Abstract
On account of its strong efferent projections to the hippocampus, recent animal studies have emphasized an important role for the nucleus reuniens (NRe) of the midline thalamus in spatial memory. However, by virtue of its reciprocal connections with the orbital and ventromedial prefrontal cortex, the NRe may also be involved in aspects of executive inhibition. To date, there has been no systematic attempt to examine the role of the NRe in inhibitory mechanisms of response control. Accordingly, we compared rats with neurotoxic lesions of the NRe with sham surgery controls on performance of the 5-choice reaction time task, a test of visuospatial attention and inhibitory control. When tested post-operatively, rats with NRe lesions were unable to actively inhibit premature responses when the intertrial interval was varied. However, the same rats with NRe lesions showed normal inhibition of perseverative responses, and under some conditions were less perseverative than shams. The NRe lesion was also associated with a reduction in omissions and fast reward collection latencies, which persisted 2 months following surgery. The NRe lesion did not affect response accuracy or latency to respond correctly throughout the course of experimental testing. Together, these results signify the important role of the NRe in impulse inhibition, especially when slight changes are made to the temporal demands of the environment, and reveal the potential contribution of the NRe in motivational processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thalamus is a complex structure whose component nuclei are interconnected with distinct portions of the telencephalon, including nearly the entire cerebral cortex, the hippocampus, and striatum. In human patients, thalamic damage is commonly associated with diencephalic amnesia, a memory impairment thought to result from a disconnection of thalamic and hippocampal structures (von Cramon et al. 1985; Zola-Morgan et al. 1986; Bogousslavsky et al. 1988a; Graff-Radford et al. 1990). Thalamic damage also leads to deficits in inhibitory control, which is generally attributed to the thalamic deafferentation of prefrontal structures relevant to executive control (Bogousslavsky et al. 1988b; Janowsky et al. 1989; McGilchrist et al. 1993; Pepin and Auray-Pepin 1993; Daum and Ackermann 1994; Van Der Werf et al. 1999). Characterizing the specific role of individual thalamic nuclei in humans is difficult because thalamic damage is usually caused by infarction, tumor or trauma, and is therefore diffuse and indiscriminate (Squire and Moore 1979; Bogousslavsky et al. 1988b; Partlow et al. 1992). Using a targeted lesion approach, animal studies have investigated the role of several thalamic nuclei, such as the anterior, medial, and intralaminar group of nuclei, in aspects of spatial memory (Aggleton and Mishkin 1983; Zola-Morgan and Squire 1985; Byatt and Dalrymple-Alford 1996; Sziklas and Petrides 1999; Burk and Mair 2001; Mitchell and Dalrymple-Alford 2005; Mitchell et al. 2007), but have placed relatively little emphasis on their contribution to the executive function of inhibitory control (Chudasama and Muir 2001; Chudasama et al. 2001). In this respect, the nucleus reuniens (NRe) of the ventral midline thalamus is of particular interest as its role in spatial navigation requires both memory for location and the inhibition of inefficient searching strategies (Davoodi et al. 2009; Dolleman-van der Weel et al. 2009; Hembrook and Mair 2011).
In rats, the NRe is the largest of the midline thalamic nuclei. It is located directly above the third ventricle and is located within the anterior one-third of the rat thalamus (Swanson 1998; Bokor et al. 2002; Groenewegen and Witter 2004). It is the principal source of thalamic input to the hippocampus (Herkenham 1978; Wouterlood et al. 1990; Su and Bentivoglio 1990; Vertes et al. 2006), exerting a strong excitatory drive to the ventral CA1 region (Dolleman-Van der Weel et al. 1997; Bertram and Zhang 1999). The NRe also sends strong projections to the orbital and ventromedial regions of the prefrontal cortex (Ohtake and Yamada 1989; Hoover and Vertes 2007), which are reciprocated (Vertes 2002, 2004; McKenna and Vertes 2004). Thus, in addition to influencing the activity of the ventral hippocampus, the NRe can potentially modulate the orbital and ventral regions of the prefrontal cortex, an area associated with multiple aspects of inhibitory control (Ragozzino et al. 1999; Schoenbaum et al. 2002, 2003; Passetti et al. 2002; Chudasama and Robbins 2003; Chudasama et al. 2003; McAlonan and Brown 2003; Bissonette et al. 2008).
Here we used neurotoxic lesions in rats to investigate the role of the NRe in the inhibitory control of inappropriate responses (Chudasama et al. 2003; Dalley et al. 2011). Previous evidence points to the NRe as having a role in the inhibitory control of impulsive, anticipatory behavior in a jumping test (see Flämig and Klingberg 1978), as well as the inhibition of inappropriate searching strategies in a standard watermaze task (Dolleman-van der Weel et al. 2009). However, the effect of NRe lesions in controlled tests of inhibitory control has not been systematically examined. In the present study, we used the 5-choice reaction time task (5-choice task), a prefrontal-dependent test of visuospatial attention and inhibitory control. In the 5-choice task, rats must accurately detect and respond to brief visual stimuli which are presented in random spatial locations. To do this optimally, they must inhibit inappropriate responses in anticipation of the visual stimulus (premature responses), as well as inhibit responses following a correct response (perseverative responses). Deficits in these forms of inhibition lead to impulsiveness and compulsiveness, respectively. We show for the first time that NRe lesions lead to contrasting effects on these two aspects of inhibitory control, increasing premature responses, while sometimes markedly attenuating repeated perseverative responses. This dissociation was demonstrated in animals that in the same testing session showed no deficits to their visual attention.
