Abstract
The phantom sound perception mechanism by which a sound perception occurs without any external sound source is still enigmatic. According to our previous fMRI study, a small region in the parietal operculum 3 was hyperactivated as a function of tinnitus periodicity in subjects with acoustic trauma tinnitus sequelae. This region was localized in the vicinity of neural correlates of middle-ear tympano-ossicular chain movements due to pressure variations. Disturbed proprioceptors are known to trigger illusory perceptions; therefore, we hypothesized that a disturbance of middle-ear proprioceptors may originate phantom sound perceptions. We designed an fMRI study that aimed to stimulate middle-ear proprioceptors by repetitive vibrations using various rates of click trains. In this study, we report that exposure to specific rates of stimuli for a few minutes at comfortable intensity level in healthy subjects distinctly triggered transient tinnitus-like aftereffects. The fMRI neural correlates of the aftereffects were unequivocally localized in the same parietal region as in acoustic trauma tinnitus sufferers. Our results strongly suggest that a middle-ear kinesthetic/proprioceptive illusion exists at the origin of acoustic trauma tinnitus via a somatosensory pathway encompassing the trigeminal system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tinnitus is a tonal auditory perception that typically occurs in the absence of external stimuli. This distressing phantom auditory perception with an estimated prevalence of approximately 10 % (Baguley et al. 2013) usually leads to problems ranging from mild discomfort such as sleep disturbance to strong anxiety and depression. The mechanism mediating tinnitus remains unknown.
Our former 3T fMRI study (Job et al. 2012) aiming to determine abnormal activation of brain regions in subjects with acoustic trauma sequelae suggested that the proprioception of the middle ear may play a key role in the origin of acoustic trauma-induced tinnitus (ATIT). Subjects with ATIT sequelae compared to normal hearing controls performing a simple auditory task had a hyperactivated region in the right parietal operculum (BA 43/40) (Fig. 2a, red voxels, black arrow) that was correlated with tinnitus periodicity. This region is defined as the operculum 3 (OP3) according to Eickhoff (2006, 2007) and the Anatomy toolbox (Jülich center, Germany). The parietal operculum region (OP) has only recently been studied in depth. This OP region appears to contain mirror-like somatosensory representation of the entire body (Eickhoff et al. 2006; Krubitzer et al. 1995; Burton et al. 1995). Activations have been reported for different body parts and under numerous experimental conditions such as light touch vibration (Ledberg et al. 1995; Disbrow et al. 2000), pain perception (Coghill et al. 1994; Mazzola et al. 2012), vibrotactile attention (Burton and Sinclair 2000), and kinesthetic illusion (Naito et al. 1999). Recently, we observed the representation of passive middle-ear movements by air pressure variation in the operculum (BA43) (Fig. 2a, green voxels) (Job et al. 2011). Consequently, we hypothesized that a middle-ear joint kinesthetic disturbance that is conveyed by middle-ear proprioceptors may occur at the origin of immediate ATIT.
The disturbance of proprioceptors (i.e., muscle spindle intrafusal fibers) present in body joints triggers illusory perceptions (Proske and Gandevia 2009), especially proprioceptors that are artificially disturbed by mechanical vibrations (pulse trains at specific repetition rates), and the joints reacted as if the muscle were stretched (Eklund 1972; Goodwin et al. 1972). In the absence of muscular contraction, the disturbance of muscle spindle proprioceptors triggered erroneous signals to the brain where the disturbance is interpreted as a movement of the joint (i.e., illusory movement). Muscle spindles are exquisitely sensitive to vibrations (Burke et al. 1976; Roll et al. 1989). Middle-ear muscles possess a few intrafusal fibers (Kierner et al. 1999) that may allow the tympano-ossicular chain to produce illusory perceptions. Additionally, muscle spindle intrafusal fibers possess sympathetic innervation (Grassi et al. 1993; Matsuo et al. 1995; Passatore et al. 1996) that may be activated in many ways (e.g., emotional stress, anxiety, and drugs), resulting in over-excitability of muscle spindles (Roatta et al. 2002).
Here, we tested on volunteers without tinnitus, the hypothesis (i) that middle-ear proprioceptors’ stimulation by various vibration rates (simulating mechanical vibrations) would produce a phantom sound aftereffect after the end of the stimulus and (ii) that the neural correlates of this phantom sound would involve the OP3 brain region.
