Abstract
Objective
To evaluate the correlation between total serum and transcutaneous bilirubin and to determine the reliability of transcutaneous bilirubinometry for screening and monitoring of neonatal jaundice among preterms.
Study Design
Ninety nine infants with gestational ages ≤34 weeks were prospectively enrolled. Babies were classified into three groups as; 24–28, 29–31, and 32–34 weeks. Total serum bilirubin and simultaneous transcutaneous bilirubin were measured before the onset of phototheraphy, during and at 24 h after discontinuing phototherapy.
Results
Total serum bilirubin significantly correlated with transcutaneous bilirubin in the whole cohort (r = 0.867, p < 0.001) and in each group before, during and after phototheraphy. Hypotension was the only variable which effects the difference between two methods at postnatal first day of life (p = 0.039).
Conclusion
Transcutaneous bilirubin levels were highly correlated with total serum bilirubin levels even in 24–28 GW babies. Transcutaneous bilirubin may be useful for screening and monitoring of jaundice in very preterm newborns.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Neonatal jaundice is a frequent problem for neonates especially for preterm babies. Approximately 80% of term and preterm babies develop jaundice in the first week of life [1, 2]. Therefore accurate determination of bilirubin levels and evaluation of its severity is an important aspect of screening and management. The National Institute for Health and Clinical Excellence (NICE) neonatal jaundice guidelines [1] recommend checking bilirubin level in all infants with visible jaundice. Although the serum bilirubin measurement remains the gold standard, heel stick or venous blood drawn for sampling are painful and time consuming procedures for particularly preterm infants. Invasive procedures also increase the risk of infection and anemia which are important morbidities for preterm infants.
Transcutaneous bilirubin (TcB) measurement is painless and easy to perform as an alternative method [3,4,5]. Routine use of TcB measurement for neonatal jaundice screening among babies >35 weeks and after postnatal 24-h was recommended, both before hospital discharge and for determination of the need for phototherapy [1, 6]. Several studies have documented that it has a good correlation and reliability also in preterm infants [4, 7, 8]. Before phototherapy, although there is a good association between serum bilirubin levels, following the initiation of phototherapy, it is reported that the correlation between TcB and total serum bilirubin (TsB) measurements may adversely get affected. This may be due to the rapid decrease of dermal bilirubin via photoisomerization in dermal interstitial spaces and subcutaneous capillaries. Because of dermal bilirubin kinetics during phototherapy, the validity and the reliability of TcB has been questioned during and after phototherapy [9,10,11,12,13,14].
There is limited data and no clear recommendations about the use of transcutaneous bilirubinometer in preterm infants undergoing phototheraphy. The results of the studies are inconsistent [14,15,16]. On the other hand, hence the preterm neonates have risk factors predisposing to neurotoxicity at lower bilirubin levels, frequent monitoring of those infants during phototheraphy is definitely important. Currently, TcB assessment is widely used only before initiating phototherapy.
In this prospective observational study, we assessed TcB measurements concurrently with TSB values in venous samples drawn at various time points before, during and after phototherapy. We aimed to detect the correlation between TsB and TcB levels among preterms less than 32 GWs in order to determine the reliability of transcutaneous bilirubinometry for screening and monitoring of neonatal jaundice in preterm babies.
Methods
Study population and data collection
This study was conducted in the neonatal intensive care unit (NICU) of Zeynep Kamil Maternity and Children Training and Research Hospital, a tertiary referral center. Ninety nine infants with gestational ages ≤34 weeks were prospectively enrolled in the study. Gestational age was determined by the first day of the last menstruel period, or prenatal ultrasonography performed at 17–18 weeks. Neonates were excluded if they were diagnosed with chromosomal or congenital malformation, direct hyperbilirubinemia, sepsis, and TORCH infection or previously exposed to phototherapy.
