Abstract
Our objective was to assess within a feasibility study the correlation and agreement of transcutaneous carbon dioxide (PtcCO2) monitoring with venous carbon dioxide (PvCO2) in infants with bronchiolitis in the emergency room (ER) and pediatric department. Sixty infants (aged 3.6 ± 3.3 months) admitted to our ER with bronchiolitis were included. PtcCO2 measurements (SenTec Digital Monitoring System) collected prospectively were compared with simultaneous PvCO2 drawn for patient care. Analysis included 100 measurements. The correlation of PtcCO2 and PvCO2 (r = 0.71, p < 0.001) was good, and the agreement (mean difference ± standard deviation of the differences 1.9 ± 7.0 mmHg) was adequate; average PtcCO2 was slightly lower than PvCO2. Changes in PtcCO2 and PvCO2 for consecutive measurements within each patient correlated (r = 0.41, p < 0.01). The level of PtcCO2 correlated with disease severity clinical score (p < 0.001).
Conclusions: PtcCO2 monitoring was feasible in the ER and pediatric department and was found to have a good correlation and adequate agreement with PvCO2 in infants with bronchiolitis. Because the standard deviation of the differences was relatively high, though comparable to the literature, we suggest that PtcCO2 should not replace blood gas but rather serve as a complementary tool for trending and for real-time continuous assessment of the CO2 levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bronchiolitis is one of the most common respiratory viral infections among children younger than 2 years [16] and one of the most common causes of hospital admission during the winter months. Approximately 3 % of otherwise healthy infants are hospitalized annually due to viral bronchiolitis for respiratory monitoring and supportive care [2].
Continuous noninvasive monitoring in the pediatric ward is important because the respiratory condition of infants with bronchiolitis may deteriorate [17]. This will be even more important and even crucial if high-flow nasal cannula will become an optional mode of treatment for bronchiolitis in the emergency room (ER) and the pediatric ward [5, 12, 20]. The medical team treating sicker infants, needing nasal support within the pediatric ward, will need objective continuous noninvasive measures that will predict the need for endotracheal intubation and timely transport to the intensive care unit [1, 12, 13]. Currently, these infants are followed by pulse oximetry oxygen saturation and repeated blood gases to assess carbon dioxide retention as a warning sign for impending respiratory failure.
Arterial blood gas (ABG) sampling represents the gold standard for acquiring blood gases and acid-base status [28], but repeated ABGs are not feasible in the pediatric wards, where the use of venous blood gases (VBG) is more prevalent. Adequate correlation between venous CO2 (PvCO2) and arterial CO2 was shown by several studies [8, 10, 28]. However, venous gas sampling may cause discomfort and pain and may not be accurate if the infants cry (hyperventilation) or hold their breath (apnea) during the puncture. Furthermore, it does not allow continuous monitoring, an important tool, that may help early recognition of impending respiratory failure and timely transfer to a Pediatric Intensive Care Unit (PICU).
Transcutaneous PCO2 (PtcCO2) measurement represents a simple and noninvasive technique for continuous monitoring of ventilation [6, 7, 21, 22, 27]. Holmgren and Sixt evaluated children with asthmatic symptoms and concluded that the transcutaneous technique can be used for monitoring acute bronchial obstruction and for evaluating the effects of treatment in children of different ages [11]. Measuring PtcCO2 with a new device that does not cause thermal injuries to the skin may enable adequate continuous noninvasive monitoring [21, 24]. To the best of our knowledge, no study was performed previously on noninvasive monitoring of PtcCO2 in infants with bronchiolitis in the ER or the pediatric ward.
The aim of the current study was to assess within a feasibility study the correlation and agreement of PtcCO2 monitoring with PvCO2 in infants with viral bronchiolitis in the ER and pediatric department.
Material and methods
Study design
This was a prospective, observational study, conducted at Bnai Zion Medical Center, Haifa, Israel, between January 2011 and March 2013. Infants aged 0–18 months with viral bronchiolitis were connected to a PtcCO2 monitoring device, and these measurements were compared to PvCO2 sampling drawn for patient care. The study was approved by the institutional review board in our center, and parents of all infants signed an informed consent prior to participation in the study. Our primary outcome measure was the correlation and agreement between PtcCO2 and PvCO2. Our secondary outcomes were to assess the trending ability of PtcCO2 measurements and to evaluate the correlation of PtcCO2 with disease severity.
