Abstract
Brain lateralization is a widespread phenomenon although its expression across primates is still controversial due to the reduced number of species analyzed and the disparity of methods used. To gain insight into the diversification of neuroanatomical asymmetries in non-human primates we analyze the endocasts, as a proxy of external brain morphology, of a large sample of New World monkeys and test the effect of brain size, home range and group sizes in the pattern and magnitude of shape asymmetry. Digital endocasts from 26 species were obtained from MicroCT scans and a set of 3D coordinates was digitized on endocast surfaces. Results indicate that Ateles, Brachyteles, Callicebus and Cacajao tend to have a rightward frontal and a leftward occipital lobe asymmetry, whereas Aotus, Callitrichinae and Cebinae have either the opposite pattern or no directional asymmetry. Such differences in the pattern of asymmetry were associated with group and home range sizes. Conversely, its magnitude was significantly associated with brain size, with larger-brained species showing higher inter-hemispheric differences. These findings support the hypothesis that reduction in inter-hemispheric connectivity in larger brains favors the lateralization and increases the structural asymmetries, whereas the patterns of shape asymmetry might be driven by socio-ecological differences among species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain asymmetry is thought to be a distinctive feature of the human lineage (Falk 1980; Holloway and de la Coste-Lareymondie 1982; Gómez-Robles et al. 2013, 2016). Asymmetry is the propensity for structure and function to be specialized to one brain hemisphere or the other (Ocklenburg and Güntürkün 2012). Inter-hemispheric differences are expressed as different properties including the external morphology, as well as the size, shape and cell composition of specific regions. The evolutionary origin of the differences between the left and right hemispheres in humans has usually been related to the development of a hemispheric dominance for specific traits, such as hand preference and language (e.g., Vallortigara 2006). This led to explore more intensively the asymmetry in areas such as the planum temporale and Broca's area, which show a leftward asymmetry in modern humans (i.e. Homo sapiens) in agreement with their functional dominance. Recent studies based on neuroimages indicate that inter-hemispheric differences, such as the petalias, are widespread not only in humans but also in apes and other non-human primates, although the similarity in the patterns of asymmetry among these species is still a matter of debate (e.g., Ocklenburg and Güntürkün 2012). Whereas some authors indicate that the rightward frontal and leftward occipital petalia is shared by modern humans and great apes, others suggest that humans present a unique pattern (Pilcher et al. 2001; Phillips and Sherwood 2007; Neubauer et al. 2020; Xiang et al. 2020).
The analysis of neuroanatomical asymmetries in primates other than hominids also showed mixed results (Phillips and Sherwood 2007; Pilcher et al. 2001). For instance, no directional asymmetries in frontal or occipital lobes had been found either in Cebus or in Saimiri species (Hopkins and Marino 2000; Pilcher et al. 2001) until more recent studies of brain magnetic resonance images reported a left frontal petalia for Cebus apella (Phillips and Sherwood 2007). Similarly, the view that Old World monkeys are characterized by a lack of asymmetry in the external morphology of the brain (Hopkins and Marino 2000) contrasts with the finding of a frontal rightward and posterior leftward petalia in a large sample of endocasts of Papio (Atkinson et al. 2016). The disparity in the type of data and variables used along with the reduced number of species included in most studies hinder the comparative analysis across primate species and it could partially account for these contrasting results.
Despite these inconsistencies, the finding of brain asymmetries in non-human primates, as well as in several species of other vertebrate clades, supports the idea that having a lateralized brain would have a fitness benefit (Rogers 2014; Giljov et al. 2018). At the individual level, the lateralization of functions is hypothesized to increase the efficiency of information processing as one hemisphere assumes the control without interfering with the other, which allows it to perform several tasks simultaneously, resulting in more complex cognitive processes (Mesulam 1985). The individual cognitive performance also has an impact at the population level, especially in social and foraging behaviors (Vallortigara and Rogers 2005). Other factors, such as the expansion of the brain could have further contributed to the hemispheric specialization in primates as well as other mammals (Phillips et al. 2015; Atkinson et al. 2016). Given that bigger brains have a proportionally larger cerebral cortex, lateralized tasks can be performed more efficiently via shorter and faster intra-hemispheric circuits (Ringo et al. 1994; Olivares et al. 2001; Stephan et al. 2003; Karolis et al. 2019). Under this hypothesis, the anatomical and structural differences between hemispheres are thought of as a by-product of increasing brain size (Hopkins et al. 2015).
