Abstract
Kawasaki disease (KD) is associated with coronary artery injury. Studies have shown that the endothelial progenitor cell (EPC) participates in the process of arterial repair. Data have been reported that the number of EPC increased significantly in the subacute phase of KD. However, until now, there are no data about the functions of EPC in KD patients. The present study was designed to further investigate the number and functions of EPC in KD. Ten KD patients in the acute phase and ten healthy volunteers were recruited and attributed to the KD group and control group, respectively. The circulating CD34/kinase insert domain-containing receptor double positive cells were evaluated in the two groups using flow cytometry. In vitro assays were used to measure the functions of EPC, including proliferation, adhesion, and migration activities. The plasma levels of nitric oxide (NO), tumor necrosis factor-α (TNF-α), and high sensitivity C-reactive protein (hs-CRP) were also assessed in both groups. The number of EPC in the KD group was significantly higher than that of the control group (0.021 ± 0.007% vs. 0.014 ± 0.003%, P < 0.05). The migratory response of EPC was significantly decreased in the KD group, compared with that of the control group (5.50 ± 1.78 vs. 3.40 ± 1.35 cells/high power field, P < 0.01). Similarly, the proliferative and adhesive activities of EPC in the KD group were also decreased (0.47 ± 0.08 vs. 0.66 ± 0.07, P < 0.01; 6.5 ± 2.12 vs. 11.2 ± 2.04 cells/high power field, P < 0.01). The plasma NO, TNF-α, and hs-CRP levels in the KD group were higher than those of the control group (54.10 ± 11.78 vs. 38.80 ± 11.10 μmol/l, P < 0.01; 48.20 ± 7.42 vs. 37.00 ± 11.12 pg/ml, P < 0.05; 87.10 ± 30.18 vs. 5.30 ± 3.37 mg/l, P < 0.01). The number of circulating EPC positively correlated with the level of NO (r = 0.92, P < 0.001), and the functions of EPC negatively correlated with the levels of TNF-α and hs-CRP, respectively. In Kawasaki disease, the number of EPC was enhanced and the functions of EPC were attenuated. The two-way regulation of circulating EPC in KD patients may be associated with the disorders of cytokines or messengers in KD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kawasaki disease (KD) is a kind of systemic vasculitis characterized by auto-immune features [21, 22, 28]. Studies have also shown that patients with KD have complicated inflammative reaction characterized by high level of tumor necrosis factor-α (TNF-α) and high sensitivity C-reactive protein (hs-CRP), all of which play a pivotal role in endothelial dysfunction (ED) [6, 7]. ED is a well-established risk factor of atherosclerosis in adulthood [30]. Therefore, a history of KD could possibly be one of the risk factors for early onset of atherosclerosis.
Emerging data predicated that the endothelial progenitor cell (EPC), precursor cell of mature endothelial cell, is important for maintaining the normal function and structure of arterial endothelial cells [3, 4, 11, 14]. Furthermore, Werner et al. reported that increased levels of EPC were associated with a reduced risk of death from cardiovascular causes [36]. Data have shown that the level of circulating EPC was regulated by numerous mediators, which include nitric oxide (NO), TNF-α, and hs-CRP [2]. NO plays a pivotal role in mobilizing and upregulation of circulating EPC, while TNF-α and hs-CRP participate in the impairment and downregulation of circulating EPC [10, 12, 13]. It was reported that NO concentration in KD patients was higher than that of healthy control subjects [18]. Meanwhile, TNF-α and hs-CRP also increased in KD patients [16, 24]. Data have been reported that the number of EPC increased significantly in the subacute phase of KD [26]. However, to the best of our knowledge, there are no data about the functions of EPC in KD patients.
The present study was designed to further investigate the number and functions of EPC in KD. We measured the number of EPC using flow cytometry and the functions of EPC including proliferation, adhesion, and migration in the KD group and control group. The level of plasma NO, TNF-α, and hs-CRP were also assessed in the KD group and control group.