Materials and methods
Subjects
All subjects were male Long Evans rats (Charles River Laboratories, Saint-Constant, CA) housed in pairs in a temperature controlled room (21–22°C) with a 12-h light/dark cycle. All subjects were food restricted and maintained at 85% of their free-feeding weight throughout the entire experiment with water available ad libitum. Behavioral testing took place within the light period of the cycle. At the beginning of testing, animals weighed 200–250 g. All experimental procedures were approved by the McGill University Animal Care and Use Committee and were carried out in accordance with the guidelines of the Canadian Council on Animal Care.
Apparatus
The test apparatus consisted of four 25 cm × 25 cm operant testing chambers (Lafayette, IA, USA). Each chamber was enclosed within a soundproof cabinet and was ventilated by low level noise fans, which also provided background noise throughout testing. Each testing chamber contained a 3 W houselight mounted in the center of the roof. The back wall of each testing chamber was concave and contained nine apertures, four of which were occluded with a metal shield. An infrared photocell at the entrance of each aperture detected nose-poke responses made by the rat. Illumination of the apertures was provided by a 3 W light bulb located at the rear of each aperture. On the wall opposite the apertures was a food magazine connected to a food pellet dispenser. Sucrose pellets served as food reward (Dustless Precision Pellets, Bioserve, NJ, USA). The apparatus and online data collection were controlled by a Dell Optiplex 745 computer (MCS, Montreal, CA) using the Whisker control system for research (Cardinal and Aitken 2010).
Behavioral procedures
Habituation and magazine training
Rats were initially given two sessions in which they were allowed to habituate to the testing chamber and collect sucrose pellets from the food magazine. A few sucrose pellets were also placed inside the apertures to encourage the rat to insert its nose into the aperture. In the first session (30 min), the houselight and food magazine light were illuminated and 10–15 sucrose pellets were freely available in the food magazine. In the second session (10 min), the houselight and food magazine light were illuminated and pellets were delivered into the food magazine according to a variable interval schedule of 15 s. Once rats were reliably retrieving and consuming pellets, they were ready for behavioral training.
Behavioral training
A schematic illustration of the sequence of events and performance measures during a single trial of the 5-choice task is provided in Fig. 1. Rats were trained to detect a brief visual stimulus presented pseudorandomly in one of five spatial locations, as described previously (Muir et al. 1996). At the beginning of each test session, the houselight was illuminated and a single 45 mg sucrose pellet was dispensed into the food magazine. A trial was initiated by the rat making an entry into the food magazine to collect this pellet. Following a fixed 5 s intertrial interval (ITI), the light at the rear of one aperture was illuminated for 0.5 s. A response in this aperture during the illumination of the light and within the following 4.5 s was rewarded with the delivery of a sucrose pellet, and recorded as a correct response. Thus, the total response time (limited hold) was 5 s. A response in a non-illuminated hole (incorrect response) or a failure to respond within the limited hold period (omission) was not rewarded. The next trial began following a 5 s period of darkness (time-out) during which all lights were extinguished. In addition, responses in the holes during the ITI, before the onset of the target light, were scored as premature responses, and were also punished with a 5 s time-out. Additional responses made in the apertures during the limited hold following a correct response were recorded as perseverative responses. A response in the food magazine after the delivery of a sucrose pellet, or after a time-out, initiated the next trial.
During each session, the light stimulus was presented an equal number of times in each of the five apertures in a pseudorandom order. A daily session consisted of 100 trials or was terminated after 30 min of testing. For the first session of training, the duration of the stimulus and the limited hold were set at 60 s. These variables were altered on subsequent sessions according to the individual rat’s performance until the target set of task parameters could be instituted. The target parameters were stimulus duration, 0.5 s; limited hold, 5 s; ITI and time-out, 5 s. The rats were considered to have reached criterion when these target parameters were attained over ten consecutive sessions with >75% correct responses and <20% omissions within the 30 min session time. Approximately 25 sessions were required for the rats to attain this criterion. Once rats had acquired this training criterion, they received NRe lesions or sham control surgery.
Two weeks following surgery, rats were tested across ten sessions on the standard schedule of the task (baseline). After the baseline test sessions, various manipulations to the basic test schedule were instituted. First, the stimulus duration was reduced to 0.2 s to increase the attentional challenge. Second, in order to assess the effect of stimulus unpredictability and control of responding during the ITI, rats were exposed to one session of variable long ITIs (5, 7, 9, 11 s). The session length for this manipulation was increased to 45 min. In another session, rats were subjected to variable short ITIs (0.5, 1.5, 3, 4.5 s). Each ITI was presented pseudorandomly, 25 times during the 100-trial session. Each manipulation was preceded by a baseline session. Approximately 2 months post-surgery, performance was re-evaluated on the standard baseline schedule of the task for a further 7 days.
