Abstract
Rationale
Deficits in impulse control are prevalent in several neuropsychiatric disorders that are based on impaired frontostriatal communication. The ventral medial prefrontal cortex (vmPFC) and the nucleus accumbens (NAc) are key substrates of impulse control in rats. The NAc core and shell are considered to be differentially involved suggesting a functional distinction between the connections of the vmPFC and particular NAc subregions concerning impulse control.
Objectives/methods
In the present study, simultaneous inactivation of the rats’ vmPFC and NAc core or shell by contralateral microinfusion of the GABAA receptor agonist muscimol was used to investigate their relevance for impulse control in the five-choice serial reaction time task (5-CSRTT).
Results
Disconnection of the vmPFC and NAc shell produced specific impairments in inhibitory control, indicated by significantly increased premature responding and an enhanced number of time-out responses, closely resembling the effects of bilateral inactivation of either the vmPFC or NAc shell previously reported using the same task. In contrast, disconnection of the vmPFC and NAc core only slightly increased the rate of omissions and latency of reward collection indicating attentional and motivational deficits.
Conclusions
Our results extend previous findings indicating the functional specialisation of frontostriatal networks and show a differential contribution of specific vmPFC-NAc connections to behavioural control depending on the NAc subregion. We conclude that the regulation of impulse control in rats requires an intact connection between the vmPFC and the NAc shell, while the vmPFC-NAc core projection seems to be of minor importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optimal adaptation to the environment is critical for animals’ inclusive fitness and requires the balance of behavioural inhibition and activation (Ghazizadeh et al. 2012; West and Gardner 2013). Behavioural control is highly influenced by motivational states (“impulses”). The active inhibitory mechanism, which modulates such internally or externally driven prepotent desires for reinforcement, is referred to as impulse control (Jentsch and Taylor 1999; Winstanley et al. 2006).
Deficient impulse control leads to maladaptive impulsive behaviours including inability to wait and difficulty withholding responses, generally defined as impulsive action or motor impulsivity (Brunner and Hen 1997; Dalley et al. 2011; de Wit 2009). The dominant behavioural measures of impulse control are response inhibition paradigms, such as the five-choice serial reaction time task (5-CSRTT). During 5-CSRTT performance, rats are required to withhold from premature responding to a visual, reward-predicting stimulus, generally regarded as an index of impulse control (Carli et al. 1983; Muir et al. 1996; Robbins 2002). Impulse control is based on cortico-limbic-striatal circuits, and dysfunctions of these systems are associated with several psychiatric disorders characterised by high levels of impulsivity, like ADHD (Nigg and Casey 2005), obsessive-compulsive disorder (Anticevic et al. 2013), pathological gambling (Fineberg et al. 2010), schizophrenia (Meyer-Lindenberg et al. 2002; Pantelis et al. 1997; Robbins 1990), drug abuse and other forms of addiction (Kalivas and Volkow 2005; Russo and Nestler 2013). There is evidence that frontostriatal connections are part of parallel, functionally segregated re-entrant striato-thalamo-cortical loops. In both primates and rats, frontostriatal projections are topographically organised so that functionally different subregions of the prefrontal cortex (PFC) have separate targets in the striatum (Alexander et al. 1986; Berendse et al. 1992; McGeorge and Faull 1989). The most pronounced anatomical partition of the rodent PFC is made in the medial PFC, which can be divided into dorsal (anterior cingulate and medial precentral cortices) and ventral subdivisions (prelimbic, infralimbic and medial orbital cortices) (Dalley et al. 2004; Gabbott et al. 2005; Heidbreder and Groenewegen 2003; Ongur and Price 2000). The anatomical heterogeneity of the mPFC is paralleled by functional subregional differentiation, with the network of prelimbic and infralimbic cortices, henceforth referred to as ventral medial prefrontal cortex (vmPFC), being more critically involved in impulsive behaviour (Chudasama et al. 2003; Kesner and Churchwell 2011). On the striatal level, the nucleus accumbens (NAc) as part of the ventral striatum and as core element of the mesoaccumbal dopamine (DA) system is generally implicated in reward and motivation and ideally positioned to integrate input signals of executive-cognitive information, such as impulse control, arising from the mPFC (Carlezon and Thomas 2009; Groenewegen and Trimble 2007; Mogenson et al. 1980). The vmPFC of rats is anatomically and functionally interconnected with the NAc, whereas the rodent dorsal mPFC preferentially innervates the dorsomedial striatum (Berendse et al. 1992; Brog et al. 1993; Ding et al. 2001; Gorelova and Yang 1997; McGeorge and Faull 1989; Sesack et al. 1989; Vertes 2004). Converging lines of evidence further indicate a functional relationship between mPFC and NAc in terms of behavioural inhibition, as both regions have found to be involved in inhibitory action control (Aron 2007; Christakou et al. 2004), impulsive decision-making (Costa Dias et al. 2012; Diergaarde et al. 2008), behavioural flexibility (Coppens et al. 2010; Goto and Grace 2005) and drug seeking (Bossert et al. 2012; Peters et al. 2008; Vassoler et al. 2013). Recent findings indicate that impulsive behaviour is not only top-down controlled by cortical areas, but also modulated on the subcortical level (Dalley et al. 2011). For instance, intra-NAc injections of DA receptor antagonists reverse behavioural disinhibition induced by vmPFC inactivation (Ghazizadeh et al. 2012) and block premature responding following mPFC lesions in the 5-CSRTT (Pezze et al. 2009). Findings of electrophysiological recording (Hayton et al. 2011), lesion (Chudasama et al. 2003; Muir et al. 1996; Pezze et al. 2009) and reversible inactivation (Izaki et al. 2007; Murphy et al. 2012; Narayanan et al. 2006; Paine et al. 2011) studies already implicated the rodent mPFC in impulse control. The contribution of the NAc to impulsive behaviours turned out to be even more complex, as the NAc cannot be regarded as an anatomical and functional entity (Groenewegen and Trimble 2007; Heimer 2003). Based on anatomical, neurochemical and electrophysiological criteria, the NAc in the rat brain is divided into distinct subterritories, which are also present in the human brain and show considerably different input-output features: a dorsolateral core region surrounding the anterior commissure and a shell compartment that is located ventromedially to the core (Berendse et al. 1992; Brog et al. 1993; Heidbreder and Groenewegen 2003; Meredith et al. 1996; Sokolowski and Salamone 1998; Zaborszky et al. 1985). The functional dichotomy of the NAc subregions regarding several behaviours (Bassareo et al. 2002; Corbit et al. 2001; Floresco et al. 2006; Floresco et al. 2008; Jongen-Relo et al. 2002; McFarland et al. 2004; Pothuizen et al. 2005a; Robbins and Everitt 1996; Stratford and Kelley 1997; Vassoler et al. 2013) appears also to hold true for impulse control in terms of motor impulsivity. While core lesions induce deficits in 5-CSRTT and differential reinforcement for low rates of responding tasks (DRL) (Christakou et al. 2004; Pothuizen et al. 2005b), lesions of the NAc shell lack a significant influence on anticipatory responding in response inhibition tasks (Murphy et al. 2008; Pothuizen et al. 2005b). In line with this, disconnection of the mPFC from the NAc core by lesions caused impulse control deficits in the 5-CSRTT (Christakou et al. 2004), whereas an involvement of the mPFC-NAc shell connection was not yet examined. However, DA D1 receptors in NAc shell are involved in inhibitory response control in the 5-CSRTT (Pattij et al. 2007).