Materials and methods
Patients and blood collection
We studied ten patients with KD (KD group, aged 6 months to 4 years; median 26 months; male/female = 7/3) and ten healthy children (control group, aged 6 months to 5 years; median 22 months; male/female = 6/4). There was no statistical difference between the two groups, in terms of age, body weight, albumin, globulin, and bilirubin. Healthy children were selected from children who had health examination, and we asked the parents to provide an additional blood sample (10 ml) from their children. Informed consent was obtained from the parents of all patients and all healthy children. All KD patients were hospitalized at our hospital from July 2007 to March 2009. The KD patients were enrolled within 7 days of the onset of illness, with day 1 defined as the first day of fever. All KD patients met the diagnostic criteria for KD established by the Japanese Kawasaki Disease Research Committee and were considered typical cases. The peripheral blood was collected from the KD patients within 5–7 days of the onset of the illness. All patients were scheduled to receive both aspirin (25 mg/kg/day) and intravenous immunoglobulin (i.v. IG, 2 g/kg) after the blood was collected. The initial course of i.v. IG therapy was started on days 5–7. The study protocol was approved by the ethics committee of our hospital.
Cell culture and EPC characterization
The procedure was performed as described previously [38]. Briefly, the peripheral blood mononuclear cells (PBMNCs) of enrolled subjects were isolated using Ficoll density gradient centrifugation and suspended into 25 cm2 cell culture bottle coated with fibronectin (Hematological Technologies) using endothelial cell basal medium-2 (Clonetics, San Diego, CA, USA) supplemented with EGM-2 MV (Clonetics) and incubated at 37°C in a humidified environment with 5% carbon dioxide (CO2). The culture medium was replaced every 4 days. To confirm the EPC phenotype, the attached EC-like cells were incubated with 1, 10-dioctadecyl-3, 3, 30, 30-tetramethylindo-carbocyanine perchlorate-labeled acetylated low density lipoprotein (Dil-acLDL; 10 μg/ml; Molecular Probes) at 37°C for 1 h. The cells were then fixed with 4% paraformaldehyde for 30 min at 37°C and incubated with fluorescein isothiocyanate-labeled Ulex europeus agglutin (FITC-lectin, 10 μg/ml; Sigma) for 4 h at 37°C. After being stained, the samples were observed with a phase contrast fluorescent microscope (Olympus). Cells demonstrating double positive fluorescence were identified as differentiating EPC.
EPC incubated with the serum of KD patients
We recruited ten children’s fathers as healthy adult volunteers, when they took their child to our hospital. They agreed with the experimental procedure and offered 30 ml peripheral venous blood. We used the peripheral venous blood to isolate the PBMNCs and induced the PBMNCs into EPCs as described previously [38]. Then, the EPCs were submitted to incubate with the serum of KD patients or healthy control subjects for 24 h (n = 10). After then, the EPCs were collected for function assay.
EPC function assay: proliferation, adhesion, and migration activities
The procedure of EPC function assay was performed according to a previous study, described as below [38].
EPCs were digested with 0.25% trypsin and then cultured in serum-free medium in 96-well culture plate (200 μl/well). After being cultured for 24 h, EPCs were supplemented with 10 μl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (5 g/l; Fluka) and incubated for another 4 h. Then, the supernatant was discarded by aspiration, and the EPC preparation was shaken with 200 μl dimethylsulfoxide for 10 min, before the optical density value was measured at 490 nm.
EPCs (2.5 × 104/well) were seeded on 24-well plates pre-coated with fibronectin and incubated for 30 min at 37°C and 5% CO2. After being washed three times with phosphate-buffered saline (PBS), the attached cells were counted. Adhesion activity was evaluated as the mean number of attached cells in 5 high power fields (200×) from each well.
EPC migrations were evaluated using a modified Boyden chamber method. EPC suspension (2.5 × 104 cells/well) was placed in the upper chamber with endothelial basal medium (EBM), and the chamber was placed in a 24-well culture dish containing EBM and 50 ng/ml vascular endothelial growth factor (VEGF, Hematological Technologies, Essex Junction, USA). The chamber was incubated for 24 h and the lower side of the filter was washed with PBS and fixed with 2% paraformaldehyde. For quantification, cell nuclei were stained with 4′, 6-diamidino-2-phenylindole. Cells migrating into the lower chamber were counted manually in three random microscopic fields.
