Abstract
Natural killer (NK) and CD8+ T cells play a crucial role in the control of mouse cytomegalovirus (MCMV) infection. These effector cells exert their functions by releasing antiviral cytokines and by cytolytic mechanisms including perforin activation. In addition to their role in virus control, NK cells play an immunoregulatory role since they shape the CD8+ T cell response to MCMV. To investigate the role of perforin-dependent cytolytic mechanism in NK cell modulation of CD8+ T cell response during acute MCMV infection, we have used perforin-deficient C57BL/6 mice (Prf1−/−) and have shown that virus control by CD8+ T cells in Prf1−/− mice is more efficient if NK cells are activated by the engagement of the Ly49H receptor with the m157 MCMV protein. A lack of perforin results in severe liver inflammation after MCMV infection, which is characterized by immunopathological lesions that are more pronounced in Prf1−/− mice infected with virus unable to activate NK cells. This immunopathology is caused by an abundant infiltration of activated CD8+ T cells. The depletion of CD8+ T cells has markedly reduced pathohistological lesions in the liver and improved the survival of Prf1−/− mice in spite of an increased viral load. Altogether, the results of our study suggest that a lack of perforin and absence of the specific activation of NK cells during acute MCMV infection lead to an unleashed CD8+ T cell response that is detrimental for the host.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human cytomegalovirus (HCMV) is a ubiquitous pathogen causing high morbidity risk in immunologically suppressed and immunodeficient patients. Primary cytomegalovirus (CMV) infection is efficiently controlled in immunocompetent hosts, but nevertheless, the viral genome remains in a lifelong state of latency from which periodic reactivation may occur [1]. Due to the strict species specificity, animal CMVs models, particularly mouse CMV (MCMV), are being used in studying HCMV pathogenesis [2, 3].
The host defense against CMV involves the innate and adaptive immune response. Cells of innate immune system including dendritic cells (DCs), macrophages and natural killer (NK) cells are activated rapidly upon CMV infection [4]. The major characteristic of innate immune cells is the secretion of proinflammatory and immunoregulatory cytokines. Previous studies reported that innate cytokines IL-12 and IFN-α/β promote NK and CD8+ T cell effector functions by enhancing the production of cytokines, such as IFN-γ and cytolytic granules including perforin and granzymes [5–8].
Among the innate immune cells, NK cells are major effector cells, which are involved in the control of primary CMV infection during the time that precedes the induction of the specific immune response [7, 9]. The control of MCMV by NK cells in C57BL/6 mice is primarily dependent on signaling via the activating Ly49H receptor, which specifically recognizes the MCMV protein m157 [10–12]. Therefore, the deletion of the m157 gene (Δm157 MCMV) compromises virus control by NK cells and enhances virus replication in vivo [11]. Evidence has been accumulated that, in addition to early containment of virus replication, NK cells play an important role in shaping the subsequent specific T cell response [13]. This function of NK cells has been extensively studied with regard to their capacity to modulate the antiviral CD8+ T cell response [14–21]. Several studies demonstrated that NK cells can accelerate [22] or enhance the CD8+ T cell response [23, 24]. While the NK cells stimulate CD8+ T cell response inducing the maturation of DCs and consequently T cell polarization, an increasing body of evidence suggests that NK cells may also limit the CD8+ T cell response (reviewed in [13] and [19]). Negative regulation of CD8+ T cell response is important for maintaining homeostasis of lymphocytes, whereby it is especially important in the prevention of an excessive activation of CD8+ T cells, which could eventually lead to immunopathology [15, 17, 18, 20]. Although the mechanisms governing the complex interaction between NK cells and CD8+ T cells are still not been fully elucidated, it is assumed that NK cells may impair CD8+ T cell response by direct killing of DCs [25–28] and/or activated T cells [21], as well as by the secretion of cytokines that have an inhibitory effect [17, 29]. Early upon MCMV infection, in the absence of cytolytic mechanism, expanding NK cells produce IL-10, which is important for limiting overall CD8+ T cell response [17].
In this paper, we have investigated the regulation of CD8+ T cell response to MCMV by NK cells in the absence of cytolytic mechanism by using perforin-deficient C57BL/6 mice (Prf1−/−). The lack of perforin and of specific NK cell activation via Ly49H promotes a robust CD8+ T cell response to MCMV, which fails to contain virus replication, but resulted in a severe liver inflammation followed by a high mortality rate of infected mice. The depletion of CD8+ T cells in these mice has proven to have not only positive impact on the resolution of liver inflammation, but also on the survival rate. Specific activation of NK cells after MCMV infection partially compensated for the lack of perforin and had positive effect on the outcome of the infection. Altogether, our results suggest that perforin and specific activation of NK cells are required for homeostatic regulation of the specific CD8+ T cell response to MCMV.
