Abstract
Intraductal carcinoma (IC) is a rare salivary gland tumor with low- to intermediate-grade cytological features. It is further classified into intercalated duct type and apocrine type based on its distinct histologic and immunohistochemical expression. Conventional salivary duct carcinoma (SDC) is an aggressive carcinoma with high-grade features and is usually associated with poor prognosis. In this study, immunohistochemistry and mutation analyses (including HRAS/PIK3CA mutations, RET rearrangement, and human epidermal growth factor receptor 2 [HER2] amplification) of 9 ICs (including 3 pure ICs, 6 ICs with invasive carcinoma) and 24 conventional SDCs were performed and the results were compared. Four intercalated duct-type cases were positive for SOX10 and S100 and negative for AR; five apocrine-type cases showed opposite results. All five apocrine-type cases had cysts with relatively circumscribed tumor borders and morphologically mimicking breast low-grade ductal carcinoma in situ or papillary carcinoma. RET fusion is detected in half of the 4 intercalated duct-type IC but not in the apocrine-type or conventional SDC. HER2 amplification was only observed in conventional SDC. The monoclonal antibody (clone RBT-NRAS) against NRAS Q61R is a sensitive and specific marker used for detecting HRAS Q61R mutation in the salivary gland tumors. The apocrine-type IC had different cytological grades, distinct tumor growth patterns, and no evidence of low- to high-grade transition, suggesting that apocrine-type IC should be distinguished from apocrine SDC with an in situ component.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraductal carcinoma (IC) is the term used in the 2017 World Health Organization (WHO) Classification of Head and Neck Tumours to describe a low-grade salivary gland tumor originally called as “low-grade salivary duct carcinoma” and later re-named by WHO 2005 as “low-grade cribriform cystadenocarcinoma” [1,2,3]. It is further categorized as low grade, intermediate grade, or high grade based on the degree of cytological atypia [1]. ICs are typically unencapsulated and composed of multiple cysts with intraductal proliferation of epithelial cells forming mixed cribriform, papillary, or micropapillary structures [1, 2, 4,5,6,7]. These cells are typically low-grade, cuboidal, bland ductal cells with fine chromatin, inconspicuous nucleoli, and rare mitoses [1,2,3]. Sometimes, an invasive component with the loss of p63-positive cells can be observed [7]. Immunohistochemically, the classical “low-grade” IC is positive for S100, SOX10, mammaglobin, and negative for androgen receptor (AR) or human epidermal growth factor receptor 2 (HER2) [4,5,6,7,8]. Since the immunohistochemical expression of IC is different from that of conventional salivary duct carcinoma (SDC), ICs are considered to be different types of salivary gland tumors. However, some ICs show apocrine features, increasing cytological atypia, some with nucleoli and necrosis, and strong expression of AR [4,5,6,7, 9]. Weinreb et al. first described this rare variant of IC in detail and designated it as low-grade intraductal carcinoma with marked apocrine differentiation [9]. Due to differences in cytological atypia and immunophenotypes, intraductal carcinoma is nowadays further described as intercalated duct type, apocrine type, or even hybrid type [5,6,7].
Many pathognomonic genetic changes or rearrangements have been identified in different low-grade salivary gland tumors including pleomorphic adenoma, adenoid cystic carcinoma, mucoepidermoid carcinoma, clear cell carcinoma, secretory carcinoma, polymorphous adenocarcinoma, and cribriform adenocarcinoma of minor salivary gland [10,11,12,13,14,15,16,17]. SDC is a high-grade salivary gland tumor, and its genetic changes are far more complex than those of previously mentioned low-grade salivary gland tumors. The most commonly observed somatic mutations in SDC include HER2 amplification, AR amplification, HRAS mutations, PIK3CA mutations, AKT1 mutations, TP53 mutations, and NRAS mutations [18,19,20,21]. Data on the genetic changes in IC are relatively limited due to its rarity. Recently, recurrent RET gene rearrangements have been found in individuals with IC, especially the intercalated duct type [5,6,7]. Weinreb et al. first reported NCOA4-RET fusion in one intercalated duct-type IC, and RET rearrangement was found in 47% of tested intercalated duct-type ICs but not in cases with apocrine features, which were found to harbor PIK3CA and/or HRAS hotspot mutations [5]. Later, Skálová et al. confirmed that the NCOA4-RET fusion was the major genetic change in intercalated duct-type IC, but they also discovered recurrent TRIM27-RET fusions in apocrine IC cases [6, 7]. RET rearrangement is now considered an important genetic change in IC.