Materials and methods
Preliminary behavioral experimental design
The aim of this preliminary study was to obtain a phantom auditory aftereffect using various vibration rate stimulations of a few minutes at comfortable levels and to choose stimulations used to localize the neural correlates of the aftereffect using fMRI. This study was conducted in accordance with the Declaration of Helsinki (2004), was approved by the local Ethics Committee and was referenced as N° 05-CRSS-1/CPP Sud-Est. Written informed consents from all individuals were obtained.
Participants (n = 25)
The preliminary behavioral experiment was conducted with twenty-five healthy volunteers (20 males, 5 females) aged between 21 and 53 years (median 35). No particular exclusion criteria were included except that the volunteers could not have any known hearing problems and frequent or permanent tinnitus. Due to the lack of biomarkers and objective methods to assess the presence of tinnitus, the only exclusion criteria included subjects that reported frequent/permanent tinnitus or hearing loss. Nevertheless, we checked auditory function using objective measurements of otoacoustic emissions (Kemp et al. 1990). The volunteers were mostly students or members of the institute.
Stimuli
We used Sound Forge™ Audio Studio 8.0 to synthesize and deliver clicks stimuli. The clicks were 1 ms sinusoidal broadband noise (0–22 kHz) with 44, 100 Hz sample rate and 16 bits resolution. The click trains were produced with five different rates (vibration rates) based on different inter-stimulus intervals (ISI), ISI = 8 ms (111 Hz, 111 clicks/s), ISI = 16 ms (59 Hz, 59 clicks/s), ISI = 32 ms (30 Hz, 30 clicks/s), ISI = 64 ms (15 Hz, 15 clicks/s) and ISI = 128 ms (8 Hz, 8 clicks/s), thus all of the click trains have the same spectral contents of sounds but differ only by their vibration rates. The vibration rates were chosen using a logarithmic scale to explore a large range of vibration rates that were associated with mechanoreceptors in terms of neuronal discharge rates (Taylor et al. 1999; Roll and Vedel 1982).
The running order of the five stimuli was randomized and balanced over the group of subjects and was repeated a week apart with a different running order to ensure the stability of the subject’s perception. The subjects were seated comfortably in a soundproof booth and listened via earphones (Sennheiser© HD 650) to each stimulus (i.e., train of clicks). Each stimulus was applied bilaterally and was adjusted by the subject itself to a comfortable level. The intensity levels were adjusted from the software volume (i.e., a scale ranging from 0 (no sound) to 100 uncomfortable sound). The settings for all of the subjects were lower than 55. Later, the intensity levels were measured from the scale (lower and higher score) under earphones by an artificial ear (sonometer Brüel & Kaejer type 2238 Mediator inserted in Brüel & Kaejer artificial pinnae DZ9769). The acoustic calibration of the sonometer was performed with a Brüel & Kaejer calibrator 4231. The intensity levels were comprised between 65 and 72 dB sound pressure level (LAeq).
Behavioral protocol
The protocol was an alternation of the 3-min click trains and the 5-min resting periods. The subjects had no particular instruction during the run of the stimulus except the last 30 s before the end of the stimulus, and the subjects were visually warned via the booth window to focus attention to their ears. At the end of the stimulus, subjects were asked to carefully listen for any auditory aftereffects. If they noticed perception of phantom sounds, they were instructed to raise their hand when the illusory perception vanished. The aftereffect duration was recorded using a chronometer.
During each resting period, the subject was asked to describe as acutely as possible the aftereffect, which was a difficult exercise for the subjects. At the end of the behavioral experiment, the subject had to complete a short questionnaire on their past experience of perceiving phantom sounds and their vivid experience of the aftereffect.
We found only one publication in the literature dating before the fifties that resembled our experimental design and the obtained aftereffects (Rosenblith et al. 1947). The authors used rectangular pulse train stimuli at various vibration rates coupled with earphones to trigger the phantom auditory aftereffects, but the authors based their experiments on three subjects, used a shorter duration of exposure (1–2 min) and much higher acoustic levels. This study appears to be the first time that phantom auditory perceptions were described and published.
Functional Magnetic Resonance Imaging (fMRI) experimental design
FMRI participants (n = 18)
Among the 25 volunteers, six volunteers could not participate in the fMRI protocol because of geographical constraint (4) and because of a doubt of slight permanent tinnitus that reach subjects’ consciousness after the behavioral test (2) and a volunteer was excluded from our image analysis because the brain images contain many movement artifacts. Thus, the fMRI results presented here were based on data obtained from the study of 18 subjects (15 males, 3 females) aged between 21 and 53 years (median 34).