To evaluate the correlation between TcB and TsB in babies with different gestational ages, we classified them into 3 groups as; 24–28 weeks (group I), 29–31 weeks (Group II) and 32–34 weeks (Group III), respectively. Neonatal characteristics were collected (GA, gender, mode of delivery, need for inotropes and antenatal steroid use, presence of IUGR and abnormal Doppler findings.
All of the infants constituting the study group were in postnatal 48 h. The decision for commencing phototherapy treatment and follow up was made by the attending neonatologist according to National Institue for Health and Clinical Excellence (NICE) TsB nomograms [1]. Patients were treated with continuous phototherapy by standard LED phototherapy unit. Infants’ eyes were routinely covered with opaque eye patches made of carbon and silk. The pads were 4 cm wide and 25 cm long and attached by micropore tape to the side of the head. The position of the eye patches were routinely checked at 2–3 h intervals during feedings.
TcB and TsB measurements
Transcutaneous bilirubin and simultaneous TsB levels were measured before the onset of phototheraphy. During phototheraphy, serum bilirubin measurements were repeated for every 24 h until phototherapy has terminated. Serum bilirubin levels were determined for rebound hyperbilirubinemia 24 h after discontinuing phototherapy. Transcutaneous measurements were carried out within 5 min of blood sampling for TsB by two neonatologists (ST and DA). A single JM-103 transcutaneous bilirubinometer (Draeger Air-Shields, Telford, PA, USA) was used. The device was used according to manufacturer’s recommendations. Quality control measures were performed daily. TcB were measured on glabellar skin protected from phototherapy lights by opaque eye patch. TcB levels before and after phototherapy were measured on glabellar area as well. The device-calculated mean of three measurements was recorded. TsB measurements were performed with in the clinical chemistry laboratory. This laboratory utilizes diazo method (Architect c4100, Abbott). Local ethics committee approved the study. Written informed consent were obtained from parents of all infants included in the study.
Statistical analysis
Descriptive statistics were generated for entire group. The results are presented as the means ± SD. The correlation between TcB and TsB was assessed with Pearson’s correlation coefficients calculated in the overall populations. Statistical significance is implied by a p-value of less than 0.05. Bland–Altman analysis was used to show the differences against the mean of the two compared methods. The risk estimation and confidence intervals for each cut off level were analyzed. Mann Whitney U analysis was carried out to asses the relationship between the absolute difference TsB and TcB values and the categorical variables, such as; birth weight, gender, delivery route, need for ventilatory support and inotropes, presence of reversal of blood flow in antenatal Doppler ultrasonography, antenatal steroid use, PPROM and IUGR. Power analysis revealed that in order for an effect of this size to be detected (80%) as significant at the 5% level, a sample of 417 pairs of measurements would be required.
Results
Baseline data
Overall, 417 pairs of measurements (TcB and TsB) were performed on 99 eligible infants. Of these, 99 (%23.7), 240 (%57.5) and 78 (%18.7) measurements were before, during and after phototheraphy (rebound), respectively. The infants ranged in age between 1 to 6 d of life. Mean birth weight was 1301 ± 402.8 g and mean gestational age was 30.0 ± 2.64 weeks. None of the infants required exchange transfusion. Demographic characteristics of study group is shown in Table 1.
Correlation between TcB and TsB
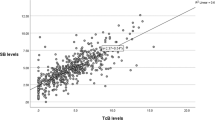
TsB values ranged from 1.5 to 13.4 mg/dL compared with 0.9 to 13.6 mg/dL for TcB values. Mean TcB levels were significantly higher than TsB levels (6.83 ± 2.13 vs 7.08 ± 2.35 mg/dL, p < 0.001). Mean difference of the pairs (TsB-TcB) was −0.24 ± 1.17 mg/dL. The correlation coefficients did not differ in the presence or absence of phototheraphy. TcB levels were significantly correlated with TsB in the whole cohort (r = 0.867, p < 0.001) (Fig. 1a).