Asymmetrical disconnection designs have successfully been used to show a functional interaction between mPFC and NAc in a variety of behavioural paradigms, including effort-based decision-making (Hauber and Sommer 2009), Pavlovian conditioning (Parkinson et al. 1999), behavioural flexibility (Block et al. 2007), working memory (Floresco et al. 1999) as well as inhibitory and attentional control (Christakou et al. 2004). Bilateral projections from the vmPFC to the NAc are predominantly ipsilateral (Berendse et al. 1992; Brog et al. 1993; Gabbott et al. 2005; McGeorge and Faull 1989; Montaron et al. 1996; Sesack et al. 1989). Thus, combined unilateral lesioning or reversibly inactivating both structures in opposite hemispheres results in the disruption of the neuronal circuitry in both hemispheres (Gaffan and Wilson 2008). Chemical agents like the GABAA agonist muscimol allow repeated acute and reversible inactivations of distinct brain regions, and hence, within-subject designs accompanied by increased test-retest reliability (Lomber 1999).
Hence, in the present study, we used an asymmetrical disconnection approach combining unilateral temporary inactivations of the vmPFC and the contralateral NAc core or shell by muscimol to investigate the relevance of the vmPFC-NAc connectivity to impulse control in the 5-CSRTT in rats. Interestingly, previous results from our laboratory revealed that transient bilateral inactivation of the vmPFC (Feja and Koch 2014) as well as the NAc shell, but not the core (Feja et al. 2014), by muscimol induced impulsive over-responding in the 5-CSRTT. The present study aimed to extend these findings hypothesising, firstly, that the vmPFC and NAc functionally interact in motor impulsivity; secondly, that specific frontostriatal connections differentially affect 5-CSRTT performance and, thirdly, that motor impulse control preferentially requires an intact vmPFC-NAc shell circuitry.
Methods
Subjects
A total of 22 adult male Lister hooded rats (Harlan, Venray, Netherlands) weighing 260–340 g at the beginning of the experiments were assigned to two testing cohorts, defined as vmPFC-NAc core (n = 12) and vmPFC-NAc shell (n = 10) groups. The animals were housed in groups of four to six in standard Macrolon cages (type IV) under controlled ambient conditions (21–22 °C, 45–55 % humidity, 12-h light/dark cycle, lights on at 7:00 a.m.). The rats were kept on their experimental body weights of approximately 85 % of those under free feeding conditions by controlled feeding of laboratory rodent chow (Altromin Standard Diet 1324 19 % crude protein, 4.0 % crude fat, 6.0 % crude fibre, 7.0 % crude ash; Altromin Spezialfutter GmbH & Co. KG, Lage, Germany) and received tap water ad libitum. Behavioural testing took place between 8:00 a.m. and 6:00 p.m. The experiments were performed in accordance with the National Institutes of Health ethical guidelines for the care and use of laboratory animals for experiments and were approved by the local animal care committee (Senatorische Behörde, Bremen, Germany).
Apparatus
The 5-CSRTT was conducted in two operant aluminium chambers (26 × 26 × 26 cm; Campden Instruments Ltd., Loughborough, UK), wherein five apertures (2.5 × 2.5 cm, 4 cm deep) were inserted 2 cm above floor level in the concavely curved rear wall. This assembly provided five response options located equidistant to the food magazine on the opposite. Inside each hole, a light-emitting diode (LED) generated visual stimuli of variable duration. Nose-poke responses of the animals were detected by infrared photocell beams at the entrance of the apertures. The rats could be placed in the box through a Plexiglas® door on the upper part of the front wall. Underneath the door, a small Plexiglas® panel provided access to the food magazine which was lighted via two LEDs and automatically supplied with casein pellets (45 mg Dustless Precision Pellets, Bio-Serv®, UK) by an electromechanical feeder. Food collection was detected by a microswitch monitoring the movement of the hinged panel. Each chamber was illuminated by a 3-W house light mounted on the ceiling. A noise-damped fan served as ventilation and background noise of about 60 dB. The grid floor facilitated the removal of excrements. For the purpose of sound attenuation, the wooden cabinet was reinforced with an insulating plate at the interior of the door. The apparatus was controlled by specific software written in Turandot (Cambridge Cognition Ltd., version 1.23) which was run on a personal computer connected to the BNC Mark 2 System (Behavioral Net Controller, Campden Instruments Ltd., Loughborough, UK).