FMRI stimuli
The stimuli were the same as in the audiometric booth and were delivered binaurally via headphones. Results of the behavioral test showed that 4 out of 5 vibration rates triggered phantom sound aftereffects with an average duration of 18 s and with different characteristics (see supplementary Fig. 5). For the purpose of the fMRI study, we chose only two vibration rates that triggered an opposite effect, the 30-Hz vibration rates produced a tinnitus-like phantom sounds (high-frequency whistling) in all of the subjects and the 8-Hz vibration rates did not produce any phantom sounds (Fig. 1a, b) (see supplementary audio 1 and 2). The stimuli sequences were programmed by Presentation® software (www.neurobs.com/presentation), triggered by the MR scanner and transmitted to MR Confon Gmbh technology® headphones to deliver the auditory stimuli. The audio presentation system provided electrodynamic headphones developed for the fMRI. It comprised an active gradient noise suppression, for an optimized undisturbed communication between subject and operator via an optical microphone and for an undisturbed reception of stimuli through headphones. The subjects were lying in supine position, and they were instructed to keep their eyes open. The subjects could see the operators piloting the experiment behind a large window via a mirror fixed on the head coil. The subjects were wearing ear foam plugs E.A.R® classic under headphones. Before scanner acquisition, in the scanner, fMRI subjects were asked to indicate if the sound level under the headphones was comfortable and subjectively as close as possible as the level set in the audiometric booth, and if needed, we adjusted the sound level and the balance between the ears. The subjects were not warned of the chosen stimuli.
Stimuli and the fMRI protocol. a Zoom of 1 s of stimuli. Top 1 ms click trains at 8 Hz (8-Hz vibration rate). Bottom 1 ms click trains at 30 Hz (30-Hz vibration rate). The stimuli were delivered binaurally via headphones at a comfortable level. b The behavioral test of the aftereffect perception (debriefing) in the fMRI condition. All of the subjects perceived an aftereffect after the 30-Hz stimulation. c Schematic representation of one of the six functional runs. During every 3 min (180 s) of the stimulation, 60 images were acquired, and 10 images were obtained during the post-stimulation period of 30 s. We used the first six images (0–18 s) to analyze the tinnitus-like aftereffects after the 30-Hz rate and the missing aftereffect at the 8-Hz rate. Thus, a total volume of 140 images was acquired with 36 images during the tinnitus-like aftereffects
The exposure to the vibration rates’ stimulation was 3 min. The 8-Hz vibration rate was set at a comfortable intensity level, and the 30-Hz vibration rate was delivered systematically 6 dB lower than the 8-Hz stimuli to ensure that an equivalent dose of acoustic energy was delivered to the ears. Despite the fact that both stimuli produced sounds, the differential proprioceptive effects on the hearing transmission system (i.e., middle ear) were investigated as the equivalent of pulse mechanical vibration rates of 30 and 8 Hz.
FMRI data acquisition
The fMRI protocol consisted of six functional runs. Each run was composed of two successive periods of 3 min (180 s) of stimulus (i.e., 8 or 30 Hz) followed by a 30-s blank (i.e., without stimulus) (Fig. 1c). The order of the stimuli presentation was balanced between runs and among subjects. Thirty seconds before the end of the 180-s stimulus, the subjects were warned by the operators by a hand sign to pay attention to their ears.
After each run, via the scanner communication system, we interviewed the subject to determine after which period the phantom sound was perceived.
We performed the fMRI scanning sessions on a 3T Philips Achieva-TX scanner (Best, The Netherlands) equipped with a 32 channel-head coil at Grenoble MRI facility-IRMaGe. The fMRI T2* BOLD-weighted sequence was adapted (i) to reduce the scanner noise, (ii) to avoid an aftereffect potentially induced by scanning sequences and (iii) to achieve a high spatial resolution of functional images. We collected six functional runs (gradient echo EPI, voxel size = 2 × 2 × 2 mm3, TR = 3,000 ms, TE = 28 ms, 24 axial slices centered on lateral sulci, SENSE factor = 3, 140 volumes) and a T1-weighted anatomical volume (3D MPRAGE, 0.9 × 0.9 × 1.2 mm3, TI = 800 ms, TR = 3,000 ms, TE = 28 ms, flip = 15°, SENSE factor = 4.4).
The MRI scanner noise was reduced by lowering the gradient slopes (SofTone mode = ‘yes’), thus participants could hear the stimuli and the aftereffect fairly well. To ensure that the scanner pulse noise did not trigger any phantom sound aftereffect itself, we used a TR of 3 s and 24 slices providing a scanner rhythm of 8 Hz that matched the control 8 Hz stimulus that did not produce any aftereffect.