IC and SDC of the salivary gland have morphologically similar counterparts in the breast. In the breast, it is well known that intraductal carcinoma is the precursor lesion of invasive carcinoma. However, the relationship between IC and SDC of the salivary gland is not clear as SDC is a high-grade and common malignancy, while IC is rare and usually low-grade in terms of histology. In this study, immunohistochemistry and mutation analysis (including HRAS/PIK3CA mutations, RET rearrangement, and HER2 amplification) of IC (both intercalated duct type and apocrine type) and conventional SDC were performed and the results were compared. A rabbit monoclonal antibody against NRAS Q61R mutant protein has been found to cross-react with HRAS and KRAS Q61R mutant proteins in colorectal carcinoma and malignant melanoma [22, 23]. Immunohistochemistry of the antibody against NRAS (Q61R) was therefore performed in all cases to verify its value in the diagnosis of HRAS mutation in salivary gland tumors.
Methods
Case selection
Patients diagnosed with low-grade salivary duct carcinoma, IC, cribriform cystadenocarcinoma, cystadenocarcinoma, and salivary duct carcinoma between 1999 and 2018 in the Department of Pathology at NTUH were evaluated by two pathologists (MSH and YJK). In this study, tumors were classified as IC when they show multiple cystically enlarged ducts and proliferation of ductal cells with appearance of mammary type atypical ductal hyperplasia or ductal carcinoma in situ. The small- to medium-sized tumor cells are bland-looking, with pale to eosinophilic cytoplasm and indistinct cell borders, having round to oval nuclei without prominent nucleoli and forming mixed cribriform, papillary-cystic, or filigreed patterns consistent with previous description [4,5,6,7]. ICs were further classified into (1) intercalated duct type when tumor cells had small eosinophilic to amphophilic cytoplasm, had cuboidal cells, and were positive for S100 immunohistochemistry and (2) apocrine type when tumor cells had granular eosinophilic cytoplasm, apocrine snouts, secretions, and were positive for AR immunohistochemistry [5,6,7]. Conventional SDC was confirmed using previous described criteria when ductal cells were high grade with architectural and cytological resemblances to in situ and invasive grade 2–3 ductal carcinoma of the breast featured by the presence of solid, cribriform, or comedonecrosis patterns, along with infiltrating ducts or nests [24]. A total of 4 intercalated duct-type ICs, 5 apocrine-type ICs, and 24 conventional SDCs were collected. SDC ex pleomorphic carcinoma was excluded from this study. Clinical data of these cases were obtained from their medical records. This study (201812013RINA) was approved by the Research Ethics Committee of National Taiwan University Hospital.
Immunohistochemistry
Immunohistochemistry was performed using an automated stainer (Ventana Benchmark; Roche Ventana, Tucson, AZ, USA). Tissue sections (thickness 4 μm) were dewaxed, rehydrated, and reacted with primary antibodies listed in Table 1. The HER2 status was scored according to the American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update [25]. The NRAS Q61R staining was scored as 0 (negative), 1 (mild), 2 (moderate), and 3 (strong). The IHC results were evaluated by two pathologists (MSH and YHL).