Additionally, several macroevolutionary studies in primates report significant associations between brain (or endocranial) volume and socio-ecological variables. Positive correlations have been found with group size (Dunbar 1998) and home range size (Clutton-Brock and Harvey 1980; Powell et al. 2017), with results varying according to the species sampled and the predictors included into the models. Whether these factors also contribute directly to brain lateralization, or indirectly via allometric changes related to brain size increase, has not yet been evaluated. Such factors could have played though a significant role, given that species with a more social behavior tend to exhibit more lateralized brains, and that hemispheric dominance for certain functions has been associated with the propensity to explore unfamiliar environments (Cameron and Rogers 1999; Ghirlanda and Vallortigara 2004). If such factors influenced the selection for hemispheric specialization, they would be expected to be associated with brain asymmetry. Notwithstanding their potential relevance, the contribution of brain size and socio-ecological variables to the diversification in the degree and pattern of asymmetry in the primate brain has been scarcely studied.
To date, the study of brain asymmetry among primates has mainly focused on a few species of hominids (Homo, Pan and Gorilla) and other catarrhine monkeys (Macaca and Papio). Conversely, the New World monkeys—which experienced a notable process of diversification in brain size and shape (Aristide et al. 2016)—remain largely unexplored. This clade exhibits high inter-specific variation in body size, degree of encephalization and diversity of social and ecological characteristics (Aristide et al. 2015, 2016). Particularly, because of their large variation in brain morphology and characteristics such as home range and group sizes, the New World monkeys represent an interesting reference system for investigating the factors underlying the evolution of brain asymmetry in primates. Here, we describe shape asymmetry of endocasts, as a proxy of external brain morphology, in representatives of the five main clades of Platyrrhini and analyze the importance of endocranial size, home range and group sizes in the diversification of the pattern and magnitude of endocranial asymmetry in shape.
The analysis of endocranial morphology using 3D digital models generated from computer tomography (i.e., endocasts) allows us to include a larger number of species in the comparative analysis. Even though the endocasts do not provide detailed information about all gyri and sulci or subcortical regions, the inner surface of the skull is a good proxy for global asymmetry of the brain (Fournier et al. 2011; Dumoncel et al. 2021). Previous studies have shown that the analysis of endocast shape allows the quantification of external brain morphology because it represents a good proxy for describing correlated changes in relative size and position of brain lobes (Aristide et al. 2016; Neubauer et al. 2020). In this way, endocasts are a valuable source of information being increasingly used for studying the evolution of the brain in extinct and extant species (Neubauer et al. 2010; Watanabe et al. 2019; Dumoncel et al. 2021; Early et al. 2020). Particularly for the latter, endocasts are used because it is not always possible to collect specimens under the conditions required to preserve the soft tissues, and in some cases the capture of wild specimens and endangered species is not allowed. Consequently, the endocasts represent a valid alternative to perform comparative studies that require sampling a large number of species. Additionally, the information gathered from the endocasts of living species in comparative studies provides a framework to discuss the findings in fossil specimens (Neubauer et al. 2010, 2020; Aristide et al. 2019). Finally, as several studies have shown, the close interaction between brain tissues and the bones that compose the neurocranium during individual ontogeny support their use as a valuable alternative for evolutionary studies (Bruner 2014; Aristide et al. 2016; Neubauer et al. 2020).
Methods
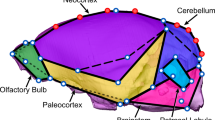
We analyzed a sample of 110 digital 3D endocasts of adult individuals of both sexes from 26 species from the five families of New World monkeys (Supplementary Table S1), deposited in Museu de Zoologia (Universidade de São Paulo, Brazil), Museu Nacional (Rio do Janeiro, Brazil) and DMM-KUPRI repository (Kyoto University, Japan). The sample size of each species has a mode of four individuals, with a few species having three or eight specimens. Almost all samples have an approximately equal number of females and males (Supplementary Table S1). The sampled species span the platyrrhine diversity in terms of body and brain size. The 3D images, in Polygon (.PLY) file format, were compiled from previous works (Aristide et al. 2016). These .PLY files were obtained from X-ray computed tomography or micro-computed tomography scans using a threshold-based 2D segmentation procedure (see details in Aristide et al. 2016). From each endocast in .PLY format, a total of 26 anatomical landmarks and 105 curve semilandmarks were digitized, including paired and unpaired reference points (Fig. 1; Aristide et al. 2016). Additionally, 200 paired surface semilandmarks were digitized on one endocast as equidistant points. Then, these surface semilandmarks were automatically projected onto each endocast using the thin-plate spline deformation and considering landmarks and curve semilandmarks as a reference frame. This projection was obtained with the function placePatch in the Morpho package for R (Schlager 2017).