General procedure
Training
The animals (n = 22) were trained to detect the occurrence of brief light stimuli in one of the five rear wall apertures. The general procedure was based on the protocol of Campden Instruments and was divided into a habituation, pretraining and baseline training phase (Campden Instruments 2005). Before training and tests, the rats were acclimatised to the laboratory for at least 30 min in their home cages.
The first experimental phase comprised two daily half-hour habituation sessions. The boxes were prepared as follows: before the first training session, the tray panel was opened to facilitate access to 15 freely available pellets in order to reinforce the magazine as location of food reward. During the second session, no panel manipulation was carried out. Besides the reward in the tray, two pellets were placed in each aperture to promote exploration of these areas. The chambers were permanently illuminated by the house light during both sessions.
The daily training session lasted 30 min or was finished after completion of 100 trials. Each session started with the simultaneous illumination of the box and the food magazine and the delivery of a single pellet into the tray. Once the rat opened the panel for food retrieval, the first trial was initiated. The magazine light faded, and a fixed intertrial interval (ITI) of 5 s started. At the end of the ITI, a light stimulus of determinate duration (stimulus duration (SD)) was randomly presented in one of the five holes. The rats had to respond with a nose poke into the appropriate aperture during the stimulus presentation or within a subsequent limited hold period (LH). A correct response was followed by the supply of a pellet into the lighted food magazine. The next trial was triggered by the panel movement. Inappropriate responses led to a punishment in terms of a predefined 5-s period of darkness (time out) without reward delivery. The task procedure offered various opportunities for such reactions:
-
Incorrect responses in a hole where no stimulus appeared,
-
Omissions in the form of absent reaction to the occurrence of the stimulus within the LH,
-
Premature responses before the onset of the stimulus during the ITI in one of the apertures and
-
Perseverative responses, meaning additional responses after a correct response and before reward collection.
Every nose-poke response in one of the apertures during the time-out phase (time-out responses) prolonged the period of darkness. Following the time-out, the box and the tray were illuminated again and the next trial was started by a nose poke into the food magazine. Within a session, the visual stimuli were randomly presented in equal number in each hole. The progressive decrement of the variables SD (60 → 1 s) and LH (60 → 5 s) over eight training levels enabled the acquisition of the 5-CSRTT.
The baseline training session was determined by the conditions of the eighth training level (SD = 1 s, LH = 5 s). After showing a stable baseline performance (>80 % accuracy and <20 % omissions with <10 % variation over five consecutive training sessions), rats underwent surgery.
Surgery
The rats were anaesthetised with chloral hydrate (360 mg/kg; Sigma-Aldrich, Steinheim, Germany) and fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). Stainless steel 21-gauge guide cannulae were implanted unilaterally 1 mm above the target injection site into the vmPFC (anteroposterior +2.7 mm, mediolateral ±0.8 mm, dorsoventral −4.0 mm from bregma) and 2 mm above the intended injection sites into the contralateral NAc core (anteroposterior +1.2 mm, mediolateral ±1.8 mm, dorsoventral −6.8 mm from bregma) or shell (anteroposterior +1.2 mm, mediolateral ±0.5 mm, dorsoventral −7.3 mm from bregma). The sides of the implantations were counterbalanced, resulting in approximately equal numbers of rats with microinfusions in the left or right hemispheres at the level of vmPFC and NAc. Jeweller screws were anchored in the skull serving to fix the cannulae which were embedded in dental cement and closed by removable 26-gauge stylets of the same length. After surgery, the rats were kept individually for 3 days with free access to food and water.
Following a total recovery period of 5 days, the animals were reintroduced to the baseline training until they re-established the presurgical baseline performance.
Microinfusion
The test design comprised four 4-day sessions for the animals. Each session started with an injection day, followed by a day without training. The second and third post-testing days were used to achieve the baseline performance and to ensure the washout process of the drug. Before infusion, the stylets were exchanged for injection cannulae (vmPFC 27 gauge; NAc 26 gauge) connected with microlitre syringes (SGE Scientific Glass Engineering, Darmstadt, Germany) via polyethylene tubes. The rats received four sets of combined unilateral microinjections of the GABAA agonist muscimol (0.05 μg/0.3 μl) and 0.9 % saline as vehicle (0.3 μl) into the vmPFC and the contralateral NAc core or shell according to a pseudorandom Latin square design. The subject groups were divided as follows:
-
Disconnection group I (vmPFC + NAc core; n = 12): vehicle + vehicle, vehicle + muscimol, muscimol + vehicle and muscimol + muscimol.
-
Disconnection group II (vmPFC + NAc shell; n = 10): vehicle + vehicle, vehicle + muscimol, muscimol + vehicle and muscimol + muscimol.
The injection rate was 0.1 μl/30 s. The injectors were left in place for 1 min to guarantee diffusion and to avoid reflux of the solution. Ten minutes after the microinjection, the rats underwent behavioural testing. The sequence of the test sessions matched with the baseline training.
Drugs
The GABAA agonist muscimol was purchased from Tocris Bioscience (Ellisville, MO, USA) and dissolved in 0.9 % saline. Aliquots of stock solutions (0.5 μg/0.3 μl) were prepared and stored at −20 °C until use. On the treatment day, aliquots were further diluted to a dose of 0.05 μg/0.3 μl. Doses were based on previous studies (Diederich and Koch 2005).
Histology
Upon termination of the experiment, the rats were euthanised with a lethal dose of chloral hydrate. The brains were removed from the skull and immersion fixed in a 4 % formalin/30 % sucrose solution for 48 h. Coronal 50-μm sections of the mPFC were cut on a cryostat (Jung CM 3000; Leica Instrument GmbH, Nussloch, Germany), mounted on gelatine-coated glass slides and Nissl stained with thionin. Then, the sections were analysed using a light microscope and injection sites plotted on standardised coronal sections of a rat brain stereotaxic atlas (Paxinos and Watson 1998).