Performance measures
Accuracy of performance was measured as the proportion of correct responses (correct responses/total responses) expressed as a percentage, without including errors of omission. Errors of omission were defined as failures to make a response during the 5 s limited hold period, expressed as a percentage of the total number of trials. This measure reflected possible failures of detection as well as motivational/motor deficits, depending on the overall pattern of effects.
The number of premature responses made in the holes during the ITI provided a measure of impulsivity. Such responses occurred inappropriately before the onset of the visual target, presumably because the rat anticipated their occurrence. In addition, the number of perseverative responses provided a measure of compulsivity. These responses persisted following a correct response and were, therefore, inappropriate to the task. Speed was assessed according to two different latencies. The latency to respond correctly (correct latency) was the time from the onset of the stimulus to when the rat made the correct response. The reward collection latency was the time between the correct response and when the rat entered the food magazine to collect food reward.
Locomotor activity
Following post-operative testing, locomotor activity was assessed using four standard home cage activity frames. Each home cage was a clear polycarbonate tub (61 cm wide × 37 cm long × 20.5 cm high) lined with sawdust, and covered with a barrier filter lid (Ancare, Bellmore, USA). Each home cage was placed within a Cage Rack SmartFrame™ (58 cm wide × 60.33 cm long × 2.11 cm high) equipped with infrared photobeams located on the interior perimeter of the frame (Lafayette, IA, USA). The rat was placed in the activity cage for 2 h. The total number of horizontal beam breaks were recorded using MotorMonitor™ software, version 5.05 (Lafayette, IA, USA), and transmitted to a Dell Optiplex 745 computer (MCS, Montreal, CA).
Data analysis
Data for each variable were subjected to a repeated measures analysis of variance using the PASW statistical package, version 18.0 (PASW Inc, Chicago, USA). Homogeneity of variance across groups was assessed using the Mauchly’s sphericity test. When data sets significantly violated this requirement for a repeated measures design, Greenhouse–Geisser was used to calculate a more conservative p value for each F ratio. The criterion for statistical significance was a probability level of p < 0.05. The between subjects factor was lesion [two levels: sham control group (shams) and lesion group nucleus reuniens (NRe)]. The within subjects factor was session (10 days for post-operative baseline 1, 7 days for post-operative baseline 2), stimulus duration at two levels (0.5 and 0.2 s), and ITI intervals as appropriate. The within subjects factor for locomotor activity was number of blocks of 15 min over 2 h (i.e., 8 blocks).
Surgical procedures
Rats received neurotoxic lesions of the ventral midline NRe of the thalamus or sham control surgery. All animals were anesthetized with isoflurane (4–5% induction, 1–3% maintenance) and placed in a Kopf stereotaxic frame fitted with atraumatic ear bars (David Kopf Instruments, Tujunga, USA). The incisor bar was set at −3.3 mm.
The NRe is an unpaired midline nucleus, which is located beneath the midline sagittal sinus. The scalp of the animal was retracted to expose the skull, and a square bone flap above the target region of the brain was removed to expose the sagittal sinus. For lesions of the NRe (n = 17), rats received a total of four injections of 0.1 μL of 0.09 M N-methyl-d-aspartic acid (NMDA; Sigma-Aldrich, CA) dissolved in 0.9% saline. Injections were made using a 0.5 μL SGE precision microsyringe (Canadian Life Science, Peterborough, CA) at the following anterior–posterior (AP), and dorsoventral (DV) co-ordinates from bregma: AP −1.72 mm; DV −7.40 mm; and AP −2.40 mm; DV −7.40 mm. All DV readings were taken from the dural surface. However, because the NRe is located ventrally and beneath the midline sagittal sinus, the mediolateral (ML) reading was taken from either side of the sagittal sinus (two injections to the left of the sinus, and two injections to the right). This ML reading worked out to be ~0.2 mm from bregma. All coordinates were taken from Paxinos and Watson (2005). Each injection was made over 1 min and the syringe remained in place for another minute to allow dispersion of the toxin before the needle was retracted. Rats that received sham control surgery (n = 11) received the same surgical procedure but received injections of 0.9% saline instead of NMDA.
Histology
At the conclusion of behavioral testing, the animals were perfused transcardially with 0.9% saline followed by 4% paraformaldehyde prepared in 0.1 M phosphate buffered saline. After dehydration by immersion in 30% sucrose, brains were sectioned using a cryostat at 40 μm thickness. Every second section was mounted on a gelatine-coated slide and stained with cresyl violet. These sections were used to identify the location of the lesion and to determine the degree of lesion-induced neuronal loss within the NRe.