We used geometric morphometrics to decompose endocranial variation in size, and the symmetric and asymmetric shape components (Dryden and Mardi 1998; Gunz et al. 2005; Neubauer et al. 2020). We first reflected the configurations of landmarks and semilandmarks of each specimen and relabeled the coordinates of mirrored configurations, so the coordinates on their left side were compared with the right side of the original configurations and vice versa (Klingenberg et al. 2002). Then, the original and the mirrored and relabeled configurations were superimposed by a Generalized Procrustes Analysis (GPA; Rohlf and Slice 1990) to remove variation in location, orientation and scale. The size of each endocast was estimated using the centroid size (CS) of each point configuration. The average between the original configuration and its superimposed relabeled reflection represents the symmetric component of shape, whereas the deviation of the original shape (or Procrustes coordinates) from its symmetrized version represents the asymmetric component (Schlager 2012; Neubauer et al. 2020). Because we also include semilandmarks in the analyses, a further step was needed to remove the non-shape variation along curves and surfaces. This was done by sliding the semilandmarks of each configuration by minimizing the bending energy toward the mean symmetric shape of the sample (obtained as the average of the mean shape configuration and its superimposed relabeled reflection). This procedure ensures that the asymmetry of the template used to project the surface semilandmarks is not transferred to all specimens (Schlager 2012; Neubauer et al. 2020).
A principal component (PC) analysis was performed on the coordinates of landmarks and semilandmarks representing the asymmetric component of shape of each specimen to describe the pattern of shape asymmetry in the sample. The zero score along this principal component represents the symmetric shape, whereas negative and positive values represent the shape differences between right and left sides, which is the pattern of asymmetry. Consequently, the scores of the first principal component summarize the main pattern of asymmetry in a sample (Neubauer et al. 2020). This pattern of shape asymmetry was illustrated using warps and heatmaps.
The magnitude of shape asymmetry (D) in the endocasts was estimated for each specimen as the difference between the symmetric shape component and the original shape coordinates (i.e., the square root of the sum of the square differences between the symmetric shape component and the superimposed Procrustes coordinates; Schlager 2012; Neubauer et al. 2020). If a configuration of points is symmetrical, the distance D with its reflection will be zero. Otherwise, D increases with the amount of shape differences between the left and right sides of the endocranial surface. Evidently, the larger the distance D the greater the magnitude of shape asymmetry. The analyses of asymmetry were performed with the functions slider3d, procSym and meshDist in Morpho package for R (Schlager 2017; R Core Team 2020).
Phylogenetic Generalized Least Squares model (PGLS; Freckleton et al. 2002) was used to explore the association between the pattern (PC) and magnitude (D) of endocranial shape asymmetry among species with the potential explanatory variables (i.e., endocranial size, home range and group sizes). Home range and group sizes were obtained from Powell et al. (2017). Two estimations of endocranial size, as a proxy to brain size, were used: the logarithm of the centroid size of the coordinates of landmarks and semilandmarks (log CS), and the logarithm of the endocranial volume (log ECV). The PGLS model takes into account the lack of independence among species due to phylogenetic structure. We modeled the regression residual variation by relaxing the Brownian motion assumption using the λ parameter, as implemented in the Caper package for R software (R Core Team 2020). This parameter is estimated by maximum likelihood and measures the phylogenetic signal in the residuals. The chrono-phylogenetic tree for the sampled species was obtained from Aristide et al. (2015). We also used this phylogeny to map as continuous variables the pattern and magnitude of endocranial shape asymmetry, and the potential explanatory variables with the contMap function based on the least-square parsimony algorithm implemented in phytools R package (R Core Team 2020).
Results
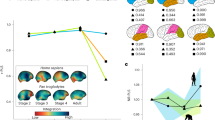
Principal components calculated from the asymmetric component of shape variables show that most of the specimens have negative scores along the first PC, which accounts for 13.65% of total variation (Fig. 2A; Supplementary Fig. S1). The pattern of shape asymmetry of the endocasts at the negative scores is characterized by the relative expansion of the left frontal lobe and the right occipital lobe (Fig. 2B). In contrast to the general trend, the specimens of Ateles, Brachyteles, Callicebus and Cacajao show positive scores along PC1 (Fig. 2A). The endocasts of these specimens at the positive side of PC1 show a rightward frontal and a leftward occipital lobe asymmetry, which resembles the human pattern of asymmetry. Along PC2, which accounts for 9.92% of variation in endocranial shape asymmetry, the scores of the specimens of each taxa are distributed between positive and negative values with a lack of directionality (Supplementary Fig. S1). This means that the taxa do not differ in the mean shape asymmetry captured by this component. Alouatta is the only genus with a clear trend to be distributed towards the negative scores of PC2.