Flow cytometry analysis
Flow cytometry analysis was performed as described previously [37]. The phycoerythrin-conjugated monoclonal antibody against human CD34 (Becton Dickinson) and rabbit polyclonal antibody against human vascular endothelial growth factor receptor-2, namely kinase insert domain-containing receptor (KDR; Neomarkers, Fremont, CA, USA), were incubated in a volume of 100 μl of peripheral blood for 15 min in the dark. The KDR antibody was detected with FITC-conjugated goat against rabbit IgG (Southern Biotech, Birmingham, UK). After incubation, cells were lysed, washed with PBS, and fixed in 4% paraformaldehyde before analysis of 50,000 events after exclusion of debris and platelets. The number of circulating EPC was evaluated by the ratio of CD34/KDR double positive cells per 100 PBMNCs. Isotype-identical antibodies served as controls. Double color flow cytometry analysis was performed by using a Beckman Coulter Epics-XL 4 flow cytometer and performed in duplicate.
Plasma TNF-α and hs-CRP detection
Blood samples were separated at 4°C and stored at −20°C. hs-CRP was measured at 550 nm with the use of the particle-enhanced immunoturbidimetric assay (Orion Diagnostica) by a specialist who was unaware of the study assignment.
Plasma levels of TNF-α were measured by enzyme-linked immunosorbent assay (R&D Systems) according to the manufacturer’s instructions. Results were compared with standard curves. Measurements were performed in duplicate.
Measurement of plasma NO concentration
We used the Greiss method to estimate nitrite, the stable metabolite of NO [19]. This assay determined the total NO based on the enzymatic conversion of NO −3 to NO −2 by nitrate reductase (Sigma) and detection of nitrite as an azo dye product of the Greiss reaction. The results are presented as micromoles NO x of NO −3 /NO −2 per liter of medium.
Statistical analysis
Results are presented as mean ± SD and analyzed with the SPSS software (version 13.0). Statistical significance was evaluated by independent t test. Bivariate correlations were calculated according to Pearson. A value of P < 0.05 was considered significant.
Results
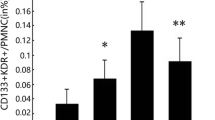
The number of EPC in the two groups
The number of EPC detected by flow cytometry in the KD group was higher than that of the control group (0.021 ± 0.007% vs. 0.014 ± 0.003%, P < 0.05, Fig. 1).
The functions of EPC in the two groups
Figure 2 shows that the in vitro cultured EPCs were characterized by double staining with Dil-acLDL and FITC-lectin. Figure 3 shows the functions of EPC in the two groups. The migratory response of EPC was significantly decreased in the KD group, compared with that of the control group (Fig. 3a). Similarly, the proliferative and adhesive activities of EPC in the KD group were also decreased (Fig. 3b, c).
The EPC induced from healthy adult volunteers incubated with the serum of KD patients showed impaired migratory, proliferative, and adhesive activity, compared with that incubated with the serum of healthy control subjects (Fig. 4a–c).
The plasma NO, TNF-α, and hs-CRP levels in the two groups
Figure 5 shows that the plasma NO, TNF-α, and hs-CRP levels in the KD group are higher than those of the control group.
Association between EPC and plasma NO, TNF-α, and hs-CRP levels
The number of EPC positively correlated with the plasma level of NO (Fig. 6). The migration and proliferation activity of EPC negatively correlated with the plasma level of TNF-α (Fig. 7). The adhesion activity of EPC did not correlate with the plasma level of TNF-α (r = −0.57, P > 0.05) and the functions of EPC negatively correlated with the plasma level of hs-CRP, respectively (Fig. 8).
Discussion
The present study investigated the number of peripheral circulating EPC and the functions of in vitro cultured EPC in patients with KD and healthy control subjects. Our major findings were that the number of peripheral circulating EPC was markedly increased in patients with KD, and the functional capacities of cultured EPC were significantly reduced in patients with KD. Elevated levels of nitric oxide were found in KD patients and positively correlated with EPC number. Meanwhile, a close correlation was found between plasma TNF-α and hs-CRP, related inversely to EPC function in our study. The present study possibly indicates that KD patients have the ability to mobilize EPC into the peripheral circulation to participate in the vascular repair process, while the poor functional state of EPC obliterates its potentiality for repair vascular injury.