Data analysis
The descriptive statistics is based on means and variance and is indicated by the standard error of the mean (±SEM). The statistical analyses were conducted by the software IBM SPSS Statistics (version 20 for Windows).
The drug effects within the testing group on the following behavioural parameters were investigated using separate two-way split-plot-factorial analysis of variance (ANOVA; within-subject factor: drug treatment, between-subject factor: disconnection group): percentage of correct responses (accuracy; 100 × number of correct responses/number of correct and incorrect responses), percentage of omitted responses (100 × number of omitted responses/total number of correct, incorrect and omitted responses), number of premature responses, number of perseverative responses, number of trials completed, number of time-out responses, latency of correct response [s] and latency of reward collection [s].
In the case of significant main effects (P < 0.05), one-way repeated measures ANOVA and post hoc Bonferroni tests for the factor drug treatment as well as independent t tests between the disconnection groups were conducted separately for each behavioural parameter.
Results
Histology
In total, 22 rats received unilateral microinjections into the vmPFC combined with contralateral microinfusions into NAc core (n = 12) or shell (n = 10). The histological analysis revealed, as indicated in Fig. 1, that 18 rats (n = 9 in each group) had acceptable injection sites accurately located in the target structures with minimal tissue damage.
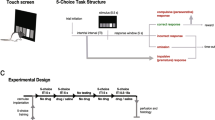
Location of the unilateral injection sites in a the ventral medial prefrontal cortex (circles corresponding to nucleus accumbens core, n = 9; triangles corresponding to nucleus accumbens shell, n = 9) and in b the contralateral nucleus accumbens core (circles, n = 9) or shell (triangles, n = 9) in the 5-choice serial reaction time task depicted on schematic drawings from the rat brain atlas of Paxinos and Watson (1998). Rostral distance (in mm) to bregma is indicated by numbers
Effects of inactivation of vmPFC-NAc core and vmPFC-NAc shell connections by muscimol on rats’ performance in the 5-CSRTT
Before testing, the rats performed at a stable baseline with high levels of accuracy (disconnection group I 91.64 ± 1.06 %; disconnection group II 92.19 ± 0.92 %), fast correct response (disconnection group I 0.69 ± 0.01 s; disconnection group II 0.68 ± 0.02 s) and reward collection latencies (disconnection group I 1.10 ± 0.05 s; disconnection group II 1.05 ± 0.03 s), low percentages of omissions (disconnection group I 12.68 ± 1.02 %; disconnection group II 9.72 ± 1.25 %) as well as low numbers of premature (disconnection group I 8.10 ± 0.90; disconnection group II 8.28 ± 1.05) and perseverative responses (disconnection group I 1.98 ± 0.35; disconnection group II 2.00 ± 0.54). Analysis of the training data demonstrated no significant differences in the pre- and post-operative sessions and the ‘drug-free days’ between testing excluding any carry-over effects of drug treatment or surgery (data not shown).
Two-way split-plot-factorial ANOVAs on the 5-CSRTT performance showed main effects of drug treatment [F (3,51) = 6.119; P = 0.001] and disconnection group [F (1,51) = 5.71; P = 0.03] as well as a statistically significant treatment × disconnection group interaction [F (3,51) = 7.704; P < 0.001] for premature responses, suggesting impaired motor impulse control. The functional heterogeneity between both frontostriatal systems (vmPFC + NAc core vs. vmPFC + NAc shell) was further substantiated by main effects of disconnection group for omissions [F (1,51) = 8.228; P = 0.011], completed trials [F (1,51) = 4.754; P = 0.045] and accuracy [F (1,51) = 5.259; P = 0.036] as well as by a statistically significant treatment × disconnection group interaction [F (3,51) = 3.223; P = 0.031] for accuracy.
Further, one-way repeated measure ANOVAs and post hoc Bonferroni tests revealed that simultaneous unilateral inactivation of vmPFC and the contralateral NAc shell specifically enhanced impulsive behaviour reflected by a significant increase in premature responding compared to vehicle (P = 0.042), while no other measured parameter was affected (Fig. 2 and Table 1). Unilateral intra-NAc shell injection of muscimol as well as combined deactivation of vmPFC and NAc shell appeared to augment time-out responses, but this effect did not reach statistical significance (Fig. 2b). By contrast, neither unilateral NAc core nor combined vmPFC and NAc core inactivation had any effect on 5-CSRTT performance. Independent t tests between disconnection groups showed significant differences between the vmPFC-NAc core and vmPFC-NAc shell connection following combined muscimol injection regarding premature responses (P = 0.008) and accuracy (P =0.006) (Fig. 2a, c), substantiating the differential impact of the two distinct frontostriatal circuits on impulse and attentional control. Further, unilateral inactivation of NAc core significantly increased the omission rate compared to NAc shell (P = 0.021) (Fig. 2d).
Effects of combined local unilateral infusions of the GABAA agonist muscimol (M; 0.05 μg/0.3 μl) and 0.9 % saline as vehicle (V) into the ventral medial prefrontal cortex (vmPFC) and the contralateral nucleus accumbens (NAc) core (n = 9) or shell (n = 9) on the rats’ performance in the 5-choice serial reaction time task. V + M signifies vehicle injection into the vmPFC + muscimol injection into the NAc core/shell; M + V signifies muscimol injection into the vmPFC + vehicle injection into the NAc core/shell. Data of a premature responses, b time-out responses, c accuracy and d omissions are means ± SEM. Statistically significant differences between drug treatment compared to vehicle are indicated by asterisk (one-way repeated measures ANOVA, post hoc Bonferroni test, P < 0.05) and between disconnection groups (vmPFC + NAc core compared to vmPFC + NAc shell) by circles (independent t test, P < 0.05)
Discussion
In terms of impulsivity, the vmPFC is considered to be primarily implicated in impulsive action while there is only limited evidence for an involvement of the NAc, which is more associated with impulsive decision-making. Previous studies revealed that NAc shell lesions showed no effect on premature responding and core lesions merely tended to increase motor impulsivity in the 5-CSRTT. However, the present data show that both the vmPFC and the NAc are involved in the neural network mediating impulse control in the 5-CSRTT in rats, with a predominant role for the connection of vmPFC and NAc shell. In contrast, the vmPFC-NAc core connection appears to be more involved in attentional and motivational aspects of behaviour.