Pattern of asymmetric variation in endocranial shape. A Box plot summarizing the distribution of scores along the first principal component (PC1) of the asymmetric component of shape. The scores of the specimens were grouped in 14 genera. B The pattern of asymmetry towards the negative scores of the first principal component is shown as deformations from the symmetrized shape. Red surfaces are relatively larger than their counterparts, whereas blue surfaces are relatively smaller compared to the opposite hemisphere. L left, R right
The magnitude of endocranial shape asymmetry, represented as the Procrustes distance between symmetrized and original configurations (D), differs among genera (Fig. 3). The lowest values are found among Callitrichinae specimens, intermediate values in Cebinae, Pithecidae and Aotus, and the largest values in Atelidae. It is remarkable the great variation found in Atelidae, which includes both the taxa characterized by the largest variation in the magnitude of asymmetry (Ateles, Brachyteles and Lagothrix) and the least variable group (Alouatta; Fig. 3).
The pattern and magnitude of endocranial shape asymmetry were mapped onto the phylogeny using as variables the scores along PC1 and the distance D, respectively (Fig. 4). Callitrichines show the lowest magnitude of asymmetry and the most negative values of PC1 scores, whereas atelines and Brachyteles have the largest magnitude of asymmetry and positive scores along PC1. More intermediate values for both variables are found in cebines, Aotus and pitheciids, with the exception of Cacajao that has a higher asymmetry and positive values of PC1 scores (Fig. 4). This association is reflected in the correlation between the scores of PC1 and the magnitude of asymmetry (D) with an r = 0.51 (p < 0.01). When the analysis is performed taking into account the phylogeny, only 23% of the variation in endocranial shape asymmetry summarized by the first PC is explained by the magnitude of asymmetry (F: 8.49, p: 0.0076).
Changes in the pattern (principal component, PC1) and magnitude (D) of endocranial asymmetry mapped onto the phylogeny. Values of terminal branches were estimated based on morphometric data. Values for PC1 and D at internal nodes and branches were reconstructed using a maximum likelihood ancestral character estimation method. The phylogenetic tree is from Aristide et al. (2015)
The values of endocranial centroid size, home range and group sizes mapped onto the phylogeny show similar patterns (Fig. 5). Taxa with small endocranial size, the callitrichines and pitheciids, also have smaller home range and group sizes, whereas atelines and cebines have larger endocranial sizes, home range and group sizes. The results of the PGLS model indicate that the pattern of asymmetry, measured as the scores of the PC1, is not associated with endocranial size whereas it has a significant association with group and home range sizes (Table 1). The PGLS model accounts for 33% of variation in the pattern of asymmetry. Conversely, the magnitude of endocranial asymmetry has a significant association only with endocast centroid size (Table 1). Similar results were obtained when the analysis was repeated using log ECV. Neither home range size nor group size have additional effects on the magnitude of endocranial asymmetry (Table 1).
Home range size (HRS) and group size (GS) by species mapped onto the phylogeny. Values of terminal nodes were obtained from Powell et al. (2017). Values for HRS and GS at internal nodes and branches were reconstructed using a maximum likelihood ancestral character estimation method. The phylogenetic tree is from Aristide et al. (2015)
Discussion
We provide here an extensive comparative analysis of the pattern and magnitude of endocranial shape asymmetry in New World monkeys. Results indicate that the majority of the specimens of the 26 species analyzed show a relative expansion of the left frontal and the right occipital lobes, although there is great variability both among and within species, with some specimens displaying the opposite pattern of asymmetry. In some species such variation results from the inversion in some individuals of the pattern of asymmetry commonly expressed in its species, whereas in others is related to the lack of a consistent direction in the pattern of shape asymmetry within the species. The most frequent asymmetry in endocranial shape found here agrees with the left frontal petalia described for the genus Cebus—currently called Sapajus—(Phillips and Sherwood 2007), although contrasts with other published studies that reported no significant asymmetries in brain width of this species, the same as in Saimiri sciureus (Hopkins and Marino 2000; Pilcher et al. 2001). Our results also show that the left-occipital protrusion previously found in some New World monkeys (LeMay 1976) is within the range of variation of this clade. Particularly, two atelids genera (Ateles and Brachyteles) and two pitheciids (Cacajao and Callicebus) predominantly show a pattern of right frontal and left-occipital protrusion, which is the most frequent pattern of petalia among great apes and humans (Balzeau and Gilissen 2010; Balzeau et al. 2011; Atkinson et al. 2016). In these four genera the left occipital is also projected more inferiorly and medially than the right one, similarly to what was observed in extant hominoid primates (Neubauer et al. 2020). It is remarkable though, that Alouatta departs from the pattern of endocranial shape asymmetry found in atelids, being similar to the more generalized pattern of New World monkeys. The particularity of this genus is also observed in other characteristics, such as the smallest relative brain size, its relatively simpler folding scheme, and an elongated and flat endocranial shape with a less flexed cranial base that makes Alouatta the most morphologically distinct among extant platyrrhine species (Hartwig et al. 2011; Aristide et al. 2016).