Detection of HRAS/PIK3CA mutations
The HRAS and PIK3CA mutations were detected by PCR and Sanger sequencing. Regions of interest were macrodissected from unstained slides of the formalin-fixed, paraffin-embedded tissue and extracted using a DNeasy tissue kit (Qiagen, Hilden, Germany). PCR was performed using a HotStarTaq Master Mix kit (Qiagen) with the primers listed in Table 2. Successfully amplified products were purified and sequenced from both ends by DNA sequencing services using a BigDye Terminator kit (Applied Biosystems, Foster City, CA, USA) and an ABI Prism 3700 DNA Analyzer (Applied Biosystems). All sequencing reactions were conducted in both forward and reverse directions, using amplicons from at least two independent PCRs. Specimens with mutations were confirmed in two rounds; only specimens that yielded the same result in both rounds were recorded as mutation positive.
FISH
All IC tissue samples were examined for RET break-apart fluorescent in situ hybridization (FISH) using ZytoLight SPEC RET Dual Color Break Apart Probe (ZytoVision, Bremerhaven, Germany) and ETV6 break-apart FISH (Abbott Molecular, USA) to rule out secretory carcinoma. FISH was performed as previously described [26]. Fifty nonoverlapping tumor cell nuclei were evaluated in each patient. They were considered positive for target gene translocation when ≥ 20% of the tumor nuclei exhibited a break-apart signal pattern. All patients were tested for HER2 amplification using ZytoLight SPEC ERBB2/CEN 17 Dual Color Probe (ZytoVision). Twenty nonoverlapping tumor cell nuclei were evaluated in each patient. HER2 amplification was defined when the ERBB2/CEN17 ratio is ≥ 2 or the average HER2 copy number is ≥ 4 signals per cell [25].
Statistical analysis
Fisher’s exact and Pearson’s χ2 tests were used to determine differences in categorical data. A two-sample Mann-Whitney U test was used to determine differences in age and size. Two-sided p < 0.05 were considered statistically significant. The analysis for the prognostic effects including HER2 mutation, T (T1 + T2 vs T3 + T4), N (N0 vs N1 + N2), age (< 65 vs ≥ 65), and sex on recurrence-free survival in the SDC group was evaluated by multivariate Cox regression analysis. SPSS 25.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses.
Results
Clinicopathological features of intercalated duct type and apocrine type IC
The main clinicopathological data of 9 IC patients are summarized in Table 3. This cohort comprises four female and six male patients, and the median age was 70 years (range from 44 to 85 years). The median tumor size was 3.3 cm (range from 0.8 to 6 cm). All tumors arose from the parotid gland except one located in the submandibular gland. Patients with intercalated duct type (44–70 years, median 58 years) were younger than those with apocrine type (50–85 years, median 80 years); the intercalated duct type also showed a female predominance. However, these differences did not reach statistical significance.
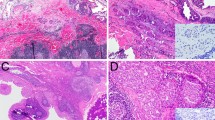
Microscopically, all nine IC patients showed variable-sized cysts lined by epithelial cells arranged in mixed micropapillary or filigreed epithelial tufts, cribriform, papillary, or solid patterns in variable proportions. Three intercalated duct-type ICs had poorly defined tumor borders and comprised multiple cysts with variable sizes, which were separated by a fibrous, nontumorous stroma (Fig. 1). One intercalated duct-type IC (no. 2) had MASC-like features with a relatively well-defined tumor border and a predominant papillary-cystic pattern (Fig. 1d). Hemorrhage and hemosiderin deposition were observed in three intercalated duct-type ICs. P63-positive myoepithelial cells were preserved in three and focally lost in one case.