Results
Histological analysis
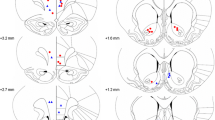
Cytoarchitectonic borders and nomenclature were taken from the atlas by Paxinos and Watson (2005). Figure 2a provides a diagrammatic reconstruction of the lesion showing the smallest and the largest extent of the NRe lesion. The photomicrographs shown in Fig. 2b and c provide a high magnification of the midline thalamic nuclei in a sham-operated rat and NRe-lesioned rat, respectively. Examination of the cresyl violet-stained sections revealed that three rats in the NRe group had a small or incomplete lesion. Another four rats showed extensive damage that included the subthalamic nucleus, centromedial nucleus, and periventricular gray. These seven rats were excluded from analysis. The remaining ten rats showed extensive neuronal loss in the NRe. The lesion started rostrally −1.7 mm from bregma and included the perireuniens except the most lateral extent on the left. At its most rostral level the lesion encroached into the rhomboid nucleus. The lesion then continued until −3.7 mm from bregma where the NRe lesion encroached into the ventral limits of the central medial thalamic nucleus, the ventral posterior nucleus of the thalamus, and the most dorsal limit of the posterior hypothalamic area. In the sham group, two rats showed cell damage within the dorsal hippocampus and minor damage to the NRe. These two rats were discarded from analysis. The final group numbers for this study were as follows: shams 9, and NRe 10.
Extent of NRe lesion. a Schematic reconstructions of representative coronal brain sections showing the largest (dark gray) and smallest (light gray) extent of the NRe lesion. Numbers indicate sections relative to bregma according to the atlas of Paxinos and Watson (2005). Representative photomicrographs of Nissl-stained coronal sections provide a magnified view of the midline group of thalamic nuclei in a sham-operated rat (b) compared with NRe-lesioned rat (c). AM anteromedial thalamic nucleus, CM centromedial thalamic nucleus, DA dorsal hypothalamic area, f fornix, MD mediodorsal thalamic nuclei, NRe nucleus reuniens, PHD posterior hypothalamic area, dorsal, Rh rhomboid, Rt reticular thalamic nucleus, VL ventrolateral thalamic nucleus, 3V third ventricle, AHC central hypothalamus, anterior, PV paraventricular nucleus, VM ventromedial thalamic nucleus
Pre-operative baseline (10 sessions)
Prior to surgery, both groups were matched on all measures of performance over 10 consecutive days of baseline testing. No significant differences were observed for accuracy (means ± SEM, sham 76 ± 0.6%; NRe 74 ± 0.7%) or omissions (means ± SEM, sham 5 ± 0.4%; NRe 8 ± 1.2%). As expected, the number of premature responses declined with increasing session [F (9,153) = 2.51; p < 0.05] but the groups did not differ from each other [F (1,17) = 0.10; p > 0.05]. There were no differences in session or group in terms of perseverative responses, correct latency, or reward collection latency [all p > 0.05].
Post-operative baseline 1: 2 weeks post-surgery
Following post-operative recovery, both groups of rats were re-introduced to the baseline schedule for 10 days. The groups did not differ significantly in accuracy of performance [F (1,17) = 0.84; p > 0.05] or in errors of omission [F (1,17) = 3.75; p > 0.05]. Relative to sham controls, rats with NRe lesions made more premature responses (means ± SEM, sham 20 ± 1.8; NRe 31 ± 1.8), and fewer perseverative responses (means ± SEM, sham 30 ± 1.9; NRe 22 ± 1.3), but the groups did not differ from each other on either measure [premature responses: F (1,17) = 2.98; p > 0.05; perseverative responses: F (1,17) = 1.89; p > 0.05]. There was no differential effect of lesion group on correct latency [F (1,17) = 1.46; p > 0.05] or latency to collect reward [F (1,17) = 0.83; p > 0.05].
Effect of reducing the stimulus duration
When the stimulus duration was reduced in one test session from 0.5 to 0.2 s, both groups of rats showed an overall reduction in accuracy [F (1,17) = 40.09; p < 0.01], but the groups did not differ from each other [F (1,17) = 0.02; p > 0.05]. Rats with NRe lesions made fewer omissions (see Fig. 3a) but they were not significantly different from sham controls [F (1,17) = 2.48; p > 0.05]. Figure 3b shows that the NRe group of rats made many premature responses relative to the sham group, but the groups did not differ significantly on this measure [F (1,17) = 2.22; p > 0.05]. Reducing the stimulus duration served to reduce the number of perseverative responses in both groups [F (1,17) = 23.07; p < 0.01], especially the NRe group of rats (see Fig. 3c), but again, there was no main effect of lesion [F (1,17) = 1.84; p > 0.05]. Although there was no effect of lesion group on latency to respond correctly [F (1,17) = 2.07; p > 0.05], relative to sham controls, rats in the NRe group were very fast to collect their reward at both stimulus durations [F (1,17) = 4.55; p < 0.05; see Fig. 3d].