KD is associated with systemic vascular endothelial dysfunction [9, 17, 25, 27, 29, 39]. Normal endothelial function plays a pivotal role in maintaining cardiovascular homeostasis. Yamakawa et al. demonstrated that coronary endothelial dysfunction was present in patients with Kawasaki disease, evaluated by intracoronary injection of acetylcholine [39]. Niboshi et al. [27] revealed that adult patients with a history of KD had systemic vascular endothelial dysfunction and also suggested that a history of KD could possibly be one of the risk factors for early onset of atherosclerosis.
Data have shown that EPC plays a pivotal role in maintaining normal endothelial function [3, 4, 11, 14]. Animal experiments have shown that enhanced peripheral circulating EPC contributed to the improved rate of reendothelialization and inhibition of neointimal formation in arteries denuded by balloon injury [11, 35]. In humans, it has been found that the number and function of EPC are positively associated with endothelial function [14]. Furthermore, Werner et al. reported that an increased level of EPC was associated with reduced risk of death from cardiovascular diseases [36]. Therefore, the number and function of EPC in patients with KD may affect the cardiovascular outcomes of these patients in adulthood.
In the present study, we found that the number of circulating EPC increased significantly in KD patients, compared with that of control subjects. A previous study has shown that the concentration of NO was associated with the mobilization of EPC [1]. To investigate the possible mechanism of the phenomenon, we measured the level of plasma NO of the two groups. We found that the plasma concentration of NO was raised in KD patients, which is in keeping with previous studies [5, 32]. Furthermore, a positive correlation was found between NO level and EPC number in KD patients.
However, the mechanisms underlying the enhanced NO production in KD patients were not completely clear. The study of Wang et al. and other groups demonstrated that the enhanced NO level in KD patients was mainly derived from inducible but not constitutive nitric oxide synthase (NOS) and that intravenous immunoglobulin suppressed inducible NOS expression in KD patients [34]. In contrast, Khajoee et al. reported that endothelial and inducible NOS gene polymorphisms were not involved in the development of coronary artery lesions in KD patients [20]. It should be stressed that the impacts of NO on vascular and endothelial function are complicated and often appear to be contradictory. At present, it is not clear whether the enhanced level of NO is beneficial or harmful in children with KD, and in addition, the mechanisms of such effect remain to be clarified.
In order to investigate the functions of EPC in KD patients, we performed in vitro cell function assay, including proliferative activity and migratory activity. We found that the functions of EPC decreased significantly, compared with that of control subjects. It is well known that the functional state of cells is associated with its efficacy in playing a physiology role. The favorable effects of EPC on the cardiovascular system may be interrupted in KD patients because of its perishing functional state.
Increasing evidence indicates that inflammation has a key role in the injury of cell functions. In the present study, we also detect the concentration of TNF-α and hs-CRP. The results showed that the concentrations of TNF-α and hs-CRP were higher than those of control subjects, which is consistent with the results of previous studies [16, 24]. Data have shown that TNF-α has a negative effect on the function of EPC [13, 31, 33]. It was also reported that plasma hs-CRP, together with the inflammatory cytokines, was negatively associated with the function of circulating EPC [10]. Our results also showed that CRP attenuated the mobilization, differentiation, and survival of EPC.
Several cytokines and growth factors are involved in the mobilization and homing of EPC [2]. Although the levels of inflammatory mediators were elevated in KD patients, the levels of the angiogenic protein VEGF were also selectively increased in KD patients [23]. VEGF is a potent stimulus for mobilizing bone marrow-derived EPC, and enhanced levels may be important in the mobilization of EPC [4]. Indeed, it may be interesting to investigate whether an imbalance exists between EPC mobilizers (e.g., NO and VEGF) and inflammatory mediators (e.g., TNF-α and hs-CRP) in KD patients. We postulated that the internal environment disorder may be responsible for the bidirectional regulation of EPC kinetics in KD patients.
