Abstract
The objective of this study was to evaluate the correlation and agreement between transcutaneous and serum bilirubin among preterm low-birth-weight neonates. Neonates born at <35 weeks of gestation with birth weight <2,000 g were enrolled prospectively. Transcutaneous bilirubin (TcB) was measured at forehead, sternum, and abdomen at 24 ± 6 and 72 ± 12 h after birth and when icterus involved arms or legs (Kramer zone 4–5). Serum total bilirubin (STB) was measured by microbilimeter (STB-M) at all these time-points and by high-performance liquid chromatography (STB-H) at one randomly chosen time-point. A total of 1,619 observations were made in 256 neonates (median gestation, 34 weeks (IQR, 32–35), birth weight 1,522 ± 288 g). Overall there was excellent correlation and agreement between TcB and STB-M with TcB on forehead being most accurate (r = 0.84, mean difference, 0.3 ± 1.9 mg/dL) followed by TcB on abdomen (r = 0.73, mean difference, 1.5 ± 2.6 mg/dL) and sternum (r = 0.72, mean difference, 1.5 ± 2.6 mg/dL). TcB performed well at all three points of measurement with best correlations being observed at icterus level 4/5. Correlation between TcB and STB-H measured by high-performance liquid chromatography was less strong but significant (r = 0.59 to 0.69 at different time points of measurement). Conclusions: TcB has good correlation and agreement with STB in preterm low-birth-weight neonates born at ≥28 weeks of gestation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperbilirubinemia needing therapeutic intervention is one of the most common morbidities in preterm neonates [7, 22]. Both developmental immaturity and management practices play a role in causing increased incidence and severity of hyperbilirubinemia. Preterm neonates are also at higher risk of bilirubin-induced brain damage because of lower serum albumin concentration with weaker bilirubin binding sites and more permeable blood–brain barrier [6]. This necessitates periodic monitoring of severity of hyperbilirubinemia by measurement of serum bilirubin. Serum total bilirubin (STB) measurement, although an objective method, is painful, time-consuming, and needs expertise. In addition, significant inter-laboratory and intra-laboratory variability has been reported with measurement of STB [14, 31]. Measurement of transcutaneous bilirubin (TcB) concentration has been recommended for initial assessment of jaundice in term neonates [15, 16]. Due to excellent correlation and agreement between TcB and STB in neonates born at term gestation, TcB measurement-based screening strategy has been associated with reduction in number of invasive blood tests to measure STB and decreased health costs [20]. Although there is some published data on role of TcB measurement in preterm neonates, studies conducted have been limited to white or East Asian neonates and the results have been inconsistent [2–5, 8, 11, 12, 17, 21, 24, 26, 28, 29, 32]. Accuracy of multi-wavelength transcutaneous bilirubinometer has not been proven in Indian neonates who may have darker skin color and higher rate of rise of bilirubin during first week of life [1, 23]. In addition only a few studies have included preterm, very low-birth-weight infants [12, 21, 27, 32]. Effects of postnatal age at measurement of TcB, different sites of measurement, and birth weight on correlation between STB and TcB has also not been reported. Main objective of this study was to evaluate correlation and agreement between TcB and STB among preterm low-birth-weight Indian neonates. In addition, we planned to examine the effect of postnatal age at measurement of TcB, different sites of TcB measurement, and birth weight on correlation between STB and TcB.
Material and methods
This prospective observational study was conducted in a tertiary care teaching hospital of north India from December 2009 to June 2011. The study protocol was cleared by ethics Committee of the hospital and written informed consent was obtained from parents of enrolled infants.
Inclusion and exclusion criteria
Neonates born at less than 35 weeks of gestation and weighing <2,000 g were eligible for enrolment. Neonates with major congenital malformation, gestation <28 completed weeks or birth weight <750 g were excluded. We planned to enroll 100 neonates each in following two groups: gestation <32 weeks with birth weight <1,500 g (VLBW group) and gestation >32 weeks or birth weight 1,500–1,999 g (LBW group). We did not include preterm neonates born at <28 weeks of gestation in the study because this is a distinct group of extremely preterm neonates and we did not expect to be able to enroll sufficient number of these neonates to have a meaningful set of observations.