Study population
Included in the study were infants admitted to the ER and hospitalized with the clinical diagnosis of viral bronchiolitis in the pediatric ward, and whose parents signed an informed consent. A disease severity clinical score, based on a validated score [2, 18], but simplified for practical daily clinical practice using the following parameters was performed for each patient: (1) respiratory rate >60 breaths/min, (2) clinical signs of respiratory distress (retractions, nasal flaring), (3) oxygen saturation <92 % on room air, (4) oxygen requirement (if SpO2 < 90 % or respiratory distress), (5) heart rate >160 beats/min, (6) findings of fine crackles, and (7) wheezing on physical examination. Each clinical parameter constitutes an equal value (0 if none and 1 if present) in the clinical score to a sum between 0 and 7 points. Excluded were infants with skin rash or eruption.
Study procedure
Monitoring of the infants, using the SenTec Digital Monitoring System (SenTec Inc. Therwil, Switzerland), was performed and recorded by a physician or a nurse who was instructed on the methodology of using the device prior to the study. The sensor was warmed to a constant surface temperature of 42 °C to improve local arterialization of the measurement site. The use of lower temperatures (42 °C as opposed to 45–47 °C used before) was shown to decrease dramatically the risk for skin burning in infants [24]. Prior to application of the sensor to the patient, the sensor was prepared and calibrated as per the manufacturer recommendations. The sensor was then applied to the patient’s chest or upper abdomen for 15 min, allowing a correct stabilization of the PtcCO2 values. We evaluated the recordings, and PtcCO2 values obtained under inadequate conditions (not enough time for calibration or when the system alarmed) were excluded from analysis.
After the stabilization period, a VBG was obtained as required by the patient’s clinical condition, while documenting the PtcCO2 value at the same time. Clinical decisions were made only according to the VBG, and PtcCO2 values were recorded for study purpose only. Patients who were hospitalized and needed further blood gases sampling for clinical assessment were either continuously monitored by the PtcCO2 device or reconnected to the device before obtaining a repeat venous sample according to the working load in the pediatric ward.
Statistical analysis
We evaluated the correlation of PtcCO2 and PvCO2 by linear regression analysis and assessed the agreement between these measurements (bias [mean difference] and precision [standard deviation of the differences]) by the Bland-Altman technique [4]. A mean difference <5 mmHg was considered acceptable and >5 mmHg unacceptable as for end-tidal CO2 (ETCO2) values [9, 14, 23]. We evaluated the correlation between the changes in PvCO2, and the simultaneous changes in PtcCO2 for consecutive measurements within each patient by linear regression analysis. We performed multiple linear stepwise regression analysis to assess the correlation of PtcCO2 and clinical disease parameters. Level of significance was set at p < 0.05. Data are presented as mean ± SD or median (range). Statistical analysis was performed using SigmaStat version 2.03 (Chicago, IL) software.
Results
During the study period, a total of 100 PvCO2 and PtcCO2 simultaneous samplings were obtained from 60 patients who presented with viral bronchiolitis. The median number of sampling per patient was 1 (range 1–4). In 28 patients, we performed more than one sampling. The first sample for each patient was obtained in the ER, while the sequential measurements were made in the ER or the pediatric department. Two samples were excluded due to technical problems. One was due to manufacturer warning (inadequate probe adhesion), and in the second case, there was not enough time for stabilization before obtaining the reading. No problems related to skin warming or sensor placement were observed after removing the sensor.
Patient characteristics and clinical conditions at study entry are presented in Tables 1 and 2, respectively.
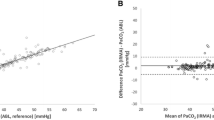
Figure 1 shows the agreement between PvCO2 and PtcCO2 by a Bland-Altman plot. The agreement was adequate, with a mean difference of 1.9 mmHg and a standard deviation of the differences of 7.0 mmHg. Figure 2 shows the positive linear correlation between PvCO2 and PtcCO2. The correlation coefficient was good (r = 0.71, p < 0.001).
We evaluated the correlation of changes in PvCO2 and the simultaneous changes in PtcCO2 for consecutive measurements within each patient (n = 28) and found an adequate correlation (r = 0.41, p < 0.01).