Materials and methods
Mice
C57BL/6 and Prf1−/− mice [30] were housed and bread under specific pathogen-free conditions at the Central Animal Facility of the Medical Faculty, University of Rijeka, in accordance with the guidelines contained in the International guiding principles for biomedical research involving animals. The Ethical Committee at the University of Rijeka approved all animal experiments described in this paper. Eight- to twelve-week-old mice were used in all experiments.
Viruses, depletion of lymphocyte subsets in vivo and the determination of viral titers and proliferation assay in vivo
Mice were injected intravenously (i.v.) with 2 × 105 PFU of the tissue culture (TC)-grown virus in a volume of 500 μl of PBS. Bacterial artificial chromosome (BAC)-derived MCMV strain MW97.01 has been shown previously to be biologically equivalent to MCMV strain Smith (VR-1399) and is hereafter referred to as wild-type (WT) MCMV [31]. In addition, mutant virus lacking m157 gene was used [11]. The depletion of CD8+ T lymphocyte subsets was done by intraperitoneal (i.p.) injection of 300 μg of mAb to CD8 (YTS 169.4) either on day 1 and 5 p.i., or on day 1 and every fifth day p.i. The efficacy of depletion was assessed by flow cytometer analysis of splenic leukocytes stained with PE-labeled anti-CD8 (Pharmingen). For determination of viral titers, mice were killed, organs were collected, and viral titers were determined by standard plaque assay as described previously [32]. For in vivo proliferation assay, mice were i.p. injected with 2 mg of bromodeoxyuridine (BrdU, Sigma) 3 h prior they were killed.
Flow cytometry and intracellular staining
Splenocytes were prepared as described previously, and Fc receptor on splenic leukocytes was blocked with 2.4G2 mAbs to reduce nonspecific staining [33]. The following Abs were purchased from eBioscience or BD Pharmingen, and cell-surface staining was performed against the following antigens: anti-CD3ε (145–2C11), anti-CD8α (53–6.7), anti-CD25 (PC61.5), anti-IFN-γ (XMG1.2) and anti-GzmB (16G6). H-2Db-restricted MCMV-specific M45 (985HGIRNASFI993) peptide was synthesized to a purity of >95 % by Jerini Peptide Technologies. The H-2Db-restricted M45 tetramer was synthesized by the National Institutes of Health. For detection of CD8+ T cell IFN-γ and GzmB expression, cells were incubated in RPMI media supplemented with 10 % of FCS (Gibco) and stimulated with 1 μg of M45 peptide [34] for 5 h at 37 °C with 1 μg/ml of brefeldin A (eBioscience) added for the last 4 h of incubation. After incubation, cells were first surface-stained, then fixed, and permeabilized using Cytofix/Cytoperm solutions (BD Pharmingen) followed by intracellular IFN-γ or GzmB staining according to the manufacturer’s protocol. For proliferation assay, liver leukocytes were first surface-stained, then fixed, and permeabilized using Cytofix/Cytoperm solutions (BD Pharmingen) and incorporated BrdU was intracellularly stained according to the manufacturer’s protocol (BrdU Flow Kit, BD Pharmingen). Flow cytometry was performed on FACSCalibur and FASCAria (BD Bioscience), and data were analyzed using the FlowJo software (Tree Star).
Preparation of tissue sections for histological analysis
Mice were killed on day 7 p.i., livers were removed, and fixed in 4 % paraformaldehyde. Paraffin-embedded 2-μm liver sections were prepared, stained with hematoxylin, and counterstained with eosin. CD3+ T cells were detected by immunohistochemistry using staining with rabbit anti-CD3 antibodies (Abcam). Biotinylated goat anti-rabbit IgG (Abcam) was used as a secondary antibody, followed by detection with a streptavidin–peroxidase complex (Roche Diagnostics). Diaminobenzidine (Dako) was used as the substrate yielding a brown precipitate. For antigen retrieval, Tris–EDTA buffer (pH 9.0) in microwave oven was used. Double immunohistochemical staining was performed with rabbit anti-mouse caspase-3 antibody, which recognizes a large fragment of active caspase-3 (Cell Signaling Technology). Biotinylated goat anti-rabbit IgG (Abcam) was used as a secondary antibody, followed by a detection with a streptavidin–alkaline phosphatase complex (Roche Diagnostics). Fuchsin (Dako) was used as the substrate yielding a red precipitate. Detection of MCMV IE1 was performed using mAb CROMA101 [35], and goat anti-mouse IgG biotin (BD Pharmingen) was used as secondary antibody followed by the detection with a streptavidin–peroxidase complex (Roche Diagnostics). Diaminobenzidine (Dako) was used as the substrate yielding a brown precipitate. For antigen retrieval, a citrate buffer (pH 6,0) in microwave oven was used. Counterstaining was performed with Shandon hematoxylin. Slides were analyzed on Olympus BX51 microscope, and images were acquired by Olympus digital camera (DP71).