Other anatomical asymmetries, especially in regions associated with handedness and language, have also been reported for New World monkeys, although with inconsistent results. In this sense, the length of the lateral sulcus (or Sylvian fissure) showed a leftward asymmetry in some species (Sapajus sp., Callitrix jachus and Saguinus oedipus) but not in others (Saimiri sciureus), and the asymmetry is alternatively found in the medial or the lateral region of the fissure depending on the study (Heilbreoner and Holloway 1988; Hopkins et al. 2000; Liu and Phillips 2009). A leftward length of the lateral sulcus has also been reported in humans, and it has been associated with the occipital bending, such that the more leftward the anterior horizontal ramus, the more rightward the bending (Hou et al. 2019). Such relation between the asymmetries in particular brain structures, such as sulci, and in the shape of the external brain surface, such as petalias, has not been analyzed in New World monkeys. The use of endocasts does not allow us to perform comparable analyses, although this needs to be explored to further contribute to the functional and anatomical origin of brain surface asymmetry across different clades.
The functional role of external brain shape changes characterized as petalias is still a matter of debate. Previous studies have reported an association between handedness and asymmetries in brain regions, such as the primary motor cortex and the lateral sulcus, in Sapajus sp. and Callithrix jacchus (Phillips and Sherwood 2005; Gorrie et al. 2008; Liu and Phillips 2009), whereas no association with asymmetries in the protrusion of frontal and occipital lobes was detected (Phillips and Sherwood 2007). It has been hypothesized that petalias may reflect a disproportionate growth of certain brain regions resulting from a hemispheric specialization for various behavioral functions, such as extractive foraging or social group complexity (Phillips and Sherwood 2007). Agreeing with these expectations, we found that differences in endocranial shape asymmetry among the genera of Platyrrhini were associated with socio-ecological variables. As much as 33% of variation in shape asymmetry was accounted for by group and home range sizes. These findings suggest that behaviors associated with socio-ecological factors might be involved in the evolution of brain asymmetry in primates. However, far more data is needed to evaluate whether this association with neuroanatomical asymmetries is related to left–right differences in cognitive or emotional processes. In contrast, no association of endocranial shape asymmetry with brain size was detected. The lack of association between the pattern of asymmetry and size, along with the significant association with the socio-ecological variables, can partially account for the differences observed between Alouatta and the other atelid species. Even though they are similar in body size, Alouatta is characterized by smaller home range and group sizes compared to the rest of its clade (Aristide et al. 2016; Powell et al. 2017).
Our study also shows that, contrary to the pattern of shape asymmetry, the total magnitude of endocranial shape asymmetry is significantly and positively associated with absolute brain size, with larger-brained species displaying higher levels of asymmetry. Moreover, the asymmetric changes were mainly localized in two regions, corresponding to the frontal and occipital lobes, which are also strongly associated to the relative enlargement of the neocortex with brain size in this clade (Aristide et al. 2016). Our finding agrees with the hypothesis that the reduction in connectivity in larger brains favors the functional lateralization and the increase of inter-hemispheric differences (Karolis et al. 2019). The hypothesis is supported by studies showing that the relative size of the corpus callosum decreases with the increase in brain volume, both within and between species (Rilling and Insel 1999). Assuming that the speed of neural impulse is constant across species, the hemispheres became increasingly isolated with the reduction in the ratio between corpus callosum and brain surface areas, originating specialized functions within each hemisphere (Ringo et al. 1994). In line with these expectations, a negative correlation between asymmetries in the surface area of common sulci and the relative size of the corpus callosum has been found in a variety of primate species, with humans having the highest levels of asymmetry and the largest brain volume (Hopkins et al. 2015). In contrast, a weak association between the magnitude of asymmetry of brain surface and brain size was found within and between hominoid species (Neubauer et al. 2020; Xiang et al. 2020). Particularly for modern humans, Xiang et al. (2020) showed that the magnitude of protrusion and bending of frontal and occipital lobes does not increase with brain size. Using a similar morphometric approach as our study, Neubaher et al. (2020) found that the amount of asymmetry in the endocasts of great apes and humans was not related to brain size. Whether such discrepancies reflect actual differences in the process that underlies the evolution of brain asymmetry among clades or are the product of differences in the methodological approaches requires the analysis of a wider sample of primate species with the same set of variables.