We also found positive linear correlation between PtcCO2 and the disease severity clinical score at study entry (p < 0.001). We tested whether this correlation would remain significant when the two other variables (PvCO2 [positive correlation as discussed above] and weight [found to have negative correlation, i.e., larger weight was associated with lower PtcCO2, p < 0.001]) that correlated with PtcCO2 on univariate analysis were entered into a model of multiple linear stepwise regression. The disease severity clinical score remained independently associated with PtcCO2 (p < 0.05). By replacing the disease severity clinical score with its components in this multiple regression model, we found that the need for oxygen (p < 0.005), heart rate (p < 0.05), and the respiratory rate (p < 0.05) were all correlated with PtcCO2.
Infant weight was found to have a negative correlation with PtcCO2 (p < 0.001) and with PvCO2 (p < 0.001). PtcCO2 and PvCO2 in infants above the median weight (n = 30) in our cohort were significantly lower compared to those of infants below the median weight (n = 29) (36.9 ± 6.0 vs. 49.4 ± 8.8 mmHg and 41.0 ± 4.8 vs. 49.9 ± 8.1 mmHg, respectively; p < 0.001). The mean difference in infants above the median weight compared with infants below the median weight was significantly larger (4.0 vs 0.4 mmHg, p < 0.005), but the standard deviation of the differences was smaller (5.2 vs. 7.7 mmHg). PtcCO2 and PvCO2 correlated in infants above and below the median weight (r = 0.70 and 0.64, respectively; both p < 0.001).
Five infants were transferred to the pediatric intensive care unit. All had relatively high levels of CO2 on admission. One infant diagnosed with atrial septal defect who developed heart failure had PvCO2 of 43 and PtcCO2 of 51 mmHg and was supported with nasal continuous positive airway pressure. One infant had PvCO2 of 44 and PtcCO2 of 45 mmHg, developed pneumothorax, and before transfer, had PvCO2 of 55 and PtcCO2 of 60 mmHg and was supported with high-flow nasal cannula. One infant had PvCO2 of 65 and PtcCO2 of 62 mmHg; one infant had PvCO2 of 51 and PtcCO2 of 54 mmHg; and one infant had PvCO2 of 60 and PtcCO2 of 48 mmHg, and before transfer, had PvCO2 of 70 and PtcCO2 of 62 mmHg and was supported with high-flow nasal cannula. Yet, our study was not powered to evaluate the correlation of specific CO2 levels and outcomes.
Discussion
We found that PtcCO2 was a feasible noninvasive method for estimating PvCO2 in patients with viral bronchiolitis in the ER and the pediatric department. PtcCO2 had a good correlation and an adequate agreement with PvCO2. We also encountered within-patient trending ability. The level of PtcCO2 correlated with the disease severity clinical score, just showing its clinical implication as another estimate for disease severity.
The use of PtcCO2 has already been evaluated in patients hospitalized in Neonatal ICU and PICU [3, 15]. In a relative recent study in the PICU [25], the SenTec device demonstrated a correlation coefficient of 0.93 and a mean difference of 4.3 ± 10.6 mmHg. Wilson et al [27] showed a mean difference of less than 2.0 ± 1.0 mmHg in patients during surgery for repair of cyanotic congenital heart disease. However, there are only a few studies in the settings of ER or in the pediatric department, and there are no previous reports, to our knowledge, in infants with bronchiolitis. In a study from 1986 comparing PaCO2 and PtcCO2 in children with acute asthmatic state who were admitted to the pediatric ward, the PtcCO2 overestimated the arterial carbon dioxide on an average of 3.0 ± 3.4 mmHg, most probably due to metabolic CO2 production in the epidermis cells [26]. Our results are consistent with previous results in other clinical conditions and settings. Although the mean difference of the PtcCO2 was relatively small in our study, the 95 % CIs were relatively wide, as reported in other studies using ETCO2 and PtcCO2 [3, 9, 14, 15, 23, 25, 26]. Thus, PtcCO2 like ETCO2, should not replace blood gas but rather serve as a complementary tool for trending and for real-time continuous assessment of the CO2 levels. We suggest correlating the PtcCO2 and the PVCO2 for monitoring in the individual patient.
While there is no definition for an adequate agreement for PtcCO2, a mean difference of <5 mmHg was considered adequate for ETCO2 [9, 14, 23]. Our results comply with that definition. Thus, as PtcCO2 performed by the medical and nursing teams in the ER and the pediatric department revealed a good correlation and an adequate agreement with PvCO2, it was found to be a feasible and reliable method for noninvasive estimation of PCO2 in bronchiolitis.