Intervention and measurement of outcomes
In each enrolled neonate TcB was measured at 24 ± 6 and 72 ± 12 h of postnatal age and when icterus involved arms or legs (Kramer zone 4 or 5 corresponding to bilirubin levels of >12 mg/dL) [13]. Visual assessment of jaundice was done by clinical care team consisting of pediatricians. Rationale behind choosing these different time-points was to ensure inclusion of wide range of serum bilirubin levels in the study. Phototherapy was started when bilirubin reached about 1 % of birth weight [18]. Bilirubin measurements after starting phototherapy were excluded as bleaching effect of phototherapy may interfere with correlation between TcB and STB [30].
TcB was measured with a multi-wavelength transcutaneous bilirubinometer (BiliChek®, coefficient of variation <5 %). The transcutaneous bilirubinometer was placed against the forehead, sternum, and abdomen of the infant in supine position and average of five readings at each of these three sites was recorded. STB was measured by a bedside spectrophotometric microbilimeter (NEO-BIL plus®, das srl, Italy) and by high-performance liquid chromatography (HPLC). STB estimation by spectrophotometry was done for all three TcB measurements. Peripheral venous blood was collected within 30 min of TcB measurement in two pre-heparinized capillaries and serum was separated by centrifugation. Average of the readings in two capillaries was obtained. Internal calibration of spectrophotometer was done daily. External calibration was done at start of the study and then at three monthly interval as per recommendation of the manufacturer.
High-performance liquid chromatography
STB estimation by HPLC was done for one of the three planned TcB measurements in each neonate. TcB measurement in a neonate for which STB was to be measured by HPLC was chosen by simple random selection method. Random number sequence was generated by computer program and sequence was placed in serially number opaque sealed envelopes to be opened at the time of enrolment. A 100-μL aliquot of serum sample was frozen at less than −20 °C for HPLC. Serum bilirubin level was quantified by modified method of Zhang following reverse-phase HPLC procedure [33]. The HPLC technician was blinded to the results of the laboratory STB and the TcB measurements.
Statistical analysis
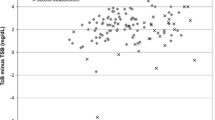
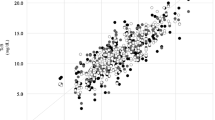
Data was entered in a Microsoft Access database and analyzed using Stata 11.1 software. Agreement between STB and TcB is presented as median difference between STB and TcB and its interquartile range in subsequent tables and as Bland–Altmann plots in figures. Bland–Altmann plots depict difference between STB (by microbilimeter or HPLC) and STB on Y-axis against mean of STB and TcB on X-axis. Distribution of points representing difference between STB and TcB against the line of zero difference visually represents the extent of agreement between STB and STB. Pair-wise correlation between STB and TcB is represented as correlation coefficient and its P value.
Results
During the study period (from 1st December 2009 to 31st December 2011) a total of 7,780 neonates (mean birth weight: 2,600 ± 588 g; median gestation: 38 completed weeks; interquartile range, 37–39) were born alive in the hospital. Consecutively, born neonates fulfilling inclusion criteria were enrolled in the study. In VLBW group, among 207 neonates born between first and last study enrolment 116 were enrolled (Fig. 1). In LBW group among 210 neonates born between first and last study enrolment 140 were enrolled.
Neonates enrolled in the study were born at a median of 34 completed weeks of gestation (interquartile range, 32–35) with a birth weight of 1,522 ± 288 g (Table 1). As per protocol, neonates were randomized at enrolment to STB estimation by HPLC at 24, 72, or at Jaundice in Kramer zone 4/5. By randomization, 87 (34 %) neonates were assigned to STB estimation by HPLC at 24 h, 84 (33 %) were assigned to HPLC measurement at 72 h and 85 (34 %) were assigned to HPLC measurement at Jaundice zone 4/5.