The main findings of this study are that acute disconnection of the vmPFC and NAc shell by simultaneous contralateral inactivation by the GABAA agonist muscimol considerably enhanced premature responding indicating deficits in impulse control. In contrast, transient disruption of the serial communication between vmPFC and NAc core did not affect impulsive action. Lesion studies have already documented the involvement of the rodent mPFC and NAc in inhibitory response control and revealed differential contributions regarding different aspects of inhibitory control and specific subregions of the mPFC and NAc (Christakou et al. 2004; Chudasama and Muir 2001; Chudasama et al. 2003; Muir et al. 1996; Murphy et al. 2008; Pezze et al. 2009; Pothuizen et al. 2005b). By use of the GABAA agonist muscimol, we and other groups have recently shown that the vmPFC, including the prelimbic (PL) and infralimbic (IL) cortices, is critically involved in controlling premature responding in the 5-CSRTT in rats (Feja and Koch 2014; Murphy et al. 2012; Paine et al. 2011). On the subcortical level of the NAc, lesions of the core but not the shell region increased anticipatory responding in response inhibition tasks (Christakou et al. 2004; Murphy et al. 2008; Pothuizen et al. 2005b). Coherently, disconnection lesions of the vmPFC and the NAc core enhanced premature and perseverative responding in the 5-CSRTT, whereas the vmPFC-NAc shell connection was not investigated (Christakou et al. 2004). However, the latest work from our laboratory highlighted the role of the NAc shell in terms of motor impulsivity and revealed for the first time that transient deactivation of the shell, but not the core, reduced impulse control in the 5-CSRTT in rats (Feja et al. 2014). The present study verifies our previous findings and confirms that in particular, the connection of vmPFC and NAc shell is implicated in the maintenance of impulse control during 5-CSRTT performance.
Asymmetrical inactivation of vmPFC and NAc shell also increased the number of time-out responses, although not reaching statistical significance. Time-out responses represent another aspect of inhibitory control, more related to cognitive flexibility (Robbins 2002). The increase of time-out responses substantiates the role of the vmPFC-NAc shell connection in behavioural inhibition. This is further supported by previous findings from our laboratory showing that bilateral injection of muscimol into the vmPFC or the NAc shell increased time-out responses in the 5-CSRTT (Feja and Koch 2014; Feja et al. 2014). Cognitive constructs such as impulsivity and behavioural flexibility are closely interrelated executive processes in the context of inhibitory control, hierarchically top-down, mediated by the PFC (Bari and Robbins 2013; Wise 2008). In this regard, vmPFC lesions or inactivations result in behavioural inflexibility in reversal learning tasks in rats (Kosaki and Watanabe 2012; Ragozzino et al. 1999; Ragozzino 2007). The neural network contributing to behavioural flexibility involves both the mPFC and NAc (Coppens et al. 2010). Set-shifting tasks indicated that the mPFC projection to the NAc is important for suppressing inappropriate responses, and asymmetrical inactivation of these structures impaired the ability to switch from one discrimination strategy to another (Block et al. 2007). Interestingly, the shell region apparently had a greater impact on the number of time-out responses than the vmPFC, as revealed by unilateral deactivations of the respective structure. Admittedly, inactivation of NAc shell, in contrast to core, does not impair performance in a set-shifting task in rats, but it was pointed out that the shell mediates the suppression of irrelevant or no-reward behaviours (Blaiss and Janak 2009; Floresco et al. 2006; Floresco et al. 2008). Thus, unilateral inactivation of NAc shell might have contributed to behavioural disinhibition during 5-CSRTT performance.
Other parameters indexing attentional (omissions), compulsive (perseverative responses), motor (correct response latency) or motivational behaviours (trials completed, reward collection latency) remained unaffected following unilateral intra-vmPFC and intra-shell or combined vmPFC and NAc shell infusions of muscimol.
Taken together, the present behavioural effects on 5-CSRTT performance induced by vmPFC-NAc shell disconnection closely resemble the deficits observed following bilateral vmPFC (Feja and Koch 2014) or NAc shell (Feja et al. 2014) inactivation in the same task, while unilateral control deactivations by muscimol of the respective regions alone did not produce significant deficits. The asymmetrical manipulation method used in this study is particularly suited to investigate the interaction between components of cortico-subcortical networks (Gaffan and Wilson 2008; Peters et al. 2008). Since neuronal projections, such as frontostriatal connections from the mPFC to the NAc, are predominantly ipsilateral (Berendse et al. 1992), learned behaviours can be preserved by an intact single hemisphere and unilateral manipulations, as in our study, often lead to minor or no cognitive impairments. Via crossed unilateral inactivation of the vmPFC and NAc core or shell, the serial communication between these structures can be bilaterally impeded (Gaffan et al. 1993; Gaffan and Wilson 2008; Setlow et al. 2002). For example, a previous study showed that disconnection of the IL and NAc shell reinstates cocaine seeking in rats after extinction learning, whereas unilateral inactivation of either IL or NAc shell does not alter seeking behaviour (Peters et al. 2008). Consequently, as the effects of the vmPFC-NAc shell disconnection on premature responding in the 5-CSRTT are more pronounced than the additive effect of the single unilateral inactivations, our findings provide evidence that the control of motor impulsivity requires serial information transfer between this specific frontostriatal system.
Unexpectedly, the transient disconnection of vmPFC and NAc core as well as unilateral manipulations of vmPFC or the core region did not produce any significant behavioural effect in the 5-CSRTT compared to control treatment.