In our study, we used PvCO2 and not arterial carbon dioxide (PaCO2) as reported in previous studies [3, 19, 26, 27]. The use of PaCO2 sampling represents the gold standard method for acquiring patients’ acid-base status [28]. Nevertheless, this method is less feasible in the ER and pediatric department as opposed to the intensive care units where infants and children may have an arterial line. The need for repeated sampling is crucial for recognizing deterioration in infants with bronchiolitis. We use venous sampling in our ER and pediatric department because it has less complications associated with arterial sampling, is more convenient, and requires less skill by the medical and nursing teams. A study published in 2004 evaluated the agreement and correlation between capillary, venous, and arterial blood gases and found that the PvCO2 is larger than the PaCO2 by a mean of 5.7 mmHg and that the correlation was excellent (r = 0.97) [28]. This study supports our clinical decision to use PvCO2 as a practical medical tool to follow children with bronchiolitis and scientifically as an optional comparative standard for PtcCO2. In our study, we found that the PtcCO2 was lower than the PvCO2 by a mean difference of 1.9 ± 7.0 mmHg, as opposed to other studies where it was higher than PaCO2 [3, 19, 26, 27]. The use of PvCO2 instead of PaCO2 explains the different direction of the bias.
PtcCO2 might be a better method for non-invasive assessment of CO2 compared with ETCO2 in bronchiolitis because nasal discharge and nasal oxygen flow (especially high-flow nasal cannula), common in bronchiolitis, might interrupt the latter. Yet, only two studies were published on the use of ETCO2 in bronchiolitis [5, 16]. Lashkeri et al. [16] examined ETCO2 by capnometry for admitted and discharged infants and found no correlation between the ETCO2 reading and the infants’ clinical score. Bressan et al [5] monitored infants with bronchiolitis treated with high-flow nasal cannula with ETCO2, but had to shortly disconnect the nasal flow to allow measurement. Both studies did not validate the ETCO2 results with blood gases. Thus, more studies and “head-to-head” comparison of the two methods is required before concluding which method is better for continuous noninvasive CO2 assessment in infants with bronchiolitis.
Continuous respiratory monitoring is important in infants hospitalized with bronchiolitis. Oxygen saturation is already a standard of care, but noninvasive monitoring of CO2 is not performed routinely, and blood gas samples are used in certain infants to help in recognizing infant fatigue and impending respiratory failure [17]. The ability to predict ventilatory deterioration as early as possible is crucial, especially in centers with no PICU, to allow transport at a timely manner to a PICU. The level of CO2 was shown to predict apnea and failure of high-flow nasal cannula and the need for endotracheal ventilation [1, 12, 13]. To our knowledge, no other study assessed the trending of PtcCO2 in bronchiolitis. Wennergren et al [26] demonstrated increasing PtcCO2 with increasing severity of asthma symptoms in infants. We found an adequate correlation within each patient, but not as good as we predicted. This could result from the small number of infants with more than one reading and the small number of readings within each patient. We had only one SenTec Digital Monitoring System used in few children, and the monitoring was not continuous; the monitor was connected just before blood gas sampling in some infants. This could affect the results. Another reason could rely on the fact that the infants admitted were transferred from the ER to the pediatric department and measurements were performed by a different team. On the other hand, a methodological bias in our data collection could result from the fact that measurements were not completely independent in some infants in whom more than one measurement was included. As trending ability may be more important than obtaining a certain PtcCO2 level, more studies evaluating the trending ability of PtcCO2 in bronchiolitis are needed before relying on that method in clinical care.
Infants with bronchiolitis in the ER and pediatric department need mainly supportive care and monitoring for the general and the cardiorespiratory condition. Currently, we use for that purpose medical history, clinical parameters, and blood gases as needed. By using multiple linear stepwise regression model, we tried to determine whether there is a correlation between the clinical parameters and the infant’s ventilation ability, using PtcCO2 measurements. The level of PtcCO2 correlated with disease severity clinical score (p < 0.001), and its individual component: oxygen requirement, heart rate, and respiratory rate. This implies a potential role for PtcCO2 as an assessment tool for disease severity in infants with bronchiolitis.