a Intercalated duct-type IC typically presented as multiple cysts lined by cuboidal tumor cells with amphophilic cytoplasm arranged in lace-like structures morphologically similar to the usual ductal hyperplasia of the breast under low power examination (40×). b Other patterns including cystic, papillary, and micropapillary patterns were also common (100×). c Tumor cells had low- to intermediate-grade features with oval nuclei, inconspicuous nucleoli, and fine chromatin and arranged in tufts or papillary structures forming irregularly shaped secondary lumens (200×). d One patient with intercalated duct-type IC had MASC-like features with eosinophilic cytoplasm; intracytoplasmic vacuoles; vesicular nuclei, arranged in a predominant cystic-papillary structure; hemorrhage; and hemosiderin deposition (200×). This patient had RET rearrangement and no ETV6 translocation by fluorescence in situ hybridization (FISH). e Intercalated duct-type IC was diffusely positive for SOX10 (400×). f Two of four patients with intercalated duct-type IC had RET rearrangement confirmed by FISH. Arrows indicate split signals
The five apocrine-type cases comprised multiple, closely compacted cysts lined with tumor cells with eosinophilic cytoplasm and vesicular nuclei with occasionally prominent nucleoli and arranged in mixed cribriform, micropapillary, filigreed, or microcystic patterns (Fig. 2). All cases were relatively circumscribed without infiltrative growth. P63-positive myoepithelial cells were completely lost in two and mostly lost in three cases. Morphologically, apocrine-type ICs were similar to low-grade ductal carcinoma in situ (DCIS) or papillary carcinoma of the breast.
Apocrine-type IC. a All cases comprised multiple cysts with relatively circumscribed tumor borders under low power field; tumor necrosis inside the cyst is not uncommon (40×). b These cysts were lined by tumor cells typically forming micropapillary, microcystic, cribriform, or filigree patterns (100×). c Tumor cells show low- to intermediate-grade features with eosinophilic cytoplasm, apical snouts, apocrine snouts, and decapitation secretions (400×). d Patients with apocrine-type IC were positive for AR (200×), e negative for SOX10 (200×), and f positive for RAS Q61R, which were detected using NRAS Q61R antibody as a surrogate marker for HRAS Q61R mutation (200×)
Immunohistochemically, the intercalated duct-type cases were positive for SOX10, S100, and mammaglobin and negative for AR, while the apocrine-type cases were negative for SOX10 and S100 and positive for AR and mammaglobin. All apocrine-type cases had strong NRAS (Q61R) staining (Fig. 2f), which correlated well with the HRAS mutation profile (3 with HRAS Q61R and 1 with Q61K mutation). Only two apocrine-type cases had mild to moderate HER2 staining.
Mutation analysis showed that two intercalated duct-type ICs had RET rearrangement. All apocrine-type IC cases had concurrent HRAS/PIK3CA mutations. All were negative for HER2 amplification or ETV6 rearrangement. During the clinical follow-up (10–96 months, median 72 months), none of the patients had tumor recurrence, distant metastasis, or tumor-related death.
Clinicopathological features of conventional SDC
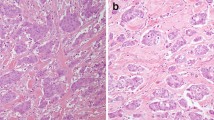
The clinicopathological data of 24 patients with conventional SDC are listed in Table 4. This cohort comprises 22 male and 2 female patients with a median of 64.5 years. The median tumor size was 3.1 cm. Two-third of the cases were located in the parotid gland, and the others were located in the submandibular gland. All SDC showed high-grade cytological features with large nuclei, prominent nucleoli, coarse chromatin, abundant eosinophilic cytoplasm, frequent mitoses, and an infiltrating growth pattern with marked stromal desmoplastic change (Fig. 3). Immunohistochemically, the majority of SDC was positive for AR (91.7%) and all were negative for SOX10 and S100. HER2 IHC was 3+ in nine (37.5%), 2+ in seven (29.2%), and 0–1+ in eight (33.3%) patients. All nine HER2 IHC 3+ and two of seven HER2 IHC 2+ patients had HER2 amplification (Fig. 3b). Three SDCs had strong (3+), and one had focal moderate (2+) staining for NRAS Q61R. Three SDCs with strong NRAS Q61R staining had HRAS Q61R mutation, the one with focal moderate (2+) staining had HRAS Q61K mutation, and those with negative or focal weak staining were negative for HRAS mutation. All four SDCs with HRAS mutations harbored concurrent PIK3CA mutations. During clinical follow-up (8 ~ 127 months, median 33 months), six cases (6/24, 25%) had tumor recurrence and five cases (5/24, 20.8%) had tumor-related death. HER2 amplification is the only factor with significant effect on tumor recurrence (p = 0.047) under multivariate analysis after adjusting T (T1 + T2 vs T3 + T4), N (N0 vs N+), age (< 65 vs ≥ 65), and sex. The small case number was inadequate for multivariate analysis on overall survival.