For immunofluorescence visualization of CD8+ T cells, frozen liver cryosections were first stained with rat anti-mouse CD8 mAb (clone YTS 169.4) followed by goat anti-rat TRITC-labeled secondary antibody (Santa Cruz Biotechnology, Inc.). Samples were analyzed with Olympus FV300 confocal laser scanning microscope.
Statistical analysis
Statistical analysis was done using Prism 5 (GraphPad). Statistically significant differences for phenotype analyses were determined by the unpaired two-tailed Student’s t test, and P values <0.05 were considered significant. Differences in viral titers were determined by two-tailed Mann–Whitney U test. Survival was analyzed with the Kaplan–Meier method. Survival curves were compared using the log-rank test.
Results
Lack of perforin affects MCMV-specific CD8+ T cell response
The results of our previous study showed that in C57BL/6 mice, the early activation of NK cells through Ly49H˗m157 interaction reduces the CD8+ T cell response to MCMV infection [18]. To further clarify how NK cells exert their immunoregulatory role, we have investigated the extent to which the cytolytic activity of NK cells affects the intensity of CD8+ T cell response to MCMV by using immunodeficient mice lacking perforin. To this aim, control mice (C57BL/6) and Prf1−/− mice were infected either with WT or Δm157 MCMV and viral titers in the spleen have been measured. A proportion of mice in each group were depleted of CD8+ T cells. Unlike in C57BL/6 mice, MCMV infection of Prf1−/− mice resulted in higher viral titers on day 3.5 p.i., irrespective of Ly49H˗m157 interaction (Fig. 1a). As expected, at this early time point, CD8+ T cells did not exert any antiviral function neither in C57BL/6, nor in Prf1−/− mice. While in C57BL/6 mice, both viruses were well controlled in spleen on day 7 p.i., in Prf1−/− mice, the infection with Δm157 virus resulted in significantly higher viral titers, compared with WT MCMV (Fig. 1b). These results suggest perforin-independent antiviral control, but only in conditions when specific activation of NK cells through Ly49H receptor is preserved.
Perforin-dependant control of MCMV infection. C57BL/6 and Prf1−/− mice were i.v. injected with 2 × 105 PFU of either WT or Δm157 MCMV, and indicated groups were treated with either PBS or depleting anti-CD8 mAbs on days 1 and 5 p.i. On day a 3.5 p.i., and b 7 p.i., mice were killed and viral titers in spleen and c liver were determined by plaque assay. Titers of individual mice (circles) and median values (horizontal bars) are shown. Data are representative from two independent experiments with three to six mice per group. Asterisks denote significant values: *P < 0.05, **P < 0.01; n.s. not significant, DL limit of detection
In accordance with our recently published data [18], in C57BL/6 mice, the depletion of CD8+ T cells resulted in a significant increase in Δm157 virus titer, while in mice infected with WT MCMV, only a moderate effect on virus titer was observed (Fig. 1b). However, in Prf1−/− mice, CD8+ T cell depletion showed a strong effect on the control of both viruses, although it was generally more pronounced in mice infected with Δm157 virus. These data suggest that immunoregulatory role of NK cells in modulation of CD8+ T cell response to MCMV depends on specific activation of NK cells through Ly49H receptor in a perforin-dependent manner.
To further explore the impact of perforin-mediated cytolytic mechanism on the quality of CD8+ T cell response to MCMV, we assessed the phenotype and functional status of CD8+ T cells on day 7 p.i. For this set of experiments, we have used the M45 epitope as an indicator for virus-specific CD8+ T cells [36, 37]. Mice lacking perforin showed increased frequencies of IFN-γ+CD8+ T cells specific for the M45 epitope (Fig. 2). Furthermore, the frequency of M45 epitope-specific CD8+ T cells was significantly higher in both mouse strains infected with the Δm157 virus compared with WT-infected groups. Since the expression of granzyme B (GzmB) and CD25 marker characterizes effector T cells, we have analyzed the expression of these markers on CD8+ T cells [38, 39]. The upregulation of both GzmB and CD25 by CD8+ T cells was more pronounced in mice infected with Δm157 MCMV. Although the lack of perforin dramatically increased the frequency of GzmB+ and CD25+CD8+ T cells, the differences between WT and Δm157 virus were still preserved. Thus, the absence of perforin and of the specific NK cell activation enhances the CD8+ T cell response to MCMV.