Infant weight was found to have a negative correlation with PtcCO2 and with PvCO2. Lower levels of PtcCO2 and PvCO2 in infants above the median weight in our cohort compared to the levels in infants below the median weight suggest better ventilation ability in larger infants with bronchiolitis. The mean difference in infants above compared to below the median weight was significantly larger, but the precision was smaller. In both groups, PtcCO2 and PvCO2 correlated. The possibility that a technical error is responsible for the inverse ratio between weight and PtcCO2 is less plausible, but we cannot rule out that the infant weight affected the mean difference. The smaller mean difference in smaller infants makes PtcCO2 suitable for the target population of hospitalized infants with bronchiolitis.
Our study is limited by the relative small number of participants with more than one measurement despite extended study duration of three winter periods. Technical constraints and the heavy workload in the ER and the pediatric department during the bronchiolitis season hampered our ability to obtain high-quality and steady measurements of continuous noninvasive PtcCO2.
We conclude that PtcCO2 is a feasible noninvasive method for estimating PvCO2 in patients with viral bronchiolitis in the ER and pediatric department. It had a good correlation and an adequate agreement with PvCO2 and possible trending ability, and it may serve as an additional tool for disease severity assessment. Because the standard deviation of the differences was relatively high, PtcCO2 should not replace blood gas but rather serve as a complementary tool for trending and for real-time continuous assessment of the CO2 levels. Larger prospective studies are warranted to evaluate the utility of continuous monitoring of PtcCO2 in the settings of the ER and pediatric department and as a predictor of clinical deterioration or improvement in infants with viral bronchiolitis, focusing on continuous monitoring, trending ability, and on the use in infants needing nasal respiratory support that does not allow the use of other noninvasive methods such as ETCO2.
Abbreviations
- ABG:
-
Arterial blood gas
- PtcCO2 :
-
Transcutaneous carbon dioxide
- VBG:
-
Venous blood gas
References
Abboud PA, Roth PJ, Skiles CL, Stolfi A, Rowin ME (2012) Predictors of failure in infants with viral bronchiolitis treated with high-flow, high-humidity nasal cannula therapy*. Pediatr Criti Care Med 13:e343–e349. doi:10.1097/PCC.0b013e31825b546f
Bar A, Srugo I, Amirav I, Tzverling C, Naftali G, Kugelman A (2008) Inhaled furosemide in hospitalized infants with viral bronchiolitis: a randomized, double-blind, placebo-controlled pilot study. Pediatr Pulmonol 43:261–267. doi:10.1002/ppul.20765
Bernet V, Doell C, Cannizzaro V, Ersch J, Frey B, Weiss M (2008) Longtime performance and reliability of two different PtcCO2 and SpO2 sensors in neonates. Pediatr Anesth 18:872–877
Bland MJ, Altman D (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327:307–310
Bressan S, Balzani M, Krauss B, Pettenazzo A, Zanconato S, Baraldi E (2013) High-flow nasal cannula oxygen for bronchiolitis in a pediatric ward: a pilot study. Eur J Pediatr 172:1649–1656. doi:10.1007/s00431-013-2094-4
Carter B, Wiwczarukt D, Hochmann M, Osborne A, Henning R (2001) Performance of transcutaneous PCO2 and pulse oximetry monitors in newborns and infants after cardiac surgery. Anaesth Intens Care 29:260–265
Dullenkopf A, Bernardo SD, Berger F, Fasnacht M, Gerber AC, Weiss M (2003) Evaluation of a new combined SpO2/PtcCO2 sensor in anaesthetized paediatric patients. Pediatr Anesth 13:777–784
Gennis PR, Skovron ML, Aronson ST, Gallagher EJ (1985) The usefulness of peripheral venous blood in estimating acid-base status in acutely III patients. Ann Emerg Med 14:845–849
Hagerty JJ, Kleinman ME, Zurakowski D, Lyons AC, Krauss B (2002) Accuracy of a new low-flow sidestream capnography technology in newborns: a pilot study. J Perinatol 22:219–225. doi:10.1038/sj.jp.7210672