Contralateral inactivation of vmPFC and NAc core tended to increase the omission rate as well as the reward collection and correct response latencies indicating marginal attentional and locomotor deficits and a slightly reduced motivation for food. Moreover, unilateral deactivation of the core significantly augmented the omission rate compared to the respective manipulation of the shell, which might indicate an impact of NAc core on attention. However, as mentioned above, the attentional aspects of the 5-CSRTT performance (accuracy, omissions) were not significantly altered by muscimol treatment compared to control.
Previously, we have shown that the core region in contrast to the shell plays an important role in the regulation of locomotion and general responsiveness with a bilaterally inactivated core severely impaired 5-CSRTT performance (Feja et al. 2014). Particularly, the strong decrease in the number of completed trials after deactivation of NAc core, but not shell, represents a consequence of motivational dysfunction and points towards a differential role of both subregions in motivated behaviour in the 5-CSRTT. This is supported by evidence that muscimol injections into the core reduce breakpoint in a progressive ratio schedule in rats (Moscarello et al. 2010), while shell inactivation enhances motivational behaviour in that task (Stratford and Wirtshafter 2012; Wirtshafter and Stratford 2010). However, lesions of the core do not reduce food motivation in a delayed reinforcement task (Cardinal and Cheung 2005) and muscimol does not affect food intake when injected into the NAc core (Stratford and Kelley 1997) and even increases eating behaviour following infusion into the shell (Basso and Kelley 1999; Lopes et al. 2007; Reynolds and Berridge 2002; Soderpalm and Berridge 2000; Stratford and Kelley 1997; Stratford and Wirtshafter 2011).
High scores of impulsivity in the 5-CSRTT inversely correlate with attentional accuracy (Blondeau and Dellu-Hagedorn 2007; Dalley et al. 2008; Puumala and Sirvio 1998). Considering the central role of frontostriatal impairments to the pathophysiology of ADHD, incorporating attentional and impulsive dysfunctions (Nigg and Casey 2005), it seems obvious that this relationship could also be valid for the vmPFC-NAc shell connection, as simultaneous inactivation of vmPFC and NAc shell produced a significant decrease of response accuracy compared to vmPFC-NAc core disconnection. But since the effect of the vmPFC-NAc shell disconnection on accuracy did not differ from control treatment, we suppose the impaired accuracy should rather be considered a consequence of rash-spontaneous impulsive behaviour of the rats leading to some kind of ‘careless mistake’.
Meanwhile, there is a scientific consensus that impulsive behaviour is not only cortically top-down controlled but also regulated by subcortical areas (Dalley et al. 2011). Most interestingly, motor impulse control seems to be more depending on an intact NAc shell than on the vmPFC, as bilateral inactivation of the shell enhances premature responding at almost the same rate as the vmPFC-NAc shell disconnection, while bilateral deactivation of the vmPFC only produces approximately half the number of anticipatory responses (Feja and Koch 2014; Feja et al. 2014). Accordingly, we hypothesise that the NAc, particularly the shell region, might function as kind of a bottleneck for impulse control, receiving serial parallel information input from the vmPFC, integrating these input signals of impulse control with emotional (basolateral amygdala), contextual (hippocampus) and arousal contents (midline thalamus) and conveying the multiplexed information to downstream brain sites involved in feeding and drinking (lateral hypothalamus), motivation (ventral tegmental area, substantia nigra) and locomotion (caudal mesencephalon) (Carlezon and Thomas 2009; Groenewegen and Trimble 2007; Mogenson et al. 1980).
Although it may be difficult to directly compare the gained knowledge with deficits following human cortical damage or with findings from animal lesion studies, the use of reversible inactivation techniques is an effective analytical tool in the area of basic biological and pharmacological research, especially for dissecting the implications of distinct neuroanatomical structures or systems in specific brain functions. One limitation of temporary inactivation is the spatial localisation of drug effects. In our study, we could not precisely define the degree of inactivation, as we did not measure the spread of muscimol. However, previous autoradiography studies estimated the spread of muscimol in rat brains and showed diffusion of radioactive muscimol restricted to NAc core or shell following injection of similar volumes and even higher concentrations compared to ours (Martin 1991; Martin and Ghez 1999; Pothuizen et al. 2005a). These pieces of evidence suggest that muscimol diffusion in our experiments was restricted to either core or shell. Our behavioural data further indicated region specificity of injections, as they revealed clear and distinct differences between the vmPFC-NAc core and vmPFC-NAc shell groups during 5-CSRTT performance.
However, it cannot be excluded that the injections may have involved adjacent non-accumbal or non-vmPFC areas, such as parts of the ventral pallidum, the dorsal striatum or the anterior cingulate cortex (AC), especially since infusions of fluorophore-conjugated muscimol in our previous study showed an asymmetrical diffusion along the dorsoventral axis up the cannula shaft (Feja et al. 2014). In line with this, a previous study has shown that the disconnection of the AC from the NAc core produces no deficits on the 5-CSRTT performance (Christakou et al. 2001).
This suggests that the lacking impact of the vmPFC-NAc core disconnection on motor impulsivity might be attributable to a concomitantly affected AC. Regarding the vmPFC-NAc shell connection, the interaction with other structures involved in impulse control, such as the mediodorsal nucleus of the thalamus (MD), should be taken into account. Within the NAc, the shell projects predominantly to the MD and lesions of this structure have been shown to increase premature responding in the 5-CSRTT (Chudasama and Muir 2001; Groenewegen et al. 1999). Besides, the vmPFC-NAc shell disconnection might have induced an increase of DA levels in the NAc shell resulting in deficient impulse control. This is supported by a previous study showing that vmPFC inactivation results in the disinhibition of phasic excitations at the level of the NAc shell that can thereby be driven by dopaminergic input from the ventral tegmental area (VTA) promoting behavioural cue responding (Ghazizadeh et al. 2012). An increase in extracellular DA levels in the shell might also occur in consequence of inactivation of this region due to its feedback loop involving the VTA. In normal conditions, terminal DA release in the NAc is tonically inhibited via GABAA receptors in the VTA (Ikemoto et al. 1997; Rahman and McBride 2002).