Augmented CD8+ T cell activation status in the absence of Ly49H signaling and perforin. C57BL/6 and Prf1−/− mice were i.v. injected with 2 × 105 PFU of either WT or Δm157 MCMV. On day 7, p.i. splenocytes were isolated and stimulated in the culture with MCMV-specific M45 peptide prior to intracellular staining for IFN-γ or GzmB, or processed immediately for staining with anti-CD25 mAbs. The frequencies of IFN-γ+, GzmB+ and CD25+ CD8+ T cells (CD3ε+CD8+) are shown. Data are presented as mean ± SEM of four mice per group from one of two independent experiments. Asterisks denote significant values: *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired, two-tailed Student’s t test)
CD8+ T cells affect control of Δm157 MCMV in the liver of Prf1−/− mice
Having in mind that MCMV can induce hepatitis, which is especially severe in Prf1−/− mice [40], our next aim was to investigate immune surveillance of MCMV infection in the liver of Prf1−/− and control mice. On day 7 p.i., both WT and Δm157 MCMV were well controlled in the liver of C57BL/6 mice (Fig. 1c). Unlike in the spleens of C57BL/6 mice (Fig. 1b), CD8+ T cells did not contribute to the control of Δm157 virus in the liver. Prf1−/− mice were also able to control WT MCMV in the liver, and the depletion of CD8+ T cells did not affect the control of this virus. However, infection of Prf1−/− mice with Δm157 MCMV resulted in significantly higher viral titers compared with WT MCMV. The depletion of CD8+ T cells additionally increased the titer of Δm157 virus in liver (Fig. 1c). Altogether, these results suggest that without perforin and specific NK cell activation, CD8+ T cells to some extent compensate virus control via a noncytolytic mechanism.
CD8+ T cells cause a severe hepatic immunopathology in Prf1−/− mice after MCMV infection
Next, we have assessed the impact of NK cell activation via Ly49H and of CD8+ T cell response on hepatitis of MCMV-infected Prf1−/− mice. We have analyzed H&E-stained liver sections prepared from mice which were infected for 7 days. MCMV-infected Prf1−/− mice developed severe pathohistological lesions that included multiple foci of hepatocellular destruction and mononuclear cell infiltrations composed mostly of lymphocytes and histiocytes, whereas in C57BL/6 mice, the lesions were milder and signs of regeneration were observed (Fig. 3a). Of note is that the absence of early NK cell activation through Ly49H˗m157 interaction significantly increased liver lesions in Prf1−/− mice. Since previous studies showed an enhanced activation of CD8+ T cells after MCMV infection of Prf1−/− mice [41], we have assumed that a vast proportion of lymphocytes in mononuclear infiltrations might be CD8+ T cells. In order to address this issue, we have performed immunofluorescent staining of frozen liver sections with anti-CD8 monoclonal antibodies. We have observed a massive infiltration of CD8+ T cells in the liver of Prf1−/− mice infected with Δm157 virus (Fig. 3b). In WT-infected Prf1−/− mice, the infiltration of CD8+ T cells in the liver was less pronounced but still prominent. Contrary to Prf1−/− mice, MCMV infection did not induce an accumulation of CD8+ T cells in the liver of control C57BL/6 mice. Altogether, these data indicate that absence of perforin and of the specific NK cell activation results in severe liver damage and a massive infiltration of CD8+ T cells.
Pathohistological lesions in liver of Δm157 MCMV-infected Prf1−/− mice. C57BL/6 and Prf1−/− mice were i.v. injected with 2 × 105 PFU of either WT or Δm157 MCMV, or left uninfected. On day 7, p.i. mice were killed and livers were processed for further analysis. a Representative paraffin-embedded liver sections analyzed for the presence of pathohistological lesions are shown (H&E; ×20). b Representative images of immunofluorescence detection of CD8+ T cells in liver are shown. Data are representative of two independent experiments with three to six mice per group. CV central vein
Prf1−/− mice infected with Δm157 MCMV show an enhanced activation of MCMV-specific CD8+ T cells in the liver
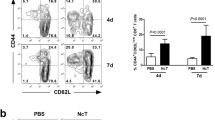
To investigate whether CD8+ T cells in the liver of MCMV-infected Prf1−/− mice are virus specific, we have performed flow cytometry analysis of liver lymphocytes on day 7 p.i. In accordance with the results obtained by immunofluorescent staining (Fig. 3b), infection of Prf1−/− mice with Δm157 virus resulted in significantly higher number of CD8+ T cells in the liver compared to Prf1−/− mice infected with WT MCMV and C57BL/6 mice, regardless of the virus used for infection (Fig. 4a). To confirm that these CD8+ T cells are specific for MCMV, we used the MHC class I tetramer filled with M45 peptide specific for the acute phase of MCMV infection. In line with the number of total CD8+ T cells, the number of M45-specific CD8+ T cells was significantly higher in the liver of Prf1−/− mice infected with Δm157 virus compared with WT MCMV-infected Prf1−/− mice, as well as C57BL/6 mice, regardless of the virus (Fig. 4a). Furthermore, the proliferation capacity of virus-specific CD8+ T cells was higher in the liver of Prf1−/− mice infected with Δm157 virus compared with WT MCMV-infected Prf1−/− mice (Fig. 4b). In addition, the frequency of IFN-γ+CD8+ T cells and GzmB+CD8+ T cells after in vitro restimulation with the M45 peptide was also higher in Prf1−/− mice infected with Δm157 MCMV compared to Prf1−/− mice infected with WT MCMV (Fig. 4b). Thus, the lack of perforin and of the specific NK cell activation results in a massive accumulation of activated virus-specific CD8+ T cells in the liver of infected animals.