STB measurement by microbilimeter was done in all neonates at all the three time-points. A total of 1,619 observations were made in 256 neonates. Overall there was excellent correlation and agreement between TcB and STB-M (Figs. 2 and 3) with TcB on forehead being most accurate (r = 0.84, mean difference, 0.3 ± 1.9 mg/dL) followed by TcB on abdomen (r = 0.73, mean difference, 1.5 ± 2.6 mg/dL) and sternum (r = 0.72, mean difference, 1.5 ± 2.6 mg/dL). TcB performed well at all three points of measurement with best correlations being observed at icterus level 4/5. Correlation between TcB and STB-H measured by HPLC was less strong but significant (r = 0.59 to 0.69 at different time points of measurement).
TcB performed well for both LBW (birth weight ≥1,500) and VLBW (birth weight <1,500 g) neonates although values of correlation coefficient were lower in the latter group (TcB on forehead, abdomen, and sternum, 0.86 vs. 0.78, 0.81 vs. 0.57, and 0.82 vs. 0.64).
Measurement at 24 h
At 24 h, most of neonates (86.7 %) had no visible yellow staining of skin. Measurement of STB by microbilimeter (STB-M) was done for all neonates at 24 ± 6 h of age. Transcutaneous bilimeter was unable to measure TcB in two neonates on forehead and in four neonates on sternum and abdomen each. STB by HPLC (STB-H) was measured in 81 neonates. Based on STB-M measurement at this time-point, phototherapy was started in 29 (11.3 %) neonates. Mean TcB on forehead, sternum, and abdomen was 7.4 ± 2.1, 8.2 ± 2.6, and 8.8 ± 2.8 mg/dL, respectively (Table 2). Mean STB-M was 7.1 ± 1.9 mg/dL and mean STB-H was 7.5 ± 2.7 mg/dL. Closest agreement and correlation between TcB and STB was observed for measurements obtained from forehead (Table 3).
Measurement at 72 h
At 72 h, most of neonates (93.8 %) had some degree of jaundice. Measurement of STB by microbilimeter was done for 225 neonates at 72 ± 12 h of age. Transcutaneous bilimeter was unable to measure TcB in two neonates on forehead and in eight neonates on sternum and abdomen each. STB by HPLC was measured in 90 neonates. Based on STB-M measurement at this time-point, phototherapy was started in 93 (41.3 %) neonates. Mean TcB on forehead, sternum, and abdomen was 11.3 ± 3.3, 12.2 ± 4.0, and 12.2 ± 3.8 mg/dL, respectively (Table 2). Mean STB-M was 10.9 ± 2.7 mg/dL and mean STB-H was 11.5 ± 2.9 mg/dL. Closest agreement between TcB and STB-M was observed for measurements obtained from forehead (Table 3).
Measurement at Jaundice in Kramer zone 4/5
Among 225 neonates who underwent study measurement at 72 h, 93 were started on phototherapy based on results of STB measurement at that time. Sixty-one neonates either died or never developed jaundice extending to zone 4/5 and therefore did not need this measurement. The remaining 71 neonates underwent TcB measurement. Measurement of STB by microbilimeter was done for 71 neonates at mean age of 121.5 ± 29.8 h. Transcutaneous bilimeter was unable to measure TcB in one neonate on sternum and four neonates on abdomen. STB by HPLC was measured in 19 neonates. Based on STB-M measurement at this time-point, phototherapy was started in 29 (40.9 %) neonates. Mean TcB on forehead, sternum, and abdomen was 12.0 ± 3.5, 12.2 ± 4.8, and 12.5 ± 3.9 mg/dL, respectively. Mean STB-M was 12.0 ± 3.0 mg/dL and mean STB-H was 12.1 ± 3.4 mg/dL. Closest agreement and correlation between TcB and STB-M again observed for measurements obtained from forehead (Table 3).
Discussion
In this study, we evaluated accuracy of TcB in preterm low-birth-weight neonates. TcB values were obtained at three time-points (at 24 h, at 72 h, and at jaundice in Kramer zone 4/5) and at three different sites (forehead, sternum, and abdomen). Good correlation and agreement were observed between TcB and STB at all the three time points. TcB values at forehead were most accurate.