It should be pointed out that the present study was subject to several limitations. One of the limitations of this study is that we did not investigate the dynamics of EPC in KD patients from the onset of disease to the convalescent period. Secondly, we proposed that increased NO production contributed to increased EPC number in KD patients. The definitive cause-and-effect relation between them, however, requires further studies in which other vasoactive agents such as granulocyte colony-stimulating factor and vascular endothelial growth factor should be examined. Thirdly, the data of the present study cannot give us an exact explanation of the bidirectional regulation of EPC in KD patients.
Further studies should be performed to investigate the EPC kinetics and its mechanisms in KD patients and other patients with vasculitis. Data have shown that the EPC colony-forming unit (EPC-CFU) in patients with antineutrophil cytoplasmic antibody-associated vasculitis (AAV) decreased significantly in untreated active phase and even in remission phase [15, 40]. On the other hand, de Groot et al. reported [8] that the number of EPC in patients with AAV evaluated by counting Dil-acLDL and FITC-lectin double positive cells increased significantly after the institution of immunosuppressive therapy and disease remission. Different methods are used in counting EPC-CFU or Dil-acLDL and FITC-lectin double positive cells to assess EPC numbers, which may possibly contribute to the various results. In addition, the number EPC-CFU represents not only the number of EPC but also the functions of EPC. We thought that the number of EPC-CFU reduced in patients with AAV could possibly indicate that the functions of EPC were impaired in different phases of AAV diseases. In a certain degree, the results are in line with ours.
Our study is important for understanding the mechanisms of coronary arterial injury in KD patients. Endothelial dysfunction is a hallmark of KD, and recent evidence suggests that bone marrow-derived EPC participates in postnatal blood vessel repair. The relative deficiency of circulating EPC in KD patients may contribute to the coronary dilation or aneurysm, whereas chronic pharmacological augmentation of circulating EPC could offer a novel therapeutic strategy. Further studies are needed to understand the therapeutic implications of circulating EPC in KD.
References
Aicher A, Heeschen C, Mildner-Rihm C et al (2003) Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9:1370–1376
Aicher A, Zeiher AM, Dimmeler S (2005) Mobilizing endothelial progenitor cells. Hypertension 45:321–325
Asahara T, Murohara T, Sullivan A, Silver M et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Asahara T, Takahashi T, Masuda H et al (1999) VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18:3964–3972
Awata T, Kurihara S, Takata N et al (2005) Functional VEGF C-634G polymorphism is associated with development of diabetic macular edema and correlated with macular retinal thickness in type 2 diabetes. Biochem Biophys Res Commun 333:679–685
Bacon PA (2005) Endothelial cell dysfunction in systemic vasculitis: new developments and therapeutic prospects. Curr Opin Rheumatol 17:49–55
Borzutzky A, Gutierrez M, Talesnik E et al (2008) High sensitivity C-reactive protein and endothelial function in Chilean patients with history of Kawasaki disease. Clin Rheumatol 27:845–850
de Groot K, Goldberg C, Bahlmann FH et al (2007) Vascular endothelial damage and repair in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 56:3847–3853
Dhillon R, Clarkson P, Donald AE et al (1996) Endothelial dysfunction late after Kawasaki disease. Circulation 94:2103–2106
Diller GP, van Eijl S, Okonko DO et al (2008) Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation 117:3020–3030
He T, Smith LA, Harrington S et al (2004) Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke 35:2378–2384
Henrich D, Seebach C, Wilhelm K et al (2007) High dosage of simvastatin reduces TNF-alpha-induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by IL-1beta in vitro. J Surg Res 142:13–19
Herbrig K, Haensel S, Oelschlaegel U et al (2006) Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann Rheum Dis 65:157–163
Hill JM, Zalos G, Halcox JP et al (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348:593–600