The strong CD8+ T cell response to Δm157 MCMV is further augmented in absence of perforin. a C57BL/6 and Prf1−/− mice were i.v. injected with 2 × 105 PFU of either WT or Δm157 MCMV. Liver lymphocytes were isolated and stained for M45 tetramer-specific CD8+ T cells. Total number of lymphocytes, total number of CD8+ T cells (CD3ε+CD8α+) and total number of M45-specific CD8+ T cells per liver are shown. b Prf1−/− mice were i.v. infected as in a. On day 7 p.i. mice were i.p. injected with 2 mg of BrdU and killed 3 h later. For IFN-γ and GzmB assay, liver lymphocytes were stimulated in the culture with MCMV-specific M45 peptide prior to intracellular staining. The frequencies of BrdU+M45 tetramer+CD8+ T cells, IFN-γ+CD8+ T cells and GzmB+CD8+ T cells specific for M45 peptide in total liver lymphocytes are depicted. Data are presented as mean ± SEM of at least three mice per group from two independent experiments. Asterisks denote significant values: *P < 0.05, **P < 0.01 (unpaired, two-tailed Student’s t test)
Prf1−/− mice infected with Δm157 MCMV after depletion of CD8+ T cells show a reduced liver pathology and improved survival
In order to investigate whether the observed massive liver damage in Prf1−/− mice infected with the Δm157 virus could be a consequence of CD8+ T cell function, we have analyzed the effect of CD8+ T cell depletion on liver pathology. Depletion of CD8+ T cells significantly reduced pathohistological lesions in liver of Prf1−/− mice on day 7 p.i. compared with the group of undepleted Δm157 MCMV-infected Prf1−/− mice (Fig. 5a, b). In addition, in the group of Δm157 MCMV-infected and CD8+ T cell-depleted Prf1−/− mice, we have observed much less caspase-3-positive cells compared with the infected undepleted group (Fig. 5c). Double staining of infected tissue for MCMV IE1 and caspase-3 demonstrated that cells undergoing apoptosis were mostly uninfected. Thus, this finding indicates an immunopathological liver damage by dysregulated CD8+ T cells.
The CD8+ T cell depletion markedly reduced pathohistological liver lesions and improved survival of Prf1−/− mice infected with Δm157 MCMV. Prf1−/− mice were i.v. injected with 2 × 105 PFU of ∆m157 MCMV, and indicated groups were treated with either PBS or depleting anti-CD8 mAbs on days 1 and 5 p.i. Mice were killed at day 7 p.i. a Representative paraffin-embedded liver sections analyzed for the pathohistological lesions are shown (H&E; ×20). b Immunohistochemistry staining of CD3+ cells on paraffin-embedded liver sections visualized with diaminobenzidine (×40). c Double staining for MCMV protein IE1 (brown) and active caspase-3 (red) performed on paraffin-embedded liver sections. Arrows show cells positive for caspase-3 (×40). d Prf1−/− mice were i.v. injected with 2 × 105 PFU of Δm157 MCMV. On day 1 p.i. and subsequently every fifth day, mice were treated with either PBS or depleting anti-CD8 mAbs and monitored daily for survival. Data are representative from two independent experiments. P value was determined by the log-rank survival test
To further analyze the impact of an unleashed CD8+ T cell response on the mortality rate of Δm157 MCMV infection in Prf1−/− mice, CD8+ T cell depleted or undepleted Δm157 MCMV-infected Prf1−/− mice were monitored daily for survival (Fig. 5d). Although the depletion of CD8+ T cells increased viral titers in the liver of Δm157 MCMV-infected Prf1−/− mice (Fig. 1c), the survival rate of these mice was higher than in the group of mice with preserved CD8+ T cells (Fig. 5d). These results indicate that in the absence of perforin and of the specific NK cell activation, unleashed CD8+ T cell response causes detrimental liver damage.
Discussion
We have investigated the role of perforin in NK cell regulation of CD8+ T cell response to MCMV infection. The results of our previous study demonstrated an enhanced CD8+ T cell response in mice infected with the virus, which fails to specifically activate NK cells [18]. Here we show that besides a specific activation through the Ly49H-m157 interaction, this immunoregulatory role of NK cells depends on perforin-mediated cytolytic mechanism. One of the major differences between normal C57BL/6 and Prf1−/− mice in virus control in spleen was that in the absence of perforin, CD8+ T cells were more efficient if NK cells are specifically activated, suggesting that noncytolytic mechanisms contribute to the control of WT MCMV infection of Prf1−/− mice.