As visual assessment of severity of jaundice can be erroneous, TcB measurement has been recommended as a screening tool to assess severity of neonatal hyperbilirubinemia [15, 16]. Accuracy of TcB obtained with multi-wavelength transcutaneous bilimeter has been well proven in neonates born at 35 or more weeks of gestation [10, 19]. However, studies investigating accuracy of TcB in preterm neonates have reported inconsistent results among neonates born at <28 weeks of gestation. Willems et al. reported that BiliCheck was as reliable in very preterm infants of gestational age less than 30 weeks [32]. Schmidt et al. reported excellent correlation between TcB and STB in preterm neonates born at 24–34 weeks of gestation [26]. However, TcB underestimated STB by 2–3 mg/dL. Ho et al. recommended that BiliCheck is a useful screening tool for neonatal jaundice only in the neonates with gestation above 32 weeks [8]. Namba and Kitajima measured TcB over forehead in preterm and VLBW infants and concluded that the TcB measurements were safe and accurate in these infants [21]. However, they reported a low reliability in neonates whose birth weights were lower than 1,000 g or whose gestational ages were shorter than 28 weeks. Rubaltelli et al. recommended BiliCheck as a reliable substitute of STB determination in newborns more than 30 weeks of gestation [24]. Knupfer et al. in their study concluded that reliable TcB values are obtained only in newborns older than 30 weeks of gestation [12]. In present study, good correlation and agreement between TcB and STB has been observed. We could not find any consistent difference in correlation or agreement between two birth weight groups (1,500–2,000 g and <1,500 g) enrolled in the present study. As a result TcB measurement may be used for initial screening of hyperbilirubinemia in preterm neonates. However, bilirubin values near treatment threshold need to be confirmed by STB measurement.
Use of TcB measurement has been shown to decrease need of blood sampling in term and late preterm neonates. Mishra et al. reported 34 % lower need of blood sampling with TcB-based screening strategy as compared to protocol-based visual assessment in neonates born at 35 or more weeks of gestation [20]. Willems et al. measured TcB and STB in preterm neonates born at less than 30 weeks of gestation and developed a model to estimate the possible reduction in need of blood sampling. In absence of skin edema or hypoperfusion, TcB measurement had potential to reduce blood sampling by up to 40 % [32]. In our study, phototherapy was started in 10 % neonates who underwent STB estimation at 24 h of age and in about 40 % neonates who underwent STB estimation at 72 h of age or at icterus level 4/5. Based on these figures, we speculate that TcB measurement can reduce need of blood sampling in moderately preterm neonates also.
Forehead is the most commonly recommended site for TcB measurement. However, performance of transcutaneous bilimeter in other body sites has not been assessed conclusively in preterm neonates. Sajjadian et al. measured TcB in preterm neonates and observed that correlation between STB and TcB was better at forehead than at sternum [25]. On the other hand, Stillova et al. reported better correlation between TcB and STB at sternum [27]. However, bilirubin was measured only at the time of clinical indication in a small group of preterm neonates. Similarly, Holland et al. reported that TcB measured at forehead is more likely to underestimate the STB concentration, whereas the TcB measured at sternum is more likely to overestimate the STB concentration [9]. In the present study, TcB at all the sites correlated well with the STB. However, TcB measured at forehead was closest to STB.
Effect of postnatal age on correlation between TcB and STB is difficult to separate from effect of increase in bilirubin concentration. In the present study, value of correlation coefficient is higher for measurements obtained at icterus level 4/5 or at 72 h of postnatal age than the value at 24 h of age. In a previous study among term and late preterm neonates TcB has been shown to perform best for STB values around 7–10 mg/dL [19]. As treatment is started in preterm neonates at values beyond 12–14 mg/dL, decreased performance of TcB with rising STB may not be reflected in studies enrolling only preterm neonates.