Activating GABAA receptors in the shell by muscimol may hyperpolarise the MSN projecting to the VTA leading to disinhibition of DA neurons targeting the NAc shell. Consistently, blockade of GABAA receptors within the VTA increases the discharge rate of DA neurons innervating the NAc (Ikemoto et al. 1997).
In conclusion, our results extend previous findings pointing out the functional heterogeneity of frontostriatal systems and show a differential contribution of the vmPFC-NAc connection to behavioural control depending on the involved accumbal subregion. We suggest that the maintenance and regulation of motor impulse control particularly requires an intact connection between the vmPFC and the NAc shell, while the vmPFC-NAc core projection seems to be of minor importance.
References
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, Bloch MH, Li CS, Pittenger C (2013) Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry
Aron AR (2007) The neural basis of inhibition in cognitive control. Neuroscientist 13:214–228
Bari A, Robbins TW (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79
Bassareo V, De Luca MA, Di Chiara G (2002) Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci 22:4709–4719
Basso AM, Kelley AE (1999) Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci 113:324–336
Berendse HW, Galis-de Graaf Y, Groenewegen HJ (1992) Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol 316:314–347
Blaiss CA, Janak PH (2009) The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behav Brain Res 200:22–32
Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB (2007) Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex 17:1625–1636
Blondeau C, Dellu-Hagedorn F (2007) Dimensional analysis of ADHD subtypes in rats. Biol Psychiatry 61:1340–1350
Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y (2012) Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci 32:4982–4991
Brog JS, Salyapongse A, Deutch AY, Zahm DS (1993) The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338:255–278
Brunner D, Hen R (1997) Insights into the neurobiology of impulsive behavior from serotonin receptor knockout mice. Ann N Y Acad Sci 836:81–105
Campden Instruments Limited (2005) Instruction Manual for MKII Rat 5CSRT. Campden Instruments, Loughborough
Cardinal RN, Cheung TH (2005) Nucleus accumbens core lesions retard instrumental learning and performance with delayed reinforcement in the rat. BMC Neurosci 6:9
Carlezon WA Jr, Thomas MJ (2009) Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56(Suppl 1):122–132
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Christakou A, Robbins TW, Everitt BJ (2001) Functional disconnection of a prefrontal corticaldorsal striatal system disrupts choice reaction time performance: implications for attentional function. Behav Neurosci 115:812–825
Christakou A, Robbins TW, Everitt BJ (2004) Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci 24:773–780
Chudasama Y, Muir JL (2001) Visual attention in the rat: a role for the prelimbic cortex and thalamic nuclei? Behav Neurosci 115:417–428
Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW (2003) Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146:105–119
Coppens CM, De Boer SF, Koolhaas JM (2010) Coping styles and behavioural flexibility: towards underlying mechanisms. Philos Trans R Soc Lond B Biol Sci 365:4021–4028
Corbit LH, Muir JL, Balleine BW (2001) The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci 21:3251–3260
Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, Stevens CA, Musser ED, Carpenter SD, Grayson DS, Mitchell SH, Nigg JT, Fair DA (2012) Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol
Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784
Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69:680–694
Dalley JW, Mar AC, Economidou D, Robbins TW (2008) Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90:250–260
de Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14:22–31
Diederich K, Koch M (2005) Role of the pedunculopontine tegmental nucleus in sensorimotor gating and reward-related behavior in rats. Psychopharmacology (Berl) 179:402–408
Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ (2008) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63:301–308
Ding DC, Gabbott PL, Totterdell S (2001) Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat. Brain Res 917:81–89
Feja M, Hayn L, Koch M (2014) Nucleus accumbens core and shell inactivation differentially affects impulsive behaviours in rats. Prog Neuropsychopharmacol Biol Psychiatry 54C:31–42
Feja M, Koch M (2014) Ventral medial prefrontal cortex inactivation impairs impulse control but does not affect delay-discounting in rats. Behav Brain Res 264:230–239
Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E (2010) Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35:591–604
Floresco SB, Braaksma DN, Phillips AG (1999) Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci 19:11061–11071
Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O (2006) Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci 26:2449–2457
Floresco SB, McLaughlin RJ, Haluk DM (2008) Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience 154:877–884
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005) Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492:145–177
Gaffan D, Murray EA, Fabre-Thorpe M (1993) Interaction of the amygdala with the frontal lobe in reward memory. Eur J Neurosci 5:968–975
Gaffan D, Wilson CR (2008) Medial temporal and prefrontal function: recent behavioural disconnection studies in the macaque monkey. Cortex 44:928–935
Ghazizadeh A, Ambroggi F, Odean N, Fields HL (2012) Prefrontal cortex mediates extinction of responding by two distinct neural mechanisms in accumbens shell. J Neurosci 32:726–737
Gorelova N, Yang CR (1997) The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience 76:689–706
Goto Y, Grace AA (2005) Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 8:805–812
Groenewegen HJ, Galis-de Graaf Y, Smeets WJ (1999) Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat 16:167–185
Groenewegen HJ, Trimble M (2007) The ventral striatum as an interface between the limbic and motor systems. CNS Spectr 12:887–892
Hauber W, Sommer S (2009) Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex 19:2240–2247
Hayton SJ, Olmstead MC, Dumont EC (2011) Shift in the intrinsic excitability of medial prefrontal cortex neurons following training in impulse control and cued-responding tasks. PLoS ONE 6:e23885
Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27:555–579
Heimer L (2003) A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry 160:1726–1739
Ikemoto S, Kohl RR, McBride WJ (1997) GABA(A) receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem 69:137–143
Izaki Y, Fujiwara SE, Akema T (2007) Involvement of the rat prefrontal cortex in a delayed reinforcement operant task. Neuroreport 18:1687–1690
Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146:373–390
Jongen-Relo AL, Kaufmann S, Feldon J (2002) A differential involvement of the shell and core subterritories of the nucleus accumbens of rats in attentional processes. Neuroscience 111:95–109
Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413