FMRI analysis
According to the preliminary behavioral study in the audiometric booth, the duration of the aftereffects reported by the subjects was 18 ± 26 s; therefore, the first six images (0–18 s) post stimulus were used to model the aftereffect. Using SPM8 software (The Welcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm/), image pre-processing was performed in each subject’s referential and included motion correction, slice timing and registration to the anatomical image. A linear generalized model was then computed with the following four regressors: (i) the vibration rate period at 30 Hz, (ii) the aftereffect (0–18 s) following the 30-Hz stimulus, (iii) the vibration rate period at 8 Hz, and (iv) the post period (0–18 s) following the 8-Hz stimulus. Contrast images corresponding to post-control stimulus condition were computed separately for 30 and 8 Hz, normalized to the MNI space and spatially smoothed with a 2-mm isotropic kernel. In a first analysis aiming to provide our results in the same referential as in the study by Job, a standard normalization procedure was used. For inference at the group level, the contrasts were then entered into a random-effect analysis to derive the regions more activated after the stop of the stimulus (height threshold p < 0.0001, extend threshold k > 10).
In a second group analysis, we kept the benefit of high-resolution fMRI using DARTEL registration tool to improve the location of our hyperactivated region. DARTEL registration (Ashburner 2007) is one of the most powerful tools that permit accurate inter-individual brain realignment (Klein et al. 2009). We applied this transformation to the individual contrast images and repeated the group-level analysis.
Finally, in a third analysis, we reproduced the same procedure with the five last images post stimulus (18–30 s) to model the extinction of the aftereffect.
ROI analysis
To compare the aftereffects following 30 and 8 Hz, we selected four ROIs likely to be related to phantom sound perception, parietal operculum OP3 and primary auditory cortex (AI) bilaterally. Following our hypothesis, the right and left ROIs in OP3 were selected to identify potential hyperactivation symmetry in this region, and the ROIs in the primary auditory cortex were selected based on the hyperactivation reports found in the literature (see “Introduction”). We used the MarsBaR toolbox (http://www.fil.ion.ucl.ac.uk/spm/ext/#MarsBar) to define these ROIs. The left and right AI ROIs were drawn unambiguously following Heschl’s gyri (Da Costa et al. 2011) based on the mean anatomical images of our group of subjects because DARTEL registration permits accurate inter-individual brain realignment (Fig. 2). The left and right OP3 locations were adapted from a previous report (Job et al. 2012) to account for the anterior shift induced by DARTEL registration (box centered at [40 −13 17] with the following widths [9 4 4]). We applied each ROI as a mask on the contrast images corresponding to 30 and 8 Hz post-control stimulus, respectively, and we extracted the maximum of activation for each subject and each contrast. A paired t test between both conditions of vibration rates was used in the 0–18 s post-stimulation period to assess whether the hyperactivity following 30 Hz was significantly higher than following 8 Hz (p < 0.05). We used the same test during the extinction period (18–30 s) in which we expected a return to basal intensity values.
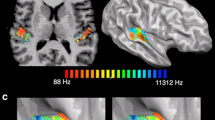
FMRI hyperactivation in the right parietal operculum 3 (OP3). a Background studies. Red voxels in the OP3 denoted by a black arrow correspond to the hyperactivation obtained in Job et al. (2012) in an acute acoustic trauma subject with tinnitus sequelae compared to controls during an auditory task. Green voxels in the OP3 correspond to the superimposition of the fMRI results of passive activation of the middle ear by mild ± 40 daPa air pressure variations (Job et al. 2011). b OP3 hyperactivation in the coronal and sagittal plane obtained during transient tinnitus-like aftereffects (T = 5.29, p < 0.0001, cluster level p = 0.025) after the 30-Hz vibration rate (same normalization procedure as in the background studies). c The same OP3 hyperactivation as in b after accurate inter-individual brain coregistration (DARTEL tool), permitting the precise localization in OP3 and eliciting an additional cluster in the left primary sensory cortex (SI). Coordinates (x y z) are in the Montreal Neurological Institute (MNI) space
Results
FMRI results
We compared pre–post-BOLD signal changes associated with two vibration rates (i.e., 30 and 8 Hz). The BOLD signal change significantly increased in a single area, the area corresponding to the right operculum 3 during the phantom auditory percept (0–18 s, post 30-Hz vibration rate) but not after the 8-Hz vibration rate (see supplementary Fig. 6). Supporting our hypothesis, this area is located at ([MNI 42 −18 22], T = 5.29, p < 0.0001, cluster level corr. p = 0.025) (Fig. 2b), which corresponds to the same area that was reported in the study by Job and colleagues (Job et al. 2012) at [MNI 42 −18 18] (Fig. 2a).