TcB levels significantly correlated with TsB levels in all groups before, during and after phototheraphy. The r-values for TcB versus TsB in Groups I,II and III before, during and after phototheraphy are shown in Table 2. The data classified according to gestational ages is shown as a scatterplot in Fig. 1b–d.
The correlation between TcB and TSB values in Group I,II and III before, after and for each day of PT are shown in Fig. 2. The correlation coefficients between TcB and TSB values in Group II and III is found to be slightly higher than Group I.
Diagnostic Value of TcB
Data for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+) and negative likelihood ratio (LR-) before, during and after phototherapy for the diagnosis of hyperbilirubinemia requiring phototherapy are shown in Table 3.
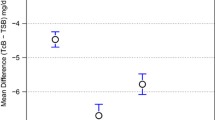
The Bland Altman plot, the mean and standart deviation (SD) for TsB-TcB before phototheraphy, during each individual phototheraphy day and after phototheraphy is shown in Table 4. The mean ± SD shows the tendency for TcB to overestimate the bilirubin values compared with TsB which is assumed as gold standard.
The risk estimation and confidence intervals for each cut off level are analyzed and shown in Table 5.
TsB, TcB and other variables
There was no significant relationship between gestational age, gender, birth weight, delivery route, presence of antenatal steroid use, abnormal Doppler findings, PPROM and IUGR and the absolute difference between TsB and TcB values. While hypotension was the only variable which effects the difference between TsB and TcB values at postnatal first day of life (p = 0.039), after the second day of life hypotension had no significant influence on discordance between two methods (p > 0.05).
Discussion
In this study, we showed that TcB levels highly correlated with TSB among very preterm newborns before,during and after PT. As the study group was classified according to gestational age, a statistically significant correlation was found among three groups even in 24–28 GW babies.
Although the reliability and validity of TcB in term infants has been shown in many studies and its use is recommended for screening [5, 8, 9], there are a few studies evaluating diagnostic accuracy of TcB for very preterm infants. Nagar et al. [16], showed that the correlation coefficient between TcB and TsB in infants less than 32 gestational week is similar to near term and term populations (r': 0.89 p < 0.001). They concluded that the transcutaneous bilirubinometry devices reliably estimated bilirubin levels in preterm infants and could be used in clinical practice to reduce blood sampling. A recent paper evaluating TcB accuracy for infants <30 weeks of gestational age reported a correlation coefficient of 0.81, and suggested that it is useful for preterm infants [17]. In our study, correlation coefficient was found to be significant for the entire cohort (r = 0.867, p < 0.001).
There are some studies evaluating the accuracy and safety of transcutaneous bilirubinometry in preterm infants with different gestational ages. Knüpfer et al, concluded that the correlation between TsB and TcB is stronger for infants >30 weeks of gestational age without phototheraphy and had no respiratory disorders. Mean values of TcB were found to be significantly higher than TSB values. The correlation coefficient between TcB and TsB was 0.64 for the whole group, and 0.73 in infants without PT [14, 18]. Amruta et al. [19] showed that TcB and TSB levels had a good correlation among preterms receiving PT. In the same study the correlation coefficient between TcB and TSB was found to be 0.97 and 0.88 for neonates with gestational ages of 28–32GWs and 32–37GWs, respectively. In their study, Rubio et al. [17] stated that TcB measurements may be useful for screening jaundice in the first 2 weeks of life among very preterm babies. In our study, the TcB and TSB levels were found to be highly correlated among different gestational age groups. Although this correlation varied a little according to the gestational age, each coefficient represented a strong correlation between TcB and TsB (p < 0.05).