In sum, the differences in endocranial shape asymmetry found here suggest that the patterns of brain asymmetry in New World monkeys could be more variable than previously thought. Moreover, we showed that some clades that had not been studied before not only show a consistent directional asymmetry in shape but they display a right frontal and left-occipital protrusion, which was thought to be characteristic of great apes and humans. The analysis of several species also contributed to test different hypotheses about the diversification of endocranial asymmetries in a phylogenetic context. In particular, the diversification in the pattern of endocranial shape asymmetry in the New World monkeys was associated with socio-ecological factors, whereas the variation in magnitude seems to be a by-product of selection for increasing brain size along some clades. Consequently, our findings remark that the hypotheses tested are not mutually exclusive but different factors might drive the diversification in the pattern and magnitude of morphological asymmetry.
Availability of data and material
All data generated and analyzed during this study are included in this published article (Supplementary Tables S1 and S2).
Code availability
Code available upon request.
References
Aristide L, dos Reis SF, Machado AC, Lima I, Lopes RT, Perez SI (2015) Encephalization and diversification of the cranial base in platyrrhine primates. J Hum Evol 81:29–40. https://doi.org/10.1016/j.jhevol.2015.02.003
Aristide L, dos Reis SF, Machado AC, Lima I, Lopes RT, Perez SI (2016) Brain shape convergence in the adaptive radiation of New World monkeys. Proc Natl Acad Sci USA 113(8):2158–2163. https://doi.org/10.1073/pnas.1514473113
Aristide L, Strauss A, Halenar-Price LB, Gilissen E, Cruz FW, Cartelle C, Rosenberger AL, Lopes RT, dos Reis SF, Perez SI (2019) Cranial and endocranial diversity in extant and fossil atelids (Platyrrhini: Atelidae): a geometric morphometric study. Am J Phys Anthropol 169:322–331. https://doi.org/10.1002/ajpa.23837
Atkinson EG, Rogers J, Cheverud JM (2016) Evolutionary and developmental implications of asymmetric brain folding in a large primate pedigree. Evolution 70(3):707–715. https://doi.org/10.1111/evo.12867
Balzeau A, Gilissen E (2010) Endocranial shape asymmetries in Pan paniscus, Pan troglodytes and Gorilla gorilla assessed via skull based landmark analysis. J Hum Evol 59(1):54–69. https://doi.org/10.1016/j.jhevol.2010.03.013
Balzeau A, Grimaud-Hervé D, Gilissen E (2011) Where are inion and endinion? Variations of the exo-and endocranial morphology of the occipital bone during hominin evolution. J Hum Evol 61(4):488–502. https://doi.org/10.1016/j.jhevol.2011.07.002
Bruner E (2014) Human paleoneurology. Springer, Berlin. https://doi.org/10.1007/978-3-319-08500-5
Cameron R, Rogers LJ (1999) Hand preference of the common marmoset (Callithrix jacchus): problem solving and responses in a novel setting. J Comp Psychol 113(2):149. https://doi.org/10.1037/0735-7036.113.2.149
Clutton-Brock TH, Harvey PH (1980) Primates, brains and ecology. J Zool 190(3):309–323. https://doi.org/10.1111/j.1469-7998.1980.tb01430.x
Dryden IL, Mardia KV (1998) Statistical shape analysis. Willey, Chichester
Dumoncel J, Subsol G, Durrleman S, Bertrand A, de Jager E, Oettlé AC, Lockhat Z, Suleman FE, Beaudet A (2021) Are endocasts reliable proxies for brains? A 3D quantitative comparison of the extant human brain and endocast. J Anat 238:480–488. https://doi.org/10.1111/joa.13318
Dunbar RIM (1998) The social brain hypothesis. Evol Anthropol 6(5):178–190. https://doi.org/10.1002/(SICI)1520-6505(1998)6:5%3c178::AID-EVAN5%3e3.0.CO;2-8
Early CM, Iwaniuk AM, Ridgely RC, Lawrence MW (2020) Endocast structures are reliable proxies for the sizes of corresponding regions of the brain in extant birds. J Anat 237:1162–1176. https://doi.org/10.1111/joa.13285
Falk D (1980) Language, handedness, and primate brains: did the australopithecines sign? Am Anthropol 82(1):72–78. https://doi.org/10.1525/aa.1980.82.1.02a00040
Fournier M, Combès B, Roberts N, Braga J, Prima S (2011) Mapping the distance between the brain and the inner surface of the skull and their global asymmetries. Med Imaging Image Process 7962:79620Y. https://doi.org/10.1117/12.876795
Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160:712–726. https://doi.org/10.1086/343873