A previously published study indicated that perforin is essential in the control of MCMV infection in spleen, whereas the noncytolytic mechanism mediated by IFN-γ is responsible for the control of MCMV infection in the liver [42]. In contrast to these organ-specific differences of the control of MCMV infection, other groups demonstrated that NK cells use perforin and IFN-γ for antiviral control in both organs [43, 44]. Our results confirm and extend these observations, showing the importance of specific activation of NK cells in the control of MCMV infection in Prf1−/− mice. However, noncytolytic mechanisms failed to fully compensate for the lack of activation of NK cells via Ly49H-m157 interaction, resulting in high Δm157 viral titers in the spleen and liver of Prf1−/− mice on day 7 p.i. Unlike in the spleen of Prf1−/− mice, where CD8+ T cells play an important role in the containment of WT MCMV infection, they have no role in the liver of the same mice. Yet, in the absence of specific NK cell activation, i.e., in Prf1−/− mice infected with the virus lacking m157, CD8+ T cells took over the virus control, suggesting that noncytolytic compensatory mechanisms are operative only in the absence of a specific activation of NK cells.
Previous work in hematopoietic cell transplantation (HCT) models by Podlech and colleagues demonstrated that the depletion of CD8+ but not CD4+ T cells during immune reconstitution after HCT leads to a disseminated MCMV infection in almost all organs and to a high level of mortality [45, 46]. This model showed that CD8+ T cells do not cause immunopathology, but actually prevent viral pathology. At first glance, our results in MCMV-infected Prf1−/− mice present an opposite scenario, since the depletion of CD8+ T cells was found to be protective, indicating immunopathology rather than viral pathology as the cause of death. However, aberrant CD8+ T cell response that causes liver immunopathology was observed only when specific NK cell activation and perforin were missing in infected mice. This is in line with previously published work showing that a higher cytokine production by expanded CD8+ T cells caused severe immunopathology and lethality of Prf1−/− mice [47, 48]. Moreover, the present study indicates that the activated CD8+ T cells in liver of Prf1−/− mice infected with Δm157 MCMV can induce apoptosis of uninfected parenchymal cells in CD8+ T cell-dependent manner. Specifically, the depletion of CD8+ subset significantly prevented liver damage and the frequency of apoptotic cells in these mice, in spite of an increased virus titer, suggesting that tissue damage was mostly a consequence of an immunopathological reaction, rather than a cytolytic effect of the virus. The fact that pathohistological lesions are less pronounced in mice infected with a virus able to activate NK cells indicates a critical role of specific NK cell activity in the prevention of unleashed CD8+ T cell response in the acute phase of infection. We speculate that an unleashed proliferation and activation of CD8+ T cells and a massive infiltration of liver are accompanied by extensive cytokine production, which can be deleterious for healthy cells, but at the same time could still have antiviral capacity. The depletion of CD8+ T cells in these mice eliminates the major source of cytokines and therefore improves the survival in spite of a significantly higher virus load. Using similar model of MCMV infection in Prf1−/− mice, Lee and colleagues showed that NK cells, stimulated through the Ly49H receptor, produce IL-10, which limits the magnitude of CD8+ T cell response [17].
In conclusion, our data further emphasize that NK cells influence the induction and maturation of the CD8+ T cell response to CMV. For this homeostatic regulation of antiviral CD8+ T cell response, perforin-dependent cytolytic mechanism and specific activation of NK cells are required. In their absence, a massive accumulation of activated virus-specific CD8+ T cells occurs, resulting in liver immunopathology. More detailed studies are needed to reveal the ultimate mechanism of liver damage in this model.