Harrison AM, Lynch JM, Dean JM, Witte MK (1997) Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care unit patients. Crit Care Med 25:1904–1908
Holmgren D, Sixt R (1992) Transcutaneous and arterial blood gas monitoring during acute asthmatic symptoms in older children. Pediat Pulmonol 14:80–84
Kelly GS, Simon HK, Sturm JJ (2013) High-flow nasal cannula use in children with respiratory distress in the emergency department: predicting the need for subsequent intubation. Pediatr Emerg Care 29:888–892. doi:10.1097/PEC.0b013e31829e7f2f
Kneyber MC, Brandenburg AH, de Groot R, Joosten KF, Rothbarth PH, Ott A, Moll HA (1998) Risk factors for respiratory syncytial virus associated apnoea. Eur J Pediatr 157:331–335
Kugelman A, Zeiger-Aginsky D, Bader D, Shoris I, Riskin A (2008) A novel method of distal end-tidal CO2 capnography in intubated infants: comparison with arterial CO2 and with proximal mainstream end-tidal CO2. Pediatrics 122:e1219–e1224. doi:10.1542/peds.2008-1300
Lacerenza S, De Carolis MP, Fusco FP, La Torre G, Chiaradia G, Romagnoli C (2008) An evaluation of a new combined Spo2/PtcCO2 sensor in very low birth weight infants. Anesth Analg 107:125–129. doi:10.1213/ane.0b013e3181733e47
Lashkeri T, Howell JM, Place R (2012) Capnometry as a predictor of admission in bronchiolitis. Pediatr Emerg Care 28:895–897
Lazner MR, Basu AP, Klonin H (2012) Non-invasive ventilation for severe bronchiolitis: analysis and evidence. Pediatr Pulmonol 47:909–916. doi:10.1002/ppul.22513
Lowell DI, Lister G, Von Koss H, McCarthy P (1987) Wheezing in infants: the response to epinephrine. Pediatrics 79:939–945
Marsden D, Chiu M, Paky F, Helms P (1985) Transcutaneous oxygen and carbon dioxide monitoring in intensive care. Arch Dis Child 60:1158–1161
Mayfield S, Bogossian F, O’Malley L, Schibler A (2014) High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health 50:373–378. doi:10.1111/jpc.12509
Paiva R, Krivec U, Aubertin G, Cohen E, Clément A, Fauroux B (2009) Carbon dioxide monitoring during long-term noninvasive respiratory support in children. Intens Care Med 35:1068–1074
Palmisano BW, Severinghaus JW (1990) Transcutaneous PCO2 and PO2: a multicenter study of accuracy. J Clin Monit 6:189–195
Rozycki HJ, Sysyn GD, Marshall MK, Malloy R, Wiswell TE (1998) Mainstream end-tidal carbon dioxide monitoring in the neonatal intensive care unit. Pediatrics 101:648–653
Storre JH, Steurer B, Kabitz H-J, Dreher M, Windisch W (2007) Transcutaneous PCO2 monitoring during initiation of noninvasive ventilation. CHEST 132:1810–1816
Urbano J, Cruzado V, Lopez-Herce J, del Castillo J, Bellon JM, Carrillo A (2010) Accuracy of three transcutaneous carbon dioxide monitors in critically ill children. Pediatr Pulmonol 45:481–486. doi:10.1002/ppul.21203
Wennergren G, Engström I, Bjure J (1986) Transcutaneous oxygen and carbon dioxide levels and a clinical symptom scale for monitoring the acute asthmatic state in infants and young children. Acta Paediatr 75:465–469
Wilson J, Russo P, Russo J, Tobias JD (2005) Noninvasive monitoring of carbon dioxide in infants and children with congenital heart disease: end-tidal versus transcutaneous techniques. J Intens Care Med 20:291–295
Yıldızdaş D, Yapıcıoğlu H, Yılmaz H, Sertdemir Y (2004) Correlation of simultaneously obtained capillary, venous, and arterial blood gases of patients in a paediatric intensive care unit. Arch Dis Child 89:176–180
Acknowledgments
Funding source
No external funding was secured for this study.
Financial disclosure
SenTec Inc. supported the study by supplying a SenTec monitor and supplies for the study.
Conflict of interest
We have no conflicts of interest to disclose, and we had no financial relationship with the organization that sponsored the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Peter de Winter
Rights and permissions
About this article
Cite this article
Gal, S., Riskin, A., Chistyakov, I. et al. Transcutaneous PCO2 monitoring in infants hospitalized with viral bronchiolitis. Eur J Pediatr 174, 319–324 (2015). https://doi.org/10.1007/s00431-014-2407-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-014-2407-2