These coordinates were obtained using the same standard normalization procedure. Benefiting from DARTEL registration tool, we obtained a more accurate location of this area (Fig. 2c). The peak of hyperactivation in the right OP3 was slightly shifted in the anterior direction [MNI 40 −13 17] but definitely located in the parietal lobe, stretching from the operculo-insular junction to the more lateral parietal operculum. DARTEL registration also revealed that the left primary somatosensory cortex (SI) in the face area ([MNI −54 −13 40], T = 4.48, cluster level corr. p = 0.005) was significantly hyperactivated.
To quantify the aftereffect or the signal change after the application of different vibration rates, we performed an ROI analysis in the right OP3 region. Additionally, to test the possible asymmetry of responses and the potential involvement of primary auditory areas during phantom sound perception, we quantified the BOLD signal change in the left OP3 and in the bilateral Heschl’s gyri. In Fig. 3, the BOLD signal change (comparison pre–post-stimulation) in the operculum ROIs was significant only on the right side during the transient phantom auditory perception (≈0–18 s post-stimulation) under the 30-Hz condition (paired t test, t = 2,90, p = 0.005 unilat.) compared to the 8-Hz control condition (paired t test, t = 1,02, p = 0,161 unilat.). The BOLD signal change at 30 Hz was significantly different than the BOLD signal change at 8 Hz at the right OP3 (paired t test, t = 2,38, p = 0.014 unilat.). When the phantom auditory perceptions vanished (18–30 s post-stimulation), hyperactivity was no longer present (paired t test, t = 0,12, p = 0,480 unilat.) under the 30-Hz condition. In Heschl’s gyri, we did not observe any significant hyperactivity.
Regions of interest (ROI) analysis in the bilateral operculum 3 (OP3) and bilateral primary auditory (AI) cortex (Heschl’s gyrus). Graphs represent the signal changes in the ROIs (mean ± SEM based on maximal voxels intensity increase) following the 30-Hz vibration rate (tinnitus-like aftereffect) and the 8-Hz vibration rate (no aftereffect). Only the right OP3 (top right, left part) elicited a statistically significant transient signal increase with 30-Hz rate (paired t test, t = 2,90, p = 0.005 unilat.) that was significantly different from the signal increase from the 8-Hz rate (paired t test, t = 2,38, p = 0.014 unilat.) The tinnitus-like aftereffect was transient during the first 18 s and vanished during the 18–30 s period (top right, right part). The signal change was expressed in arbitrary units (au). *p < 0.05, **p < 0.01
Discussion
We tested our first hypothesis that transient tinnitus-like phantom sounds are elicited in the stimulating somatosensory receptors of the middle ear. We demonstrated that transient tinnitus-like aftereffects occur when using various vibration rates of click trains and without loud stimulation. Next, we tested our second hypothesis that the hyperactivated region in the parietal operculum OP3 in ATIT subjects (Job et al. 2012) could be related to phantom sound perceptions. Using high-resolution fMRI, we observed transient hyperactivity in the same OP3 region as in the study by Job, during transient tinnitus-like perceptions and a preferential activation to the right hemisphere occurred despite bilateral stimulations. This latter observation is intriguing and suggests that an ear-independent differential hemispheric involvement occurs in phantom auditory perceptions. It would be possibly linked to the fact that sound processing is known to be preferentially ruled by the right hemisphere (Hugdahl 2000; Devlin et al. 2003); therefore, sound, even illusory sound, may be preferentially processed by the right hemisphere. In addition, we observed hyperactivity in the SI region in both sides, clearly significant in left side, corresponding to the sensory representation in face area, confirming that a somatosensory stimulation had occurred. Contribution to kinesthesia by cutaneous vibration receptors (Collins et al. 2005) of the eardrum would be one possible explanation of the SI activation because Meissner corpuscules (i.e., cutaneous vibration receptors) optimally respond to vibrations of approximately 30 Hz (Weerakkody et al. 2007).
The absence of specific reports of OP3 hyperactivation in the field of fMRI tinnitus may be partially due to very little available knowledge regarding this brain region, a small OP3 hyperactivated region and the absence of high-resolution studies of this area in fMRI tinnitus studies. Inaccurate brain coregistration and large voxel smoothing gave rise to imprecise localization, particularly around the sulci. For instance, in Maudoux’s study (Maudoux et al. 2012), the hyperactivated region in tinnitus subjects is reported as part of the auditory cortex [i.e., superior & transverse temporal gyrus (41/42/22)]; however, the strongest significant local maxima (t = 13.97) were clearly located in the right parietal cortex in operculum [MNI 62 −18 23].