Holmen C, Elsheikh E, Stenvinkel P et al (2005) Circulating inflammatory endothelial cells contribute to endothelial progenitor cell dysfunction in patients with vasculitis and kidney involvement. J Am Soc Nephrol 16:3110–3120
Huang SM, Weng KP, Chang JS et al (2008) Effects of statin therapy in children complicated with coronary arterial abnormality late after Kawasaki disease: a pilot study. Circ J 72:1583–1587
Ikeda M, Furukawa H, Imamura H et al (2002) Surgery for gastric cancer increases plasma levels of vascular endothelial growth factor and von Willebrand factor. Gastric Cancer 5:137–141
Ikemoto Y (2003) Nitric oxide in Kawasaki disease. J Pediatr 142:594–595 author reply 595-596
Jun CD, Choi BM, Hoon R et al (1994) Synergistic cooperation between phorbol ester and IFN-gamma for induction of nitric oxide synthesis in murine peritoneal macrophages. J Immunol 153:3684–3690
Kariyazono H, Ohno T, Khajoee V et al (2004) Association of vascular endothelial growth factor (VEGF) and VEGF receptor gene polymorphisms with coronary artery lesions of Kawasaki disease. Pediatr Res 56:953–959
Kawasaki T (1967) Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 16:178–222
Kawasaki T, Kosaki F, Okawa S, Shigematsu I et al (1974) A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 54:271–276
Maeno N, Takei S, Masuda K et al (1998) Increased serum levels of vascular endothelial growth factor in Kawasaki disease. Pediatr Res 44:596–599
Maury CP, Salo E, Pelkonen P (1989) Elevated circulating tumor necrosis factor-alpha in patients with Kawasaki disease. J Lab Clin Med 113:651–654
Mitani Y (2000) Coronary endothelial dysfunction after Kawasaki disease. J Am Coll Cardiol 35:821–823
Nakatani K, Takeshita S, Tsujimoto H et al (2003) Circulating endothelial cells in Kawasaki disease. Clin Exp Immunol 131:536–540
Niboshi A, Ozawa S, Hamaoka K (2008) Kawasaki disease and atherosclerosis. Nippon Rinsho 66:387–392
Nomura I, Abe J, Noma S et al (2005) Adrenomedullin is highly expressed in blood monocytes associated with acute Kawasaki disease: a microarray gene expression study. Pediatr Res 57:49–55
Noto N, Okada T, Karasawa K et al (2008) Age-related acceleration of endothelial dysfunction and subclinical atherosclerosis in subjects with coronary artery lesions after Kawasaki disease. Pediatr Cardiol 30:262–268
Poredos P (2002) Endothelial dysfunction in the pathogenesis of atherosclerosis. Int Angiol 21:109–116
Seeger FH, Haendeler J, Walter DH et al (2005) p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation 111:1184–1191
Tsukahara H, Kikuchi K, Matsuda M et al (1997) Endogenous nitric oxide production in Kawasaki disease. Scand J Clin Lab Invest 57:43–47
Valgimigli M, Rigolin GM, Fucili A et al (2004) CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation 110:1209–1212
Wang CL, Wu YT, Lee CJ et al (2002) Decreased nitric oxide production after intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr 141:560–565
Werner N, Junk S, Laufs U et al (2003) Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res 93:e17–e24
Werner N, Kosiol S, Schiegl T et al (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353:999–1007
Xu MG, Wang JM, Chen L et al (2008) Berberine-induced mobilization of circulating endothelial progenitor cells improves human small artery elasticity. J Hum Hypertens 22:389–393
Xu MG, Wang JM, Chen L et al (2008) Berberine-induced upregulation of circulating endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology 112:279–286
Yamakawa R, Ishii M, Sugimura T et al (1998) Coronary endothelial dysfunction after Kawasaki disease: evaluation by intracoronary injection of acetylcholine. J Am Coll Cardiol 31:1074–1080
Zavada J, Kideryova L, Pytlik R et al (2008) Circulating endothelial progenitor cells in patients with ANCA-associated vasculitis. Kidney Blood Press Res 31:247–254
Acknowledgment
This work was financially supported by the Guang Dong Province Medical Science Foundation (no: B2009226).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ming-Guo Xu and Li-Na Men contributed to the paper equally.
Rights and permissions
About this article
Cite this article
Xu, MG., Men, LN., Zhao, CY. et al. The number and function of circulating endothelial progenitor cells in patients with Kawasaki disease. Eur J Pediatr 169, 289–296 (2010). https://doi.org/10.1007/s00431-009-1014-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-009-1014-0