References
Pass RF (2001) Cytomegalovirus. In: Knipe DM, Howley PM (eds) Fields virology, vol 2, 4th edn. Lippincott Williams and Wilkins, Philadelphia, pp 2675–2706
Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S (2003) Pathogenesis of murine cytomegalovirus infection. Microbes Infect 5(13):1263–1277. doi:10.1016/j.micinf.2003.09.007
Reddehase MJ, Podlech J, Grzimek NK (2002) Mouse models of cytomegalovirus latency: overview. J Clin Virol 25(Suppl 2):S23–S36. doi:10.1016/S1386-6532(02)00087-2
Jonjic S, Bubic I, Krmpotic A (2006) Innate immunity to cytomegaloviruses. In: Reddehase MJ (ed) Cytomegaloviruses: molecular biology and immunology. Caister Academic Press, Wymondham, pp 285–319
Curtsinger JM, Johnson CM, Mescher MF (2003) CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol 171(10):5165–5171. doi:10.4049/jimmunol.171.10.5165
Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF (2005) Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol 174(8):4465–4469. doi:10.4049/jimmunol.174.8.4465
French AR, Yokoyama WM (2003) Natural killer cells and viral infections. Curr Opin Immunol 15(1):45–51. doi:10.1016/S095279150200002X
Trinchieri G (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3(2):133–146. doi:10.1038/nri1001
Lodoen MB, Lanier LL (2006) Natural killer cells as an initial defense against pathogens. Curr Opin Immunol 18(4):391–398. doi:10.1016/j.coi.2006.05.002
Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL (2002) Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296(5571):1323–1326. doi:10.1126/science.1070884
Bubic I, Wagner M, Krmpotic A, Saulig T, Kim S, Yokoyama WM, Jonjic S, Koszinowski UH (2004) Gain of virulence caused by loss of a gene in murine cytomegalovirus. J Virol 78(14):7536–7544. doi:10.1128/JVI.78.14.7536-7544.2004
Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM (2002) Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA 99(13):8826–8831. doi:10.1073/pnas.092258599
Biron CA (2012) Yet another role for natural killer cells: cytotoxicity in immune regulation and viral persistence. Proc Natl Acad Sci USA 109(6):1814–1815. doi:10.1073/pnas.1120528109
Zingoni A, Cerboni C, Ardolino M, Santoni A (2009) Modulation of T cell-mediated immune responses by natural killer cells. In: Zimmer J (ed) Natural killer cells at the forefront of modern immunology. Springer, New York, pp 315–327. doi:10.1007/978-3-642-02309-5_17
Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, Fleming P, Tabarias H, Hill GR, van der Most RG, Scalzo AA, Smyth MJ, Degli-Esposti MA (2010) Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med 207(6):1333–1343. doi:10.1084/jem.20091193
Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brustle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Haussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS (2012) Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci USA 109(4):1210–1215. doi:10.1073/pnas.1118834109
Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA (2009) Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med 206(10):2235–2251. doi:10.1084/jem.20082387
Mitrovic M, Arapovic J, Jordan S, Fodil-Cornu N, Ebert S, Vidal SM, Krmpotic A, Reddehase MJ, Jonjic S (2012) The NK cell response to mouse cytomegalovirus infection affects the level and kinetics of the early CD8(+) T-cell response. J Virol 86(4):2165–2175. doi:10.1128/JVI.06042-11
Mitrovic M, Arapovic J, Traven L, Krmpotic A, Jonjic S (2012) Innate immunity regulates adaptive immune response: lessons learned from studying the interplay between NK and CD8+ T cells during MCMV infection. Med Microbiol Immunol 201(4):487–495. doi:10.1007/s00430-012-0263-0
Su HC, Nguyen KB, Salazar-Mather TP, Ruzek MC, Dalod MY, Biron CA (2001) NK cell functions restrain T cell responses during viral infections. Eur J Immunol 31(10):3048–3055. doi:10.1002/1521-4141(2001010)31:10<3048:AID-IMMU3048>3.0.CO;2-1
Soderquest K, Walzer T, Zafirova B, Klavinskis LS, Polic B, Vivier E, Lord GM, Martin-Fontecha A (2011) Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J Immunol 186(6):3304–3308. doi:10.4049/jimmunol.1004122
Robbins SH, Bessou G, Cornillon A, Zucchini N, Rupp B, Ruzsics Z, Sacher T, Tomasello E, Vivier E, Koszinowski UH, Dalod M (2007) Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog 3(8):e123. doi:10.1371/journal.ppat.0030123
Slavuljica I, Busche A, Babic M, Mitrovic M, Gasparovic I, Cekinovic D, Markova Car E, Pernjak Pugel E, Cikovic A, Lisnic VJ, Britt WJ, Koszinowski U, Messerle M, Krmpotic A, Jonjic S (2010) Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest 120(12):4532–4545. doi:10.1172/JCI43961
Stadnisky MD, Xie X, Coats ER, Bullock TN, Brown MG (2011) Self MHC class I-licensed NK cells enhance adaptive CD8 T-cell viral immunity. Blood 117(19):5133–5141. doi:10.1182/blood-2010-12-324632
Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C (2002) Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med 195(3):343–351. doi:10.1084/jem.20011149
Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G (2002) Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 195(3):327–333. doi:10.1084/jem.20010938