Kesner RP, Churchwell JC (2011) An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem 96:417–431
Kosaki Y, Watanabe S (2012) Dissociable roles of the medial prefrontal cortex, the anterior cingulate cortex, and the hippocampus in behavioural flexibility revealed by serial reversal of three-choice discrimination in rats. Behav Brain Res 227:81–90
Lomber SG (1999) The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. J Neurosci Methods 86:109–117
Lopes AP, da Cunha IC, Steffens SM, Ferraz A, Vargas JC, de Lima TC, Neto JM, Faria MS, Paschoalini MA (2007) GABAA and GABAB agonist microinjections into medial accumbens shell increase feeding and induce anxiolysis in an animal model of anxiety. Behav Brain Res 184:142–149
Martin JH (1991) Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 127:160–164
Martin JH, Ghez C (1999) Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods 86:145–159
McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004) Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24:1551–1560
McGeorge AJ, Faull RL (1989) The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29:503–537
Meredith GE, Pattiselanno A, Groenewegen HJ, Haber SN (1996) Shell and core in monkey and human nucleus accumbens identified with antibodies to calbindin-D28k. J Comp Neurol 365:628–639
Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF (2002) Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci 5:267–271
Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97
Montaron MF, Deniau JM, Menetrey A, Glowinski J, Thierry AM (1996) Prefrontal cortex inputs of the nucleus accumbens-nigro-thalamic circuit. Neuroscience 71:371–382
Moscarello JM, Ben Shahar O, Ettenberg A (2010) External incentives and internal states guide goal-directed behavior via the differential recruitment of the nucleus accumbens and the medial prefrontal cortex. Neuroscience 170:468–477
Muir JL, Everitt BJ, Robbins TW (1996) The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6:470–481
Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, Dalley JW, Robbins TW (2012) Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology (Berl) 219:401–410
Murphy ER, Robinson ES, Theobald DE, Dalley JW, Robbins TW (2008) Contrasting effects of selective lesions of nucleus accumbens core or shell on inhibitory control and amphetamine-induced impulsive behaviour. Eur J Neurosci 28:353–363
Narayanan NS, Horst NK, Laubach M (2006) Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience 139:865–876
Nigg JT, Casey BJ (2005) An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol 17:785–806
Ongur D, Price JL (2000) The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10:206–219
Paine TA, Slipp LE, Carlezon WA Jr (2011) Schizophrenia-like attentional deficits following blockade of prefrontal cortex GABAA receptors. Neuropsychopharmacology 36:1703–1713
Pantelis C, Barnes TR, Nelson HE, Tanner S, Weatherley L, Owen AM, Robbins TW (1997) Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain 120(Pt 10):1823–1843
Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ (1999) Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci 19:2401–2411
Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM (2007) Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl) 191:587–598
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic, San Diego
Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28:6046–6053
Pezze MA, Dalley JW, Robbins TW (2009) Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D(2/3) receptor antagonist sulpiride. Psychopharmacology (Berl) 202:307–313
Pothuizen HH, Jongen-Relo AL, Feldon J (2005a) The effects of temporary inactivation of the core and the shell subregions of the nucleus accumbens on prepulse inhibition of the acoustic startle reflex and activity in rats. Neuropsychopharmacology 30:683–696
Pothuizen HH, Jongen-Relo AL, Feldon J, Yee BK (2005b) Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci 22:2605–2616
Puumala T, Sirvio J (1998) Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience 83:489–499
Ragozzino ME (2007) The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci 1121:355–375
Ragozzino ME, Detrick S, Kesner RP (1999) Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci 19:4585–4594
Rahman S, McBride WJ (2002) Involvement of GABA and cholinergic receptors in the nucleus accumbens on feedback control of somatodendritic dopamine release in the ventral tegmental area. J Neurochem 80:646–654
Reynolds SM, Berridge KC (2002) Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci 22:7308–7320
Robbins TW (1990) The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull 16:391–402
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380
Robbins TW, Everitt BJ (1996) Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 6:228–236
Russo SJ, Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625
Sesack SR, Deutch AY, Roth RH, Bunney BS (1989) Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290:213–242
Setlow B, Holland PC, Gallagher M (2002) Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive Pavlovian second-order conditioned responses. Behav Neurosci 116:267–275
Soderpalm AH, Berridge KC (2000) Food intake after diazepam, morphine or muscimol: microinjections in the nucleus accumbens shell. Pharmacol Biochem Behav 66:429–434
Sokolowski JD, Salamone JD (1998) The role of accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav 59:557–566
Stratford TR, Kelley AE (1997) GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci 17:4434–4440
Stratford TR, Wirtshafter D (2011) Opposite effects on the ingestion of ethanol and sucrose solutions after injections of muscimol into the nucleus accumbens shell. Behav Brain Res 216:514–518
Stratford TR, Wirtshafter D (2012) Effects of muscimol, amphetamine, and DAMGO injected into the nucleus accumbens shell on food-reinforced lever pressing by undeprived rats. Pharmacol Biochem Behav 101:499–503
Vassoler FM, White SL, Hopkins TJ, Guercio LA, Espallergues J, Berton O, Schmidt HD, Pierce RC (2013) Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J Neurosci 33:14446–14454
Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58
West SA, Gardner A (2013) Adaptation and inclusive fitness. Curr Biol 23:R577–R584
Winstanley CA, Eagle DM, Robbins TW (2006) Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 26:379–395
Wirtshafter D, Stratford TR (2010) Evidence for motivational effects elicited by activation of GABA-A or dopamine receptors in the nucleus accumbens shell. Pharmacol Biochem Behav 96:342–346
Wise SP (2008) Forward frontal fields: phylogeny and fundamental function. Trends Neurosci 31:599–608
Zaborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, Palkovits M (1985) Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience 14:427–453
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feja, M., Koch, M. Frontostriatal systems comprising connections between ventral medial prefrontal cortex and nucleus accumbens subregions differentially regulate motor impulse control in rats. Psychopharmacology 232, 1291–1302 (2015). https://doi.org/10.1007/s00213-014-3763-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3763-3