Although, it is concluded that the transcutaneous bilirubinometry devices reliably estimate bilirubin levels in preterm infants for screening of neonatal jaundice, the data about the reliability of transcutaneous bilirubin measurement during and after phototheraphy is inconsistent [16]. Knüpfer et al. suggested that Bilicheck only may give reliable results in infants without PT [14]. Similarly De Luca et al. [4], emphasizes the usefulness of TcB measurement in preterm babies before onset of PT. However monitoring of jaundice in preterm infants is still important during phototheraphy in terms of protecting vulnerable brain and reducing the number of blood draws. Several studies to date showed that the unexposed skin can be used to evaluate the bilirubin in babies under light exposure. Some authors reported that shaded measurement sites showed better correlation between TSB and TcB values [10, 20]. Zecca et al., showed that there is a good agreement between serum bilirubin and patched transcutaneous bilirubin, while unpatched transcutaneous bilirubin underestimates serum levels [21]. In a recent study including 167 preterm infants, the sensitivity and NPV of TcB was found to be >95% with or without PT. In this study, we further showed that the reliability and validity of TcB values with corresponding TsB values were high in every individual day of PT and in 24 h after PT in areas which were covered by opaque patches.
Maisels et al. [22] evaluated the function of TcB to estimate the probability of having TSB level at or above the PT initiation cutpoint. They found that when the TcB level is at least 3 mg/dl below the TSB level, the probability that the TSB is not at or above the recommended PT level is high. Similarly in our study, except for the infants with a PMA of 24–276/7, if the TcB is at least 2 mg/dl below the TSB cutpoint for PT, the probability that these babies’ TSB levels would remain under PT threshold was found to be significantly higher.
There is lack of data about the effect of clinical and demographic characteristics on agreement between TsB and TcB values. While Knüpfer et al. described a decreasing correlation in lower gestational weeks, two other studies showed that gestational age does not effect the TcB measurements [4, 23]. Specific conditions like skin maturity, temperature at the TcB assay and capillary pH may have an effect on the analysis of correlation between TcB and TSB [24]. In our study the hypotension was the only variable affecting the difference between TSB and TcB values at postnatal 1st day of life. As previous studies suggest, TcB for screening of jaundice in extremely preterm infants may be useful in only stable medical conditions, and it should be kept in mind that TcB measurements may differ due to conditions affecting tissue perfusion like hypotension.
Our study has some limitations. First, we enrolled only Caucasian white neonates. Because our patient population comprises 99% of Caucasian white neonates, it is hard to conduct a multi-racial study. Second, the data about specific conditions which may affect TcB readings like skin maturity, temperature at the TcB assay and capillary pH were not recorded. There are a few studies evaluating the usefulness of TcB among very preterm neonates providing both correlation coefficients and Bland-Altman plots. The strength of our study is that we provided correlation coefficients and the data for the Bland-Altman difference plots. These data collectively could give more reasonable results which may be helpful for clinical decision making.
Our study showed that TcB may be useful for screening and monitoring of jaundice in very preterm newborns in first week of life. The TcB measurements highly correlated with TSB even in 24–28 GW babies during PT. Transcutaneous bilirubinometry use in these very preterm babies may avoid the need for invasive bilirubin testing.
References
National Institute for Health and Clinical Excellence. Neonatal Jaundice. National Institute for Health and Clinical Excellence, 2010, www.nice.org.uk.
Bhutani VK, Stark AR, Lazzeroni LC, Poland R, Gourley GR, Kazmierczak S, et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr. 2013;162:477–82.
Maisels MJ, Kring E. Transcutaneous bilirubinometry decreases the need for serum bilirubin measurements and saves money. Pediatrics. 1997;99:599–601.
De Luca D, Zecca E, de Turris P, Barbato G, Marras M, Romagnoli C. Using BiliCheck for preterm neonates in a subintensive unit: diagnostic usefulness and suitability. Early Hum Dev. 2007;83:313–7.
Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:17.
American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Clinical guideline: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316.
Schmidt ET, Wheeler CA, Jackson GL, Engle WD. Evaluation of transcutaneous bilirubinometry in preterm neonates. J Perinatol. 2009;29:564–9.
Rubaltelli FF, Gourley GR, Loskamp N, Modi N, Roth-Kleiner M, Sender A, et al. Transcutaneous bilirubin measurement: a multicenter evaluation of a new device. Pediatrics. 2001;107:1264–71.