Laffont S, Seillet C, Ortaldo J, Coudert JD, Guery JC (2008) Natural killer cells recruited into lymph nodes inhibit alloreactive T-cell activation through perforin-mediated killing of donor allogeneic dendritic cells. Blood 112(3):661–671. doi:10.1182/blood-2007-10-120089
Piccioli D, Sbrana S, Melandri E, Valiante NM (2002) Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med 195(3):335–341. doi:10.1084/jem.20010934
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA (2006) Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24:99–146. doi:10.1146/annurev.immunol.24.021605.090737
Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H (1994) Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369(6475):31–37. doi:10.1038/369031a0
Wagner M, Jonjic S, Koszinowski UH, Messerle M (1999) Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J Virol 73(8):7056–7060
Jonjic S, Krmpotic A, Arapovic J, Koszinowski UH (2008) Dissection of the antiviral NK cell response by MCMV mutants. Methods Mol Biol 415:127–149. doi:10.1007/978-1-59745-570-1_8
Yokoyama WM, Kim S (2008) Analysis of individual natural killer cell responses. Methods Mol Biol 415:179–196. doi:10.1007/978-1-59745-570-1_11
Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB (2006) Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol 176(6):3760–3766. doi:10.4049/jimmunol.176.6.3760
Trgovcich J, Stimac D, Polic B, Krmpotic A, Pernjak-Pugel E, Tomac J, Hasan M, Wraber B, Jonjic S (2000) Immune responses and cytokine induction in the development of severe hepatitis during acute infections with murine cytomegalovirus. Arch Virol 145(12):2601–2618
Holtappels R, Podlech J, Pahl-Seibert MF, Julch M, Thomas D, Simon CO, Wagner M, Reddehase MJ (2004) Cytomegalovirus misleads its host by priming of CD8 T cells specific for an epitope not presented in infected tissues. J Exp Med 199(1):131–136. doi:10.1084/jem.20031582
Holtappels R, Thomas D, Reddehase MJ (2009) The efficacy of antigen processing is critical for protection against cytomegalovirus disease in the presence of viral immune evasion proteins. J Virol 83(18):9611–9615. doi:10.1128/JVI.00936-09
Bannard O, Kraman M, Fearon DT (2009) Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science 323(5913):505–509. doi:10.1126/science.1166831
Boyman O, Sprent J (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12(3):180–190. doi:10.1038/nri3156
van Dommelen SL, Sumaria N, Schreiber RD, Scalzo AA, Smyth MJ, Degli-Esposti MA (2006) Perforin and granzymes have distinct roles in defensive immunity and immunopathology. Immunity 25(5):835–848. doi:10.1016/j.immuni.2006.09.010
Andrews DM, Andoniou CE, Fleming P, Smyth MJ, Degli-Esposti MA (2008) The early kinetics of cytomegalovirus-specific CD8+ T-cell responses are not affected by antigen load or the absence of perforin or gamma interferon. J Virol 82(10):4931–4937. doi:10.1128/JVI.02127-07
Tay CH, Welsh RM (1997) Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol 71(1):267–275
Loh J, Chu DT, O’Guin AK, Yokoyama WM, Virgin HW (2005) Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol 79(1):661–667. doi:10.1128/JVI.79.1.661-667.2005
Sumaria N, van Dommelen SL, Andoniou CE, Smyth MJ, Scalzo AA, Degli-Esposti MA (2009) The roles of interferon-gamma and perforin in antiviral immunity in mice that differ in genetically determined NK-cell-mediated antiviral activity. Immunol Cell Biol 87(7):559–566. doi:10.1038/icb.2009.41
Podlech J, Holtappels R, Pahl-Seibert MF, Steffens HP, Reddehase MJ (2000) Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol 74(16):7496–7507
Podlech J, Holtappels R, Wirtz N, Steffens HP, Reddehase MJ (1998) Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol 79(Pt 9):2099–2104
Badovinac VP, Hamilton SE, Harty JT (2003) Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity 18(4):463–474. doi:10.1016/S1074-7613(03)00079-7
Sad S, Kagi D, Mosmann TR (1996) Perforin and Fas killing by CD8+ T cells limits their cytokine synthesis and proliferation. J Exp Med 184(4):1543–1547. doi:10.1084/jem.184.4.1543
Acknowledgments
We thank Jelena Boneta for performing immunofluorescent CD8+ T cell staining, Prof. Nives Jonjic for help with pathohistological analysis, and Dr. Felix M. Wensveen for discussion. J. A. is supported by the Federal Ministry of Education and Science, Bosnia and Herzegovina. This work has been supported in part by Croatian Science Foundation under the project 7132 (to AK) and by the University of Rijeka under the projects 13.06.1.1.01 (to SJ) and 13.06.1.1.02 (to AK).
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jurica Arapović and Maja Arapović have contributed equally to this work.
This article is part of the Special Issue on Cytomegalovirus.
Rights and permissions
About this article
Cite this article
Arapović, J., Arapović, M., Golemac, M. et al. The specific NK cell response in concert with perforin prevents CD8+ T cell-mediated immunopathology after mouse cytomegalovirus infection. Med Microbiol Immunol 204, 335–344 (2015). https://doi.org/10.1007/s00430-015-0409-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-015-0409-y