Ghirlanda S, Vallortigara G (2004) The evolution of brain lateralization: a game theoretical analysis of population structure. Proc R Soc Lond B 271:853–857. https://doi.org/10.1098/rspb.2003.2669
Giljov A, Karenina K, Malashichev Y (2018) Facing each other: mammal mothers and infants prefer the position favouring right hemisphere processing. Biol Lett 14(1):20170707. https://doi.org/10.1098/rsbl.2017.0707
Gómez-Robles A, Hopkins WD, Sherwood CC (2013) Increased morphological asymmetry, evolvability and plasticity in human brain evolution. Proc Biol Sci 280:20130575. https://doi.org/10.1098/rspb.2013.0575
Gomez- Robles A, Hopkins WD, Schapiro SJ, Sherwood CC (2016) The heritability of chimpanzee and human brain asymmetry. Proc Biol Sci 283:20161319. https://doi.org/10.1098/rspb.2016.1319
Gorrie CA, Waite PM, Rogers LJ (2008) Correlations between hand preference and cortical thickness in the secondary somatosensory (SII) cortex of the common marmoset, Callithrix Jacchus. Behav Neurosci 122(6):1343. https://doi.org/10.1037/a0013279
Gunz P, Mitteroecker P, Bookstein FL (2005) Semilandmarks in three dimensions. In: Slice DE (ed) Modern morphometrics in physical anthropology. Developments in primatology: progress and prospects. Springer, Boston, pp 73–98
Hartwig W, Rosenberger AL, Norconk MA, Owl MY (2011) Relative brain size, gut size, and evolution in New World monkeys. Anat Rec 294(12):2207–2221. https://doi.org/10.1002/ar.21515
Heilbroner PL, Holloway RL (1988) Anatomical brain asymmetries in New World and Old World monkeys: stages of temporal lobe development in primate evolution. Am J Phys Anthropol 76(1):39–48. https://doi.org/10.1002/ajpa.1330760105
Holloway RL, de la Cost Lareymondie MC (1982) Brain endocast asymmetry in pongids and hominids: some preliminary findings on the paleontology of cerebral dominance. Am J Phys Anthropol 58:101–110. https://doi.org/10.1002/ajpa.1330580111
Hopkins WD, Marino L (2000) Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI). Neuropsychologia 38(4):493–499. https://doi.org/10.1016/S0028-3932(99)00090-1
Hopkins WD, Pilcher DL, MacGregor L (2000) Sylvian fissure asymmetries in nonhuman primates revisited: a comparative MRI study. Brain Behav Evol 56(6):293–299. https://doi.org/10.1159/000047213
Hopkins WD, Misiura M, Pope SM, Latash EM (2015) Behavioral and brain asymmetries in primates: a preliminary evaluation of two evolutionary hypotheses. Ann N Y Acad Sci 1359(1):65–83. https://doi.org/10.1111/nyas.12936
Hou L, Xiang L, Crow TJ, Leroy F, Rivière D, Mangin JF, Roberts N (2019) Measurement of Sylvian Fissure asymmetry and occipital bending in humans and Pan troglodytes. Neuroimage 184:855–870. https://doi.org/10.1016/j.neuroimage.2018.08.045
Karolis VR, Corbetta M, De Schotten MT (2019) The architecture of functional lateralisation and its relationship to callosal connectivity in the human brain. Nat Commun 10(1):1–9. https://doi.org/10.1038/s41467-019-09344-1
Klingenberg CP, Barluenga M, Meyer A (2002) Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 56:1909–1920
LeMay M (1976) Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Ann N Y Acad Sci 280:349–366. https://doi.org/10.1111/j.1749-6632.1976.tb25499.x
Liu ST, Phillips KA (2009) Sylvian fissure asymmetry in capuchin monkeys (Cebus apella). Laterality 14(3):217–227. https://doi.org/10.1080/13576500802344404
Mesulam MM (1985) Attention, confusional states, and neglect. In: Mesulam MM (ed) Principles of behavioral neurology. Davis, Philadelphia, pp 125–168
Neubauer S, Gunz P, Hublin JJ (2010) Endocranial shape changes during growth in chimpanzees and humans: a morphometric analysis of unique and shared aspects. J Hum Evol 59:555–566
Neubauer S, Gunz P, Scott NA, Hublin JJ, Mitteroecker P (2020) Evolution of brain lateralization: a shared hominid pattern of endocranial asymmetry is much more variable in humans than in great apes. Sci Adv 6(7):eaax9935. https://doi.org/10.1126/sciadv.aax9935
Ocklenburg S, Güntürkün O (2012) Hemispheric asymmetries: the comparative view. Front Psychol 3:5. https://doi.org/10.3389/fpsyg.2012.00005
Olivares R, Montiel J, Aboitiz F (2001) Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain Behav Evol 57:98–105. https://doi.org/10.1159/000047229
Phillips KA, Sherwood CC (2005) Primary motor cortex asymmetry is correlated with handedness in capuchin monkeys (cebus apella). Behav Neurosci 119(6):1701–1704. https://doi.org/10.1037/0735-7044.119.6.1701