Effect of long variable intertrial intervals
Making the ITI long and unpredictable did not affect response accuracy in the NRe or sham group of rats [F (1,17) = 0.16; p > 0.05]. As shown in Fig. 4a, while both groups showed an increase in the number of omissions as the ITI increased [F (2,36) = 6.89; p < 0.01], rats with NRe lesions made significantly fewer omissions relative to the sham group [F (1,17) = 4.31; p = 0.05]. The number of premature responses increased significantly for all rats with increasing ITI [F (2,32) = 156.97; p < 0.01]. Relative to the sham group, however, rats with NRe lesions made more premature responses [F (1,17) = 5.19; p < 0.05; see Fig. 4b]. Despite their increase in premature responses, however, NRe-lesioned rats showed a significant decrease in the number of perseverative responses [F (1,17) = 8.38; p < 0.05; see Fig. 4c]. Furthermore, rats in the NRe group were significantly faster than sham controls in their latency to collect rewards [F (1,17) = 5.29; p < 0.05; see Fig. 4d] across all ITIs [F (2,32) = 5.25; p < 0.05]. However, the groups did not differ in their latency to respond correctly [F (1,17) = 0.28; p > 0.05].
Effect of short variable intertrial intervals
Accuracy scores improved as the ITI increased [F (2,30) = 30.7; p < 0.01], but there was no significant difference between groups [F (1,17) = 0.48; p > 0.05]. There was an overall decline in the number of omissions committed by both groups across all ITIs [F (3,51) = 101.68; p < 0.01], but rats with NRe lesions made significantly fewer omissions compared with shams [F (1,17) = 12.31; p < 0.01; see Fig. 5a]. Consistent with the previous manipulation, the NRe group made significantly more premature responses relative to sham controls [F (1,17) = 5.62; p < 0.05; see Fig. 5b]. Rats from either group did not make any premature responses at the shortest 0.5 s ITI.
The number of perseverative responses increased for all rats as the ITI increased [F (2,36) = 17.72; p < 0.01]. Although NRe-lesioned rats made fewer perseverative responses at the 3.0 and 4.5 s ITIs (see Fig. 5c), the groups did not differ significantly on this measure [F (1,17) = 0.32; p > 0.05]. Nevertheless, as shown in Fig. 5d, the NRe group of rats maintained fast reward collection latencies [F (1,17) = 4.47; p = 0.05]. Latency to respond correctly remained unaffected [F (1,17) = 0.03; p > 0.05].
Post-operative baseline 2: 2 months post-surgery
All rats were re-introduced to the standard post-operative baseline testing schedule 2 months following surgery. The ability to respond accurately to the stimulus did not differ between groups [F (1,17) = 0.06; p > 0.05]. However, rats with NRe lesions made significantly fewer omissions relative to sham controls [F (1,17) = 4.51; p < 0.05; see Fig. 6a]. Although the number of premature responses was unaffected by lesion [F (1,17) = 1.87; p > 0.05; see Fig. 6b], there was a strong tendency for rats with NRe lesions to make fewer perseverative responses compared with shams [F (1,17) = 4.03; p = 0.06; see Fig. 6c]. Consistent with the previous manipulations, the NRe group of rats continued to show fast reward collection latencies [F (1,17) = 5.71; p < 0.05; see Fig. 6d] but their latency to respond correctly was not affected [F (1,17) = 3.37; p > 0.05].
Locomotor activity
General locomotor activity for each animal was recorded over eight consecutive 15-min blocks. The activity of both groups of rats declined with increasing number of block [F (7,119) = 40.17; p < 0.01], but there was no main effect of lesion on this measure [F (1,17) = 0.36; p > 0.05].
Discussion
In this study, we show that selective lesions of the NRe of the ventral midline thalamus produce contrasting effects on two different types of inhibitory control processes as assessed in the 5-choice task. Specifically, rats with NRe lesions were unable to inhibit premature responses in anticipation of a visual stimulus when it was temporally unpredictable. In the same test sessions, these rats showed normal inhibition of perseverative responses following a correct response, and in some cases were more restrained from repeating these inconsequential responses (i.e., less perseverative) than shams. These contrasting effects of the NRe lesion on premature and perseverative responses were accompanied by two other behavioral changes. First, the NRe lesion had the effect of reducing the number of omitted trials, an effect that was not accompanied by a change in response accuracy. Second, rats with NRe lesions were very fast to collect food rewards, despite having normal response latencies. Neither of these behavioral effects can be ascribed to general changes in their physiological or behavioral state, such as hyperactivity or sedation. Together, these data demonstrate the fundamental contribution of the NRe to impulse inhibition in the 5-choice task, and implicate the NRe in mechanisms of motivational control.