a Conventional SDC showing infiltrating glands and high-grade cells with large nuclei, prominent nucleoli, eosinophilic cytoplasm, and strong staining for HER2 (400×), and b FISH revealed HER2 amplification. c HRAS/PIK3CA mutated SDC with IC component morphologically similar to high-grade breast cancer (40×). d HRAS/PIK3CA mutated SDC showing infiltrating nests and glands with marked stromal desmoplastic change and e diffusely positive for RAS Q61R using NRAS Q61R antibody as a surrogate marker for HRAS Q61R mutation (200×). f Mutation analysis of SDC and IC
Comparison of IHC and mutation profiles between IC and conventional SDC
Results of clinicopathological comparison between IC and conventional SDC are summarized in Table 5. There is no significant difference in age, sex, location, tumor size, stage between intercalated duct-type and apocrine-type ICs. A significant male predominance and more advanced stage could be observed in the conventional SDC group. Immunohistochemically, intercalated duct-type IC showed a distinct pattern different from apocrine-type IC and conventional SDC. The IHC expression of SOX10, S100, and mammaglobin in intercalated duct-type IC was similar to that of secretory carcinoma. The apocrine-type IC had an IHC expression more akin to that of conventional SDC, being negative for SOX10 and S100 and positive for AR. RET fusion was only present in intercalated duct-type IC. Concurrent HRAS and PIK3CA mutations were present in all patients with apocrine-type IC and a subset of conventional SDC (Fig. 3f). HER2 amplification was the most common change found in conventional SDC (11/24, 45.8%).
Discussion
In this study, we reported nine patients with IC with low- to intermediate-grade nuclear features. Intercalated duct and apocrine-type IC had different morphological, immunohistochemical, and genetic changes. The four intercalated duct-type ICs comprised variable-sized cysts and most were loosely arranged in a fibrous background. Apocrine-type ICs were circumscribed and composed of closely arranged cysts. According to the 2017 WHO, IC includes tumors previously diagnosed as low-grade salivary duct carcinoma or low-grade cribriform cystadenocarcinoma as most of these tumors have preserve p63-positive rimming [1]. However, p63-positive rimming cells were absent focally in one intercalated duct-type IC and widely lost in all five apocrine-type ICs. Our finding is in conjunction with the recent study which reported the largest cohort of 33 ICs in the literature and 8 cases with focal or widespread invasive growth, especially those with apocrine features [7]. Therefore, the term “intraductal carcinoma,” which implies an in situ tumor, may not fully describe this type of tumor.
IC is the term used in 2017 WHO for the tumor originally described as low-grade salivary duct carcinoma or low-grade cribriform cystadenocarcinoma which is characterized by its low- to intermediate-grade cytological features [1]. Nevertheless, it also says IC can be further categorized as low, intermediate, and high grade types [1]. We think low- to intermediate-grade IC properly represents low-grade salivary duct carcinoma or low-grade cribriform cystadenocarcinoma, whereas high-grade IC most likely represents the in situ component of conventional SDC. It may be inappropriate to use the same term “intraductal carcinoma” to describe these two different types of in situ neoplasms. Skálová et al. proposed to use “intercalated duct carcinoma, invasive or noninvasive” to replace “IC” in order to specify the tumor origin and make this entity more independent from SDC terminologically [7]. We concur their suggestion as that “intercalated duct carcinoma, invasive or noninvasive” is more precise and gives information of in situ or invasive status than other previously or currently used terms.