No significant hyperactivity was observed in the primary auditory cortex concomitant with right OP3 hyperactivation triggered during the transient phantom sound perceptions. This result may strongly indicate that the somatosensory system may be the predominant system active during phantom perceptions.
Connections exist between the auditory and somatosensory system. Inputs from the cochlea and middle ear may meet in the brainstem. Cochlear nuclei are innervated by both the ophthalmic and mandibular divisions of the trigeminal ganglion that also project to the cochlea and middle ear (i.e., tensor tympani muscle), respectively (Vass et al. 1998). Thus, excitability of the auditory pathway is up-regulated by the auditory nerve coming from the cochlea and also by the multimodal sensory inputs (Pilati et al. 2012). According to Pilati and colleagues, dorsal cochlear nucleus fusiform cells integrate information from the auditory nerve but also via granule cell axons (parallel fibers), the site of integration from multimodal sensory inputs from the trigeminal system (Shore et al. 2000; Zhou and Shore 2004), the pontine nucleus (Babalian 2005; Itoh et al. 1987) and gracile nuclei. Pilati and colleagues reported that these inputs are likely to encode proprioceptive information. We agree with this analysis and additionally believe that middle-ear movements and joint position feedbacks are also part of this encoding.
In the context of acoustic trauma, frequent or too strong triggering of the middle-ear reflex and startle reflex under repetitive impulse noise pressure (Westcott et al. 2013) may cause muscular tension/fatigue and the micro-disruption of the tympano-ossicular chain, resulting in a disturbance of middle-ear proprioceptors (i.e., intrafusal fibers). The disturbance in proprioceptive feedbacks of the joint, in the kinesthetic sense of tensor tympani muscle and stapedius muscle, may generate illusory movements of the tympano-ossicular chain. These illusory movements may mimic external sounds arriving to the ear, which may trigger an illusory sound to the brain. Moreover, tinnitus escalation may occur by the activation of muscle spindle sympathetic fibers acting via the autonomous nervous system, which have been shown to play a role in tinnitus (Vanneste and De Ridder 2013). Our conclusive results prompted us to present a revised model for ATIT, with the awareness of lacking components, which accounts for the primordial role of the somatosensory pathway (Fig. 4). Two concomitant phenomena would occur during an acoustic trauma such as cochlear hair cell impairments and middle-ear muscle spindle disturbances. The former causes hearing loss via the auditory pathway, and the latter leads to tinnitus (i.e., kinesthetic illusions) via the somatosensory pathway with a contribution of the autonomous sympathetic systems on muscle spindles. It would not exclude the presence of hyperactivities along the auditory pathway in acoustic trauma conditions, in relation to hyperacusis (pain to sound) for example. Inputs from the trigeminal system have been observed along the auditory pathway at least up to the inferior colliculus and have been shown to modulate sound-evoked responses (Jain and Shore 2006).
Schematic representation of the middle-ear kinesthetic illusion model for acoustic trauma-induced tinnitus (ATIT). Kinesthetic proprioceptors of the middle ear (i.e., intrafusal fibers) and cutaneous receptors of vibrations would be the peripheral starting point of the disturbance resulting in phantom auditory perceptions. A somatosensory pathway via the trigeminal system is proposed with a neuronal excitability modulation by sympathetic fibers (autonomous nervous system) innervating the middle-ear intrafusal fibers. Somatosensory disturbances or impairments affected the excitability of neurons along the auditory pathway starting from the inputs to the cochlear nuclei fusiform cells. Major contributions to the illusory perceptions (large black arrows), other contributions to illusory perceptions (medium black arrows), structure interactions (fine black arrows), modulations arising between the trigeminal system and other structures of the auditory pathway (dashed black line arrows)
In summary, our study demonstrates that the involvement of a somatosensory pathway explains the tinnitus-like phantom auditory perceptions whose central representation is located in the right parietal operculum OP3. Middle-ear proprioception in humans has been poorly documented and the presented model may be enriched by further investigations. Functional and anatomical connectivity using resting-state fMRI and diffusion tensor imaging applied in this OP region will provide further insights. We think our results open a new field of fundamental research based on the proprioception of the middle ear and of new therapeutic strategies for alleviating tinnitus.