The timing of the presentation of the stimuli appeared to be an important factor in unveiling NRe lesion effects. Rats with NRe lesions displayed normal levels of premature responding when tested on the baseline schedule of the task, with regularly paced trials and short stimulus duration (0.5 s). Evidently, rats adjusted to the temporal parameters of the task over 10 consecutive days of testing, and were thus able to accurately predict the onset of the stimuli. However, subjecting the rats to a session of variable ITIs elevated the number of premature responses in this lesioned group. The less predictable nature of the stimuli during the variable ITI manipulation may have prevented the NRe-lesioned rats from maintaining a controlled state of readiness for action, which led to over-responding in the apertures in anticipation of the visual signal. Importantly, the increase in premature responses was not accompanied by a reduction in response accuracy. Even when the processing time was markedly reduced with short ITIs (e.g., 1.5 s), the NRe lesion did not compromise general alertness or normal orienting behavior toward the response location (see also Hembrook and Mair 2011). Moreover, the maintained capacity of the NRe-lesioned rats to respond accurately even with short ITIs indicates that premature responding was not a consequence of disrupted stimulus detection. However, because the NRe-lesioned rats only exhibited increased premature responses with unpredictable stimuli, the inhibitory control of impulsive responses does not critically depend on the NRe in all cases. While it is possible that the NRe-lesioned rats were unable to adapt their pre-trained response strategy to a novel situation of stimulus unpredictability (see Dolleman-van der Weel et al. 2009), this conclusion cannot explain why NRe-lesioned rats performed normally when they were exposed to the novel situation of reduced stimulus duration. Our data suggest that the temporal structure of the task was key to unmasking the deficit, as it was only revealed when the stimulus was unpredictable. Other, more general indicators of activity, such as the speed of response and overall locomotor behavior, were within the normal range.
The effects of NRe lesions are consistent with the reciprocal anatomical projections between the NRe and the prefrontal cortex (McKenna and Vertes 2004; Vertes 2002, 2004). Similar to the effects of NRe lesions, a specific increase in premature responses is also observed following selective lesions to the infralimbic region of the ventromedial prefrontal cortex (Chudasama et al. 2003; see also Murphy et al. 2005). One possibility is that the NRe and the infralimbic cortex are members of the same thalamocortical circuit involved in inhibitory control of impulsive actions. However, the NRe-lesioned rats appeared to have a more specific deficit (present study), since rats with infralimbic lesions were always impulsive, regardless of the trial regularity (Chudasama et al. 2003). Perhaps the intact infralimbic cortex was compensating for the NRe lesion, which served to reduce the overall expression of the impulsive deficit during the baseline schedule of the task. This would suggest that the function of the NRe is critically dependent on an intact infralimbic cortex, although both the NRe and the infralimbic cortex are critical for animals to withhold premature responses when temporal changes are made to the 5-choice task.
It is interesting to consider mechanisms by which the NRe and the infralimbic cortex might interact with respect to impulse control. One possibility is that the increase in premature responding is attributable to a disruption of prefrontal–thalamic circuitry that is responsive to serotonergic modulation (Van Bockstaele et al. 1993; Vertes et al. 2010), which would normally adjust the level of response inhibition. This speculation is based on the findings that global serotonin depletion increases premature responding in the 5-choice task (Harrison et al. 1997a, b), and impulsive actions are mediated by serotonin 5-HT receptors in the prefrontal cortex (Passetti et al. 2003; Winstanley et al. 2003; Liu et al. 2004). Nevertheless, additional studies are required to establish the nature of the contribution of the NRe to impulsive behavior as increased premature responding is also observed following lesions to the mediodorsal thalamic nuclei (Chudasama and Muir 2001), and both nuclei have overlapping connections with the ventromedial prefrontal cortex (Groenewegen 1988; Takagishi and Chiba 1991; Vertes 2004).
Despite increased premature responding, there was no evidence of increased perseverative responding in NRe-lesioned rats. In fact, they made fewer perseverative responses than controls, particularly when the ITI was long and variable. To our knowledge, there is no previous study that has observed an improved ability to withhold inappropriate responses following a selective lesion in the 5-choice task. On the contrary, in the 5-choice task, prelimbic and orbital prefrontal cortex lesions selectively increase perseverative responses (Passetti et al. 2002; Chudasama et al. 2003). Interestingly, prelimbic and orbital prefrontal lesions do not lead to an increase in premature responses. Together, this implies that although the NRe interconnects with the prelimbic and orbital prefrontal cortex (Vertes 2004; Vertes et al. 2006; McKenna and Vertes 2004), this interaction does not affect inhibitory control of perseverative responses, at least not in the 5-choice task. Furthermore, because infralimbic cortex lesions increase premature responding in the 5-choice task without affecting perseverative responding (Chudasama et al. 2003), and it also interconnects with the NRe, our data suggest that it is the specific disruption of the infralimbic-NRe interaction that constitutes the impulsive deficit observed in the NRe group.
Although rats with NRe lesions showed normal latencies to make a correct response, the same rats exhibited organized and efficient retrieval of food reward. This was reflected in their very fast reward collection latencies. This finding is probably best understood through heightened motivation. Indeed, motivational changes might account for other aspects of our findings. For example, it is possible that motivation for food could also, in theory, dissuade the rats from responding repetitively in the apertures following a correct response, making them less perseverative. Increased motivation might also account for their impatience in waiting for the next stimulus to appear which resulted in high premature responses. Combined with the finding that the NRe group of rats made fewer omissions and showed normal levels of accuracy, the most parsimonious explanation is that NRe-lesioned rats were exhibiting higher levels of motivation to perform the 5-choice task accurately relative to the sham controls.