IC and SDC are generally considered as separate entities with different immunohistochemical profiles and driver mutations [5]. However, a subset of IC and SDC shares similar apocrine features [5,6,7, 9]. Apocrine IC was first described by Weinreb et al. in 2006 as “low-grade intraductal carcinoma” with marked apocrine differentiation and diffusely expression of AR [9]. In 2018, Weinreb et al. further reported seven widely invasive, high-grade apocrine invasive adenocarcinomas morphologically similar to conventional SDC and one low- to intermediate-grade apocrine carcinoma with a predominant IC component [5]. In this study, we reported five apocrine type ICs characterized by low- to intermediate-grade nuclear features and relatively circumscribed borders. Unlike other studies showing TRIM27-RET fusion in some apocrine type IC [6, 26], all five apocrine type ICs in our study had concurrent HRAS and PIK3CA mutations but no RET rearrangement. Despite apocrine-type IC and conventional SDC share some pathological features (tumor necrosis, AR expression, and HRAS/PIK3CA mutations), the low- to intermediate-grade cytological features and lack of widely infiltrating growth in apocrine-type IC make it different from conventional SDC. Moreover, among all these cases, there is no morphological evidence of transition from apocrine IC to conventional SDC. We believe that low- to intermediate-grade apocrine IC and high-grade SDC represent two different types of tumors rather than a spectrum of differentiation. In the salivary gland, apocrine-type IC with circumscribed growth is akin to low-grade DCIS or papillary carcinoma of the breast, while SDC is similar to breast high-grade invasive ductal carcinoma.
SDC is a high-grade and aggressive salivary carcinoma resembling high-grade ductal carcinoma of the breast. Different histologic variants such as micropapillary, apocrine, mucinous, oncocytic, sarcomatoid, or rhabdoid types have been reported [21, 24, 27]. SDC typically has high-grade cytological features like large nuclei, coarse chromatin, prominent nucleoli, and frequent mitoses [24]. Comedonecrosis and stromal desmoplastic change are also not uncommon [24]. HER2 amplification is the most common mutation in SDC with a frequency ranging from 15 to 40% [19,20,21, 24, 28]. Other somatic mutations that include HRAS, PIK3CA, AKT1, TP53, and NRAS have been reported [21]. SDC with HER2 amplification could benefit from trastuzumab along with chemotherapy [29]. In this study, HER2 amplification is the most common mutation (48.5%) followed by PIK3CA (27.8%) and HRAS (16.7%). Though HER2 amplification is the only factor with significant effect on tumor recurrence under multivariate analysis in our cohort, other studies showed the number of lymph node metastases and facial nerve involvement are the most important prognostic factors for disease-free survival and overall survival in SDC [30, 31].
NRAS mutation is common in melanoma, thyroid follicular carcinoma, and a subset of colorectal carcinoma, and the Q61R is the most common NRAS mutation [32, 33]. SP174, a rabbit monoclonal antibody originally developed to detect NRAS Q61R-mutant protein, cross-reacts with HRAS and KRAS Q61R mutant proteins in colorectal carcinoma and malignant melanoma [22, 23]. In this study, using another commercially available rabbit monoclonal antibody against NRAS Q61R (clone RBT-NRAS, for in vitro diagnostic use) to detect HRAS Q61R mutation yielded a high sensitivity (100%) and specificity. Seven patients with HRAS Q61R mutation showed diffuse and strong (3+) cytoplasmic staining, two with HRAS Q61K mutation showed at least focal moderate (2+) cytoplasmic staining, while the remaining 24 without HRAS mutation had negative (0) or very limited faint (0–1+) staining. This study demonstrated that another NRAS Q61R antibody (clone RBT-NRAS) can be used as a surrogate marker to detect HRAS Q61R mutation in the salivary gland tumors.