References
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38(1):95–113. doi:10.1016/j.neuroimage.2007.07.007
Babalian AL (2005) Synaptic influences of pontine nuclei on cochlear nucleus cells. Exp Brain Res 167(3):451–457. doi:10.1007/s00221-005-0178-8
Baguley D, McFerran D, Hall D (2013) Tinnitus. Lancet 382(9904):1600–1607. doi:10.1016/S0140-6736(13)60142-7
Burke D, Hagbarth KE, Lofstedt L, Wallin BG (1976) The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol 261(3):673–693
Burton H, Sinclair RJ (2000) Tactile-spatial and cross-modal attention effects in the primary somatosensory cortical areas 3b and 1-2 of rhesus monkeys. Somatosens Mot Res 17(3):213–228
Burton H, Fabri M, Alloway K (1995) Cortical areas within the lateral sulcus connected to cutaneous representations in areas 3b and 1: a revised interpretation of the second somatosensory area in macaque monkeys. J Comp Neurol 355(4):539–562. doi:10.1002/cne.903550405
Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH (1994) Distributed processing of pain and vibration by the human brain. J Neurosci 14(7):4095–4108
Collins DF, Refshauge KM, Todd G, Gandevia SC (2005) Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol 94(3):1699–1706. doi:10.1152/jn.00191.2005
Da Costa S, van der Zwaag W, Marques JP, Frackowiak RS, Clarke S, Saenz M (2011) Human primary auditory cortex follows the shape of Heschl’s gyrus. J Neurosci 31(40):14067–14075. doi:10.1523/JNEUROSCI.2000-11.2011
Devlin JT, Raley J, Tunbridge E, Lanary K, Floyer-Lea A, Narain C, Cohen I, Behrens T, Jezzard P, Matthews PM, Moore DR (2003) Functional asymmetry for auditory processing in human primary auditory cortex. J Neurosci 23(37):11516–11522 pii: 23/37/11516
Disbrow E, Roberts T, Krubitzer L (2000) Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV. J Comp Neurol 418(1):1–21
Eickhoff SB, Schleicher A, Zilles K, Amunts K (2006) The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex 16(2):254–267. doi:10.1093/cercor/bhi105
Eickhoff SB, Grefkes C, Zilles K, Fink GR (2007) The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex 17(8):1800–1811. doi:10.1093/cercor/bhl090
Eklund G (1972) General features of vibration-induced effects on balance. Ups J Med Sci 77(2):112–124
Goodwin GM, McCloskey DI, Matthews PB (1972) Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science 175(4028):1382–1384
Grassi C, Deriu F, Passatore M (1993) Effect of sympathetic nervous system activation on the tonic vibration reflex in rabbit jaw closing muscles. J Physiol 469:601–613
Hugdahl K (2000) Lateralization of cognitive processes in the brain. Acta Psychol (Amst) 105(2–3):211–235
Itoh K, Kamiya H, Mitani A, Yasui Y, Takada M, Mizuno N (1987) Direct projections from the dorsal column nuclei and the spinal trigeminal nuclei to the cochlear nuclei in the cat. Brain Res 400(1):145–150 pii: 0006-8993(87)90662-7
Jain R, Shore S (2006) External inferior colliculus integrates trigeminal and acoustic information: unit responses to trigeminal nucleus and acoustic stimulation in the guinea pig. Neurosci Lett 395(1):71–75
Job A, Paucod J-C, O’Beirne GA, Delon-Martin C (2011) Cortical representation of tympanic membrane movements due to pressure variation: a fMRI study. Hum Brain Mapp 32:744–749
Job A, Pons Y, Lamalle L, Jaillard A, Buck K, Segebarth C, Delon-Martin C (2012) Abnormal cortical sensorimotor activity during “Target” sound detection in subjects with acute acoustic trauma sequelae: an fMRI study. Brain Behav 2(2):187–199. doi:10.1002/brb3.21
Kemp DT, Ryan S, Bray P (1990) A guide to the effective use of otoacoustic emissions. Ear Hear 11(2):93–105
Kierner AC, Zelenka I, Lukas JR, Aigner M, Mayr R (1999) Observations on the number, distribution and morphological peculiarities of muscle spindles in the tensor tympani and stapedius muscle of man. Hear Res 135(1–2):71–77 pii: S0378-5955(99)00092-1
Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV (2009) Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46(3):786–802. doi:10.1016/j.neuroimage.2008.12.037
Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M (1995) A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci 15(5 Pt 2):3821–3839