We recognize that through the loss of its connections with the prefrontal cortex, lesions of the NRe may have disrupted a prefrontal–ventral striatal pathway known to be critical in mechanisms of food-related motivation (for review, see Kelley et al. 2005), and inhibitory control (Christakou et al. 2004). Currently, there is no direct evidence that implicates the NRe in the overall expression of the prefrontal–ventral striatal pathway. Although prefrontal–ventral striatal disconnections alter normal control of responding in the 5-choice task, they do not alter reward collection latencies (see Christakou et al. 2004). This clearly suggests that the response control deficits exhibited by prefrontal–ventral striatal disconnected rats were relatively independent of motivation for food. Future studies are needed to establish the interdependent function of the prefrontal cortex and the NRe in the 5-choice task, and its relationship with the ventral striatum.
The effects of NRe lesions should also be examined on other aspects of motivation, such as responding under a progressive ratio schedule of reinforcement or conditioned reinforcement. Such evidence would reinforce the conclusion that the NRe is involved in enhanced food-related motivation, especially food-seeking behavior. This conclusion accords with the recent finding that inactivating the NRe leads to fast choice response times in a delayed non-match to position task (Hembrook et al. 2011), relative to sample response times, presumably because the former response might lead to food.
Consistent with its connections with frontal and temporal lobe structures, the NRe is thought to play an important role in a neural circuit engaged in aspects of learning and memory (Dolleman-van der Weel et al. 2009; Hembrook and Mair 2011; Hembrook et al. 2011). Our data provide a novel perspective on the functional role of the NRe with respect to mechanisms of response control and show how the NRe has the capacity to influence food-motivated responding.
References
Aggleton JP, Mishkin M (1983) Memory impairments following restricted medial thalamic lesions in monkeys. Exp Brain Res 52:199–209
Bertram EH, Zhang DX (1999) Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience 92:15–26
Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM (2008) Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci 28:11124–11130
Bogousslavsky J, Miklossy J, Deruaz JP, Regli F (1988a) Thalamic aphasia. Neurology 38:1662
Bogousslavsky J, Ferrazzini M, Regli F, Assal G, Tanabe H, Delaloye-Bischof A (1988b) Manic delirium and frontal-like syndrome with paramedian infarction of the right thalamus. J Neurol Neurosurg Psychiatry 51:116–119
Bokor H, Csáki A, Kocsis K, Kiss J (2002) Cellular architecture of the nucleus reuniens thalami and its putative aspartatergic/glutamatergic projection to the hippocampus and medial septum in the rat. Eur J Neurosci 16:1227–1239
Burk JA, Mair RG (2001) Effects of intralaminar thalamic lesions on sensory attention and motor intention in the rat: a comparison with lesions involving frontal cortex and hippocampus. Behav Brain Res 123:49–63
Byatt G, Dalrymple-Alford JC (1996) Both anteromedial and anteroventral thalamic lesions impair radial-maze learning in rats. Behav Neurosci 110:1335–1348
Cardinal RN, Aitken MRF (2010) Whisker: a client-server high-performance multimedia research control system. Behav Res Methods 42:1059–1071
Christakou A, Robbins TW, Everitt BJ (2004) Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci 24:773–780
Chudasama Y, Muir JL (2001) Visual attention in the rat: a role for the prelimbic cortex and thalamic nuclei? Behav Neurosci 115:417–428
Chudasama Y, Robbins TW (2003) Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci 23:8771–8780
Chudasama Y, Bussey TJ, Muir JL (2001) Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci 14:1009–1020
Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW (2003) Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146:105–119
Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69:680–694
Daum I, Ackermann H (1994) Frontal-type memory impairment associated with thalamic damage. Int J Neurosci 77:187–198
Davoodi FG, Motamedi F, Naghdi N, Akbari E (2009) Effect of reversible inactivation of the reuniens nucleus on spatial learning and memory in rats using Morris water maze task. Behav Brain Res 198:130–135
Dolleman-van der Weel MJ, da Silva FHL, Witter MP (1997) Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J Neurosci 17:5640–5650
Dolleman-van der Weel MJ, Morris RGM, Witter MP (2009) Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct Funct 213:329–342
Flämig R, Klingberg F (1978) Participation of thalamic nuclei in the elaboration of conditioned avoidance reflexes of rats. IV. Lesions of the nucleus reuniens. Acta Biol Med Ger 37:1779–1782
Graff-Radford NR, Tranel D, Van Hoesen GW, Brandt JP (1990) Diencephalic amnesia. Brain 113(Pt 1):1–25
Groenewegen HJ (1988) Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 24:379–431
Groenewegen HJ, Witter MO (2004) Thalamus. In: Paxinos G (ed) The rat nervous system, 3rd edn. Academic Press, New York, pp 408–453
Harrison AA, Everitt BJ, Robbins TW (1997a) Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology 133:329–342
Harrison AA, Everitt BJ, Robbins TW (1997b) Doubly dissociable effects of median- and dorsal-raphé lesions on the performance of the five-choice serial reaction time test of attention in rats. Behav Brain Res 89:135–149
