernández-Ronquillo G, Gamboa-Gómez CI, Gómez-Díaz R, Rodríguez-Morán M, Guerrero-Romero F (2019) The triglycerides and glucose index is associated with elevated blood pressure in apparently healthy children and adolescents. Eur J Pediatr 178(7):1069–1074. https://doi.org/10.1007/s00431-019-03392-x
Thethi T, Kamiyama M, Kobori H (2012) The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr Hypertens Rep 14(2):160–169. https://doi.org/10.1007/s11906-012-0245-z
Whaley-Connell A, Sowers JR (2017) Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int 92(2):313–323. https://doi.org/10.1016/j.kint.2016.12.034
Wickman C, Kramer H (2013) Obesity and kidney disease: potential mechanisms. Semin Nephrol 33(1):14–22. https://doi.org/10.1016/j.semnephrol.2012.12.006
Wijnhoven TMA, van Raaij JMA, Spinelli A, Rito AI, Hovengen R, Kunesova M, Starc G, Rutter H, Sjöberg A, Petrauskiene A, O’Dwyer U, Petrova S, Farrugia Sant’Angelo V, Wauters M, Yngve A, Rubana I-M, Breda J (2013) WHO European childhood obesity surveillance initiative 2008: weight, height and body mass index in 6-9-year-old children. Pediatr Obes 8(2):79–97. https://doi.org/10.1111/j.2047-6310.2012.00090.x
Funding
Kim Pauwaert has received research grants from Ferring Pharmaceuticals.
Elke Bruneel has received research grants from Ferring Pharmaceuticals.
Johan Vande Walle has received consulting fees and travel reimbursements from Ferring Pharmaceuticals and payment for lectures from Ferring Pharmaceuticals and Astellas Pharma.
Karel Everaert has received research grants and payment for lectures all in contract with his institution from Ferring Pharmaceuticals, Medtronic and Astellas Pharma.
Author information
Authors and Affiliations
Contributions
Kim Pauwaert had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Vande Walle, Everaert, De Guchtenaere
Acquisition of data: Dejonckheere, Bruneel, Keersmaeckers, De Guchtenaere
Database work-up: Pauwaert, Dejonckheere, Bruneel, Van der Jeugt
Statistical analysis: Pauwaert, Dejonckheere, Bruneel, Keersmaeckers
Drafting of the manuscript: Pauwaert, Dejonckheere
Critical revision of the manuscript for important intellectual content: Everaert, Vande Walle, Roggeman, De Guchtenaere
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ghent University Hospital review board (EC 2015/1438). The Declaration of Helsinki was followed and conducted in accordance with the legal regulations in Belgium.
Informed consent
Informed consent was obtained from all individual participants and their parents included in the study.
Additional information
Communicated by Mario Bianchetti
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pauwaert, K., Dejonckheere, S., Bruneel, E. et al. The effect of a multidisciplinary weight loss program on renal circadian rhythm in obese adolescents. Eur J Pediatr 178, 1849–1858 (2019). https://doi.org/10.1007/s00431-019-03456-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03456-y