Fonseca R, Kyralessa R, Malloy M, Richardson J, Jain SK. Covered skin transcutaneous bilirubin estimation is comparable with serum bilirubin during and after phototherapy. J Perinatol. 2012;32:129–31.
Tan KL, Dong F. Transcutaneous bilirubinometry during and after phototherapy. Acta Paediatr. 2003;92:327–31.
Ozkan H, Oren H, Duman N, Duman M. Dermal bilirubin kinetics during phototherapy in term neonates. Acta Paediatr. 2003;92:577–81.
Hegyi T, Hiatt IM, Gertner IM, Zanni R, Tolentino T. Transcutaneous bilirubinometry II. Dermal bilirubin kinetics during phototherapy. Pediatr Res. 1983;17:888–91.
Nanjundaswamy S, Petrova A, Mehta R, Hegyi T. Transcutaneous bilirubinometry in preterm infants receiving phototherapy. Am J Perinatol. 2005;22:127–31.
Knupfer M, Pulzer F, Braun L, Heilmann A, Robel-Tillig E, Vogtmann C. Transcutaneous bilirubinometry in preterm infants. Acta Paediatr. 2001;90:899–903.
Tan KL, Mylvaganam A. Transcutaneous bilirubinometry in preterm very low birthweight infants. Acta Paediatr Scand. 1988;77:796–801.
Nagar G, Vandermeer B, Campbell S, Kumar M. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871–81.
Rubio A, Epiard C, Gebus M, Deiber M, Samperiz S, Genty C, et al. Diagnosis accuracy of transcutaneous bilirubinometry in very preterm newborns. Neonatology. 2017;111:1–7.
Maisels MJ. Transcutaneous bilirubinometry. Neo Rev. 2006;7:217–25.
Pendse A, Jasani B, Nanavati R, Kabra N. Comparison of transcutaneous bilirubin measurement with total serum bilirubin levels in preterm neonates receiving phototherapy. Indian Pediatr. 2017;54:641–3.
Jangaard KA, Curtis H, Goldbloom RB. Estimation of bilirubin using BiliChek, a transcutaneous bilirubin measurement device: effects of gestational age and use of phototherapy. Paediatr Child Health. 2006;11:79–83.
Zecca E, Barone G, De Luca D, Marra R, Tiberi E, Romagnoli C. Skin bilirubin measurement during phototherapy in preterm and term newborn infants. Early Hum Dev. 2009;85:537–40.
Maisels MJ, Coffey MP, Kring E. Transcutaneous bilirubin levels in newborns <35 weeks’ gestation. J Perinatol. 2015;35:739–44.
Willems WA, van den Berg LM, de Wit H, Molendijk A. Transcutaneous bilirubinometry with the BiliCheck in very premature newborns. J Matern Fetal Neonatal Med. 2004;16:209–14.
Bosschaart N, Kok JH, Newsum AM, Ouweneel DM, Mentink R, van Leeuwen TG, et al. Limitations and opportunities of transcutaneous bilirubin measurements. Pediatrics. 2012;129:689–94.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arman, D., Topcuoğlu, S., Gürsoy, T. et al. The accuracy of transcutaneous bilirubinometry in preterm infants. J Perinatol 40, 212–218 (2020). https://doi.org/10.1038/s41372-019-0445-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0445-3
- Springer Nature America, Inc.
This article is cited by
-
Transcutaneous bilirubin reliability during and after phototherapy depending on skin color
European Journal of Pediatrics (2024)
-
Transcutaneous bilirubin levels in extremely preterm infants less than 30 weeks gestation
Journal of Perinatology (2023)
-
Screening methods for neonatal hyperbilirubinemia: benefits, limitations, requirements, and novel developments
Pediatric Research (2021)
-
Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own
European Journal of Pediatrics (2021)
-
TcB, FFR, phototherapy and the persistent occurrence of kernicterus spectrum disorder
Journal of Perinatology (2020)