Hembrook JR, Mair RG (2011) Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus 21:815–826
Hembrook JR, Onos KD, Mair RG (2011) Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus (in press)
Herkenham M (1978) The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol 177:589–610
Hoover WB, Vertes RP (2007) Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212:149–179
Janowsky JS, Shimamura AP, Kritchevsky M, Squire LR (1989) Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav Neurosci 103:548–560
Kelley A, Baldo B, Pratt W (2005) Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86:773–795
Liu YP, Wilkinson LS, Robbins TW (2004) Effects of acute and chronic buspirone on impulsive choice and efflux of 5-HT and dopamine in hippocampus, nucleus accumbens and prefrontal cortex. Psychopharmacology (Berl.) 173:175–185
McAlonan K, Brown VJ (2003) Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 146:97–103
McGilchrist I, Goldstein LH, Jadresic D, Fenwick P (1993) Thalamo-frontal psychosis. Br J Psychiatry 163:113–115
McKenna JT, Vertes RP (2004) Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol 480:115–142
Mitchell AS, Dalrymple-Alford JC (2005) Dissociable memory effects after medial thalamus lesions in the rat. Eur J Neurosci 22:973–985
Mitchell AS, Baxter MG, Gaffan D (2007) Dissociable performance on scene learning and strategy implementation after lesions to magnocellular mediodorsal thalamic nucleus. J Neurosci 27:11888–11895
Muir JL, Everitt BJ, Robbins TW (1996) The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6:470–481
Murphy ER, Dalley JW, Robbins TW (2005) Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology 179:99–107
Ohtake T, Yamada H (1989) Efferent connections of the nucleus reuniens and the rhomboid nucleus in the rat: an anterograde PHA-L tracing study. Neurosci Res 6:556–568
Partlow GD, del Carpio-O’Donovan R, Melanson D, Peters TM (1992) Bilateral thalamic glioma: review of eight cases with personality change and mental deterioration. AJNR Am J Neuroradiol 13:1225–1230
Passetti F, Chudasama Y, Robbins TW (2002) The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex 12:1254–1268
Passetti F, Dalley JW, Robbins TW (2003) Double dissociation of serotonergic and dopaminergic mechanisms on attentional performance using a rodent five-choice reaction time task. Psychopharmacology (Berl.) 165:136–145
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates, 5th edn. Elsevier Academic Press, New York
Pepin EP, Auray-Pepin L (1993) Selective dorsolateral frontal lobe dysfunction associated with diencephalic amnesia. Neurology 43:733–741
Ragozzino M, Detrick S, Kesner RP (1999) Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci 19:4585–4594
Schoenbaum G, Nugent SL, Saddoris MP, Setlow B (2002) Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport 13:885–890
Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M (2003) Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem 10:129–140
Squire LR, Moore RY (1979) Dorsal thalamic lesion in a noted case of human memory dysfunction. Ann Neurol 6:503–506
Su HS, Bentivoglio M (1990) Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J Comp Neurol 297:582–593
Swanson LW (1998) Brain maps: structure of the rat brain. Elsevier, New York
Sziklas V, Petrides M (1999) The effects of lesions to the anterior thalamic nuclei on object-place associations in rats. Eur J Neurosci 11:559–566
Takagishi M, Chiba T (1991) Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res 566:26–39
Van Bockstaele EJ, Biswas A, Pickel VM (1993) Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res 624:188–198
Van Der Werf YD, Weerts JG, Jolles J, Witter MP, Lindeboom J, Scheltens P (1999) Neuropsychological correlates of a right unilateral lacunar thalamic infarction. J Neurol Neurosurg Psychiatry 66:36–42
Vertes RP (2002) Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol 442:163–187
Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58
Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ (2006) Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol 499:768–796
Vertes RP, Linley SB, Hoover WB (2010) Pattern of distribution of serotonergic fibres to the thalamus of the rat. Brain Struct Funct 215:1–28
von Cramon DY, Hebel N, Schuri U (1985) A contribution to the anatomical basis of thalamic amnesia. Brain 108(Pt 4):993–1008
Winstanley CA, Chudasama Y, Dalley JW, Theobald DEH, Glennon JC, Robbins TW (2003) Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology 167:304–314
Wouterlood FG, Saldana E, Witter MP (1990) Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 296:179–203
Zola-Morgan S, Squire LR (1985) Amnesia in monkeys after lesions of the mediodorsal nucleus of the thalamus. Ann Neurol 17:558–564
Zola-Morgan S, Squire LR, Amaral DG (1986) Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci 6:2950–2967
Acknowledgments
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), and Canadian Foundation for Innovation-Leaders Opportunity Fund (CFI-LOF) awarded to Y. Chudasama. We wish to thank David A. Leopold, Norman M. White, Andrew R. Abela and Alana Knapman for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasad, J.A., Macgregor, E.M. & Chudasama, Y. Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Struct Funct 218, 85–96 (2013). https://doi.org/10.1007/s00429-012-0378-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-012-0378-5