Strengths of this study include large sample size (1,619 TcB measurements in 256 neonates), prospective design, and assessment of accuracy of TcB against gold standard measurement of bilirubin by high-performance liquid chromatography. We also assessed the accuracy of TcB at three different sites of which presently recommended site of forehead was observed to be most accurate. We sampled each neonate at three different time-points to assess accuracy of TcB at different levels of STB and different intensities of clinical jaundice. Due to large number of TcB measurements, STB estimation by the gold standard method of HPLC was not done for all assessments, but by choosing one of the three time-points using a random number table. Smaller number of observations of STB with HPLC may also be the reason behind observing weaker correlation coefficients between TcB and STB by HPLC as compared to between TcB and STB by microbilimeter.
To conclude, this study demonstrates good correlation and agreement between TcB and STB in preterm neonates born at ≥28 weeks of gestation. Further studies are needed to evaluate role of TcB measurement for initial screening of hyperbilirubinemia in preterm neonates.
Abbreviations
- HPLC:
-
High-performance liquid chromatography
- LBW:
-
Low birth weight
- STB:
-
Serum total bilirubin
- STB-M:
-
Serum total bilirubin measured by microbilimeter
- STB-H:
-
Serum total bilirubin measured by high-performance liquid chromatography
- TcB:
-
Transcutaneous bilirubin
- VLBW:
-
Very low birth weight
References
Agarwal R, Kaushal M, Aggarwal R, Paul VK, Deorari AK (2002) Early neonatal hyperbilirubinemia using first day serum bilirubin level. Indian Pediatr 39(8):724–730
Amato M (1994) Transcutaneous, capillary, and arterial bilirubin levels. J Pediatr 125(2):332
Amato M, Huppi P, Markus D (1990) Assessment of neonatal jaundice in low birth weight infants comparing transcutaneous, capillary and arterial bilirubin levels. Eur J Pediatr 150(1):59–61
De Luca D, Zecca E, de Turris P, Barbato G, Marras M, Romagnoli C (2007) Using BiliCheck for preterm neonates in a sub-intensive unit: diagnostic usefulness and suitability. Early Hum Dev 83(5):313–317
Donzelli G, Pratesi S (2000) Transcutaneous bilirubinometry in healthy preterm newborns. Clin Biochem 33(6):505–508
Ebbesen F, Brodersen R (1982) Risk of bilirubin acid precipitation in preterm infants with respiratory distress syndrome: considerations of blood/brain bilirubin transfer equilibrium. Early Hum Dev 6(4):341–355
Hack M, Horbar JD, Malloy MH, Tyson JE, Wright E, Wright L (1991) Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Network. Pediatrics 87(5):587–597
Ho EY, Lee SY, Chow CB, Chung JW (2006) BiliCheck transcutaneous bilirubinometer: a screening tool for neonatal jaundice in the Chinese population. Hong Kong Med J 12(2):99–102
Holland L, Blick K (2009) Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol 132(4):555–561
Ip S, Chung M, Kulig J, O’Brien R, Sege R, Glicken S, Maisels MJ, Lau J (2004) An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics 114(1):e130–e153
Karolyi L, Pohlandt F, Muche R, Franz AR, Mihatsch WA (2004) Transcutaneous bilirubinometry in very low birthweight infants. Acta Paediatr 93(7):941–944
Knupfer M, Pulzer F, Braun L, Heilmann A, Robel-Tillig E, Vogtmann C (2001) Transcutaneous bilirubinometry in preterm infants. Acta Paediatr 90(8):899–903
Kramer LI (1969) Advancement of dermal icterus in the jaundiced newborn. Am J Dis Child 118(3):454–458
Lo SF, Doumas BT (2011) The status of bilirubin measurements in U.S. laboratories: why is accuracy elusive? Semin Perinatol 35(3):141–147
Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF (2009) Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: an update with clarifications. Pediatrics 124(4):1193–1198
Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation (2004). Pediatrics 114 (1):297–316
Mercanti I, Michel F, Thomachot L, Loundou DA, Nicaise C, Vialet R, Di Marco JN, Lagier P, Martin C (2007) Transcutaneous bilirubin measurement in preterm infants. Arch Pediatr 14(7):875–880
Mishra S, Agarwal R, Deorari AK, Paul VK (2008) Jaundice in the newborns. Indian J Pediatr 75(2):157–163
Mishra S, Chawla D, Agarwal R, Deorari AK, Paul VK (2010) Transcutaneous bilirubin levels in healthy term and late preterm Indian neonates. Indian J Pediatr 77(1):45–50
Mishra S, Chawla D, Agarwal R, Deorari AK, Paul VK, Bhutani VK (2009) Transcutaneous bilirubinometry reduces the need for blood sampling in neonates with visible jaundice. Acta Paediatr 98(12):1916–1919
Namba F, Kitajima H (2007) Utility of a new transcutaneous jaundice device with two optical paths in premature infants. Pediatr Int 49(4):497–501
Narang A, Kumar P, Kumar R (2001) Neonatal jaundice in very low birth weight babies. Indian J Pediatr 68(4):307–309
Pathak U, Chawla D, Kaur S, Jain S (2013) Bilirubin nomogram for prediction of significant hyperbilirubinemia in north Indian neonates. Indian Pediatr 50(4):383–389
Rubaltelli FF, Gourley GR, Loskamp N, Modi N, Roth-Kleiner M, Sender A, Vert P (2001) Transcutaneous bilirubin measurement: a multicenter evaluation of a new device. Pediatrics 107(6):1264–1271
Sajjadian N, Shajari H, Saalehi Z, Esphahani F, Alizadeh Taheri P (2012) Transcutaneous bilirubin measurement in preterm neonates. Acta Med Iran 50(11):765–770
Schmidt ET, Wheeler CA, Jackson GL, Engle WD (2009) Evaluation of transcutaneous bilirubinometry in preterm neonates. J Perinatol 29(8):564–569
Stillova L, Matasova K, Mikitova T, Stilla J, Kolarovszka H, Zibolen M (2007) Evaluation of transcutaneous bilirubinometry in preterm infants of gestational age 32–34 weeks. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 151(2):267–271
Stillova L, Matasova K, Zibolen M, Stilla J, Kolarovszka H (2009) Transcutaneous bilirubinometry in preterm neonates. Indian Pediatr 46(5):405–408
Szabo P, Wolf M, Bucher HU, Haensse D, Fauchere JC, Arlettaz R (2004) Assessment of jaundice in preterm neonates: comparison between clinical assessment, two transcutaneous bilirubinometers and serum bilirubin values. Acta Paediatr 93(11):1491–1495
Tan KL, Dong F (2003) Transcutaneous bilirubinometry during and after phototherapy. Acta Paediatr 92(3):327–331
Vreman HJ, Verter J, Oh W, Fanaroff AA, Wright LL, Lemons JA, Shankaran S, Tyson JE, Korones SB, Bauer CR, Stoll BJ, Papile LA, Donovan EF, Ehrenkranz RA, Stevenson DK (1996) Interlaboratory variability of bilirubin measurements. Clin Chem 42(6 Pt 1):869–873
Willems WA, van den Berg LM, de Wit H, Molendijk A (2004) Transcutaneous bilirubinometry with the Bilicheck in very premature newborns. J Matern Fetal Neonatal Med 16(4):209–214
Zhang J (1992) Analysis of unconjugated bilirubin in serum by reversed-phase high performance liquid chromatography. Scand J Clin Lab Invest 52(6):565–569
Funding source
Study was funded by extramural research grant from Indian Council of Medical Research (IRIS ID, 2008–02250)
Financial disclosure
None of the authors have any financial relationships relevant to this article to disclose.
Conflict of interest
The authors declare that they have no conflict of interest. None of the authors have any financial relationship with the organization that sponsored the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chawla, D., Jain, S., Kaur, G. et al. Accuracy of transcutaneous bilirubin measurement in preterm low-birth-weight neonates. Eur J Pediatr 173, 173–179 (2014). https://doi.org/10.1007/s00431-013-2142-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-013-2142-0