Phillips KA, Sherwood CC (2007) Cerebral petalias and their relationship to handedness in capuchin monkeys (Cebus apella). Neuropsychologia 45(10):2398–2401. https://doi.org/10.1016/j.neuropsychologia.2007.02.021
Phillips KA, Stimpson CD, Smaers JB, Raghanti MA, Jacobs B, Popratiloff A, Hof P, Sherwood CC (2015) The corpus callosum in primates: processing speed of axons and the evolution of hemispheric asymmetry. Proc R Soc B 282(1818):20151535. https://doi.org/10.1098/rspb.2015.1535
Pilcher DL, Hammock EA, Hopkins WD (2001) Cerebral volumetric asymmetries in non-human primates: a magnetic resonance imaging study. Laterality 6(2):165–179. https://doi.org/10.1080/713754406
Powell LE, Isler K, Barton RA (2017) Re-evaluating the link between brain size and behavioural ecology in primates. Proc R Soc B 284(1865):20171765. https://doi.org/10.1098/rspb.2017.1765
Rilling JK, Insel TR (1999) Differential expansion of neural projection systems in primate brain evolution. NeuroReport 10:1453–1459. https://doi.org/10.1097/00001756-199905140-00012
Ringo J, Doty R, Demeter S, Simard P (1994) Timing is of essence: a conjecture that hemispheric specialization arises from inter-hemispheric conduction delay. Cereb Cortex 4:331–343. https://doi.org/10.1093/cercor/4.4.331
Rogers LJ (2014) Asymmetry of brain and behavior in animals: its development, function, and human relevance. Genesis 52(6):555–571. https://doi.org/10.1002/dvg.22741
Rohlf FJ, Slice D (1990) Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Biol 39(1):40–59. https://doi.org/10.2307/2992207
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 20 Sept 2020
Schlager S (2012) Sliding semi-landmarks on symmetric structures in three dimensions. Am J Phys Anthropol 147:261. https://doi.org/10.1002/ajpa.21502
Schlager S (2017) Morpho and Rvcg-shape analysis in R. In: Zheng G, Li S, Szekely G (eds) Statistical shape and deformation analysis. Academic Press, New York, pp 217–256
Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, Fink GR (2003) Lateralized cognitive processes and lateralized task control in the human brain. Science 301(5631):384–386. https://doi.org/10.1126/science.1086025
Vallortigara G (2006) The evolutionary psychology of left and right: costs and benefits of lateralization. Dev Psychobiol 48(6):418–427. https://doi.org/10.1002/dev.20166
Vallortigara G, Rogers LJ (2005) Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci 28:575–633. https://doi.org/10.1017/S0140525X05000105
Watanabe A, Gignac PM, Balanoff AM, Green TL, Kley NJ, Norell MA (2019) Are endocasts good proxies for brain size and shape in archosaurs throughout ontogeny? J Anat 234:291–305. https://doi.org/10.1111/joa.12918
Xiang L, Crow TJ, Hopkins WD, Roberts N (2020) Comparison of surface area and cortical thickness asymmetry in the human and chimpanzee brain. Cereb Cortex. https://doi.org/10.1093/cercor/bhaa202
Acknowledgements
We thank two anonymous reviewers for the comments that helped to improve the clarity of this article.
Funding
This work is supported by Universidad Nacional de La Plata Grant # 911 to S.I.P. and P.N.G. (4113), FAPESP (2017/17357-0) to S.F.d.R.
Author information
Authors and Affiliations
Contributions
PNG: study conception and design, analysis and interpretation of data, drafting of manuscript, critical revision. MVA: study conception and design, analysis and interpretation of data, critical revision. LA: acquisition of data, analysis and interpretation of data, critical revision. RTL: acquisition of data, critical revision. SFR: acquisition of data, critical revision. SIP: study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
429_2021_2371_MOESM1_ESM.pdf
Supplementary Fig. S1. Distribution of specimens along the first two principal components (PC1 and PC2) obtained from the asymmetric shape component (PDF 43 kb)
429_2021_2371_MOESM2_ESM.xlsx
Supplementary Table S1. List of specimens included in this study. The information about the genus, species, specimen ID, endocranial volume and sex is provided (XLSX 11 kb)
429_2021_2371_MOESM3_ESM.csv
Supplementary Table S2. Morphometric and socio-ecological variables used for the comparative phylogenetic analyses (CSV 4 kb)
Rights and permissions
About this article
Cite this article
Gonzalez, P.N., Vallejo-Azar, M., Aristide, L. et al. Endocranial asymmetry in New World monkeys: a comparative phylogenetic analysis of morphometric data. Brain Struct Funct 227, 469–477 (2022). https://doi.org/10.1007/s00429-021-02371-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02371-z