Ledberg A, O’Sullivan BT, Kinomura S, Roland PE (1995) Somatosensory activations of the parietal operculum of man. A PET study. Eur J Neurosci 7(9):1934–1941
Matsuo R, Ikehara A, Nokubi T, Morimoto T (1995) Inhibitory effect of sympathetic stimulation on activities of masseter muscle spindles and the jaw jerk reflex in rats. J Physiol 483(Pt 1):239–250
Maudoux A, Lefebvre P, Cabay JE, Demertzi A, Vanhaudenhuyse A, Laureys S, Soddu A (2012) Auditory resting-state network connectivity in tinnitus: a functional MRI study. PLoS One 7(5):e36222. doi:10.1371/journal.pone.0036222
Mazzola L, Faillenot I, Barral FG, Mauguiere F, Peyron R (2012) Spatial segregation of somato-sensory and pain activations in the human operculo-insular cortex. Neuroimage 60(1):409–418. doi:10.1016/j.neuroimage.2011.12.072
Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE (1999) Illusory arm movements activate cortical motor areas: a positron emission tomography study. J Neurosci 19(14):6134–6144
Passatore M, Deriu F, Grassi C, Roatta S (1996) A comparative study of changes operated by sympathetic nervous system activation on spindle afferent discharge and on tonic vibration reflex in rabbit jaw muscles. J Auton Nerv Syst 57(3):163–167 pii: 0165183895000747
Pilati N, Ison MJ, Barker M, Mulheran M, Large CH, Forsythe ID, Matthias J, Hamann M (2012) Mechanisms contributing to central excitability changes during hearing loss. Proc Natl Acad Sci USA 109(21):8292–8297. doi:10.1073/pnas.1116981109
Proske U, Gandevia SC (2009) The kinaesthetic senses. J Physiol 587(Pt 17):4139–4146. doi:10.1113/jphysiol.2009.175372
Roatta S, Windhorst U, Ljubisavljevic M, Johansson H, Passatore M (2002) Sympathetic modulation of muscle spindle afferent sensitivity to stretch in rabbit jaw closing muscles. J Physiol 540(Pt 1):237–248 pii:PHY_14316
Roll JP, Vedel JP (1982) Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47(2):177–190
Roll JP, Vedel JP, Ribot E (1989) Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 76(1):213–222
Rosenblith WA, Miller GA, Egan JP, Hirsh IJ, Thomas GJ (1947) An auditory afterimage? Science 106(2754):333–335. doi:10.1126/science.106.2754.333
Shore SE, Vass Z, Wys NL, Altschuler RA (2000) Trigeminal ganglion innervates the auditory brainstem. J Comp Neurol 419(3):271–285
Taylor A, Ellaway PH, Durbaba R (1999) Why are there three types of intrafusal muscle fibers? Prog Brain Res 123:121–131
Vanneste S, De Ridder D (2013) Brain areas controlling heart rate variability in tinnitus and tinnitus-related distress. PLoS One 8(3):e59728. doi:10.1371/journal.pone.0059728
Vass Z, Shore SE, Nuttall AL, Miller JM (1998) Direct evidence of trigeminal innervation of the cochlear blood vessels. Neuroscience 84(2):559–567 pii: S0306-4522(97)00503-4
Weerakkody NS, Mahns DA, Taylor JL, Gandevia SC (2007) Impairment of human proprioception by high-frequency cutaneous vibration. J Physiol 581(Pt 3):971–980. doi:10.1113/jphysiol.2006.126854
Westcott M, Sanchez TG, Diges I, Saba C, Dineen R, McNeill C, Chiam A, O’Keefe M, Sharples T (2013) Tonic tensor tympani syndrome in tinnitus and hyperacusis patients: a multi-clinic prevalence study. Noise Health 15(63):117–128. doi:10.4103/1463-1741.110295
Zhou J, Shore S (2004) Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res 78(6):901–907. doi:10.1002/jnr.20343
Acknowledgments
We are grateful to Alain Roux and Hervé Mathieu for their technical assistance and the NATO group HFM-229 for their precious advises. This work was supported by Grants from Délégation Générale de l’Armement (DGA) under number 11 CA 801 (to A. J.). We thank the Grenoble MRI facility, partially funded by the French national program “Investissement d’Avenir” run by the ‘Agence Nationale pour la Recherche’ Grant No- ANR-11-INBS-0006.
Conflict of interest
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Job, A., Jacob, R., Pons, Y. et al. Specific activation of operculum 3 (OP3) brain region during provoked tinnitus-related phantom auditory perceptions in humans. Brain Struct Funct 221, 913–922 (2016). https://doi.org/10.1007/s00429-014-0944-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0944-0