In conclusion, we reported nine ICs including four intercalated duct-type and five apocrine-type cases with different SOX10, S100, and AR staining patterns. All five apocrine-type ICs were morphologically mimicking breast low-grade DCIS or papillary carcinoma featured by their circumscribed borders and delicate fibrovascular stroma in arborizing pattern. RET fusion was detected in intercalated duct-type IC, concurrent HRAS and PIK3CA mutations were detected in apocrine IC, and HER2 amplification was only observed in SDC. The monoclonal antibody (clone RBT-NRAS) against NRAS Q61R is a sensitive and specific surrogate marker for detecting HRAS Q61R mutation in salivary gland tumors.
References
Loening T, Leivo I, Simpson RHW, Weinreb I (2017) Intraductal carcinoma. In: El-Naggar AK, Chan JKC, Grandis JR et al (eds) WHO classification of head and neck tumours. IARC Press, Lyon, pp 170–171
Delgado R, Klimstra D, Albores-Saavedra J (1996) Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer 78:958–967
Brandwein-Gensler M, Gnepp DR (2005) Low-grade cribriform cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P et al (eds) Pathology and genetics head and neck tumors. IARC Press, Lyon, p 233
Kuo YJ, Weinreb I, Perez-Ordonez B (2013) Low-grade salivary duct carcinoma or low-grade intraductal carcinoma? Review of the literature. Head Neck Pathol 7:S59–S67
Weinreb I, Bishop JA, Chiosea SI, Seethala RR, Perez-Ordonez B, Zhang L, Sung YS, Chen CL, Assaad A, Oliai BR, Antonescu CR (2018) Recurrent RET gene rearrangements in intraductal carcinomas of salivary gland. Am J Surg Pathol 42:442–452
Skálová A, Vanecek T, Uro-Coste E, Bishop JA, Weinreb I, Thompson LDR, de Sanctis S, Schiavo-Lena M, Laco J, Badoual C, Santana Conceiçao T, Ptáková N, Baněčkova M, Miesbauerová M, Michal M (2018) Molecular profiling of salivary gland intraductal carcinoma revealed a subset of tumors harboring NCOA4-RET and novel TRIM27-RET fusions: a report of 17 cases. Am J Surg Pathol 42:1445–1455
Skálová A, Ptáková N, Santana T, Agaimy A, Ihrler S, Uro-Coste E, Thompson LDR, Bishop JA, Baněčkova M, Rupp NJ, Morbini P, de Sanctis S, Schiavo-Lena M, Vanecek T, Michal M, Leivo I (2019) NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors: is “intraductal” correct? Am J Surg Pathol 43:1303–1313
Hsieh MS, Lee YH, Chang YL (2016) SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum Pathol 56:134–142
Weinreb I, Tabanda-Lichauco R, Van der Kwast T, Perez-Ordoñez B (2006) Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol 30:1014–1021
Kas K, Voz ML, Röijer E, Aström AK, Meyen E, Stenman G et al (1997) Promoter swapping between the genes for a novel zinc finger protein and beta-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat Genet 15:170–174
Martins C, Fonseca I, Roque L, Pereira T, Ribeiro C, Bullerdiek J, Soares J (2005) PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol 18:1048–1055
Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, Perez-Ordoñez B, Have C, Asa SL, Leong IT, Bradley G, Klieb H, Weinreb I (2011) EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom Cancer 50:559–570
Persson M, Andrén Y, Mark J, Horlings HM, Persson F, Stenman G (2009) Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A 106:18740–18744
Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, Starek I, Geierova M, Simpson RHW, Passador-Santos F, Ryska A, Leivo I, Kinkor Z, Michal M (2010) Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 34:599–608
Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, O’Neil K, Stover K, el-Naggar A, Griffin JD, Kirsch IR, Kaye FJ (2003) t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet 33:208–213
Weinreb I, Zhang L, Tirunagari LM, Sung YS, Chen CL, Perez-Ordonez B et al (2014) Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosom Cancer 53:845–856
Weinreb I, Piscuoglio S, Martelotto LG, Waggott D, Ng CK, Perez-Ordonez B et al (2014) Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet 46:1166–1169
Chiosea SI, Williams L, Griffith CC, Thompson LD, Weinreb I, Bauman JE et al (2015) Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol 39:744–752
Di Palma S, Simpson RH, Marchiò C, Skálová A, Ungari M, Sandison A et al (2012) Salivary duct carcinomas can be classified into luminal androgen receptor-positive, HER2 and basal-like phenotypes. Histopathology 61:629–643
Khoo TK, Yu B, Smith JA, Clarke AJ, Luk PP, Selinger CI, Mahon KL, Kraitsek S, Palme C, Boyer MJ, Dinger ME, Cowley MJ, O’Toole SA, Clark JR, Gupta R (2017) Somatic mutations in salivary duct carcinoma and potential therapeutic targets. Oncotarget 8:75893–75903
Luk PP, Weston JD, Yu B, Selinger CI, Ekmejian R, Eviston TJ, Lum T, Gao K, Boyer M, O’Toole SA, Clark JR, Gupta R (2016) Salivary duct carcinoma: Clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck 38:E1838–E1847
Lasota J, Kowalik A, Felisiak-Golabek A, Inaguma S, Wang ZF, Pięciak L, Zięba S, Pęksa R, Kopczynski J, Okoń K, Waloszczyk P, Gozdz S, Biernat W, Miettinen M (2017) SP174, NRAS Q61R mutant-specific antibody, cross-reacts with KRAS Q61R mutant protein in colorectal carcinoma. Arch Pathol Lab Med 141:564–568
Felisiak-Goląbek A, Inaguma S, Kowalik A, Wasąg B, Wang ZF, Zięba S, Pięciak L, Ryś J, Kopczynski J, Sarlomo-Rikala M, Góźdź S, Lasota J, Miettinen M (2018) SP174 antibody lacks specificity for NRAS Q61R and cross-reacts with HRAS and KRAS Q61R mutant proteins in malignant melanoma. Appl Immunohistochem Mol Morphol 26:40–45
Simpson RH (2013) Salivary duct carcinoma: new developments – morphological variants including pure in situ high grade lesions; proposed molecular classification. Head Neck Pathol 7:S48–S58
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 36:2105–2122
Tsai TH, Wu SG, Hsieh MS, Yu CJ, Yang JC, Shih JY (2015) Clinical and prognostic implications of RET rearrangements in metastatic lung adenocarcinoma patients with malignant pleural effusion. Lung Cancer 88:208–214
Kusafuka K, Kawasaki T, Maeda M, Yamanegi K, Baba S, Ito Y, Inagaki H, Nakajima T (2017) Salivary duct carcinoma with rhabdoid features: a salivary counterpart of pleomorphic lobular carcinoma of the breast. Histopathology 70:164–173
Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK (2010) Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res 16:2266–2274
Limaye SA, Posner MR, Krane JF, Fonfria M, Lorch JH, Dillon DA, Shreenivas AV, Tishler RB, Haddad RI (2013) Trastuzumab for the treatment of salivary duct carcinoma. Oncologist 18:294–300
Boon E, Bel M, van Boxtel W, van der Graaf WTA, van Es RJJ, Eerenstein SEJ, Baatenburg de Jong RJ, van den Brekel MWM, van der Velden LA, Witjes MJH, Hoeben A, Willems SM, Bloemena E, Smit LA, Oosting SF, PALGA Group, Jonker MA, Flucke UE, van Herpen CML (2018) A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from the Netherlands. Int J Cancer 143:758–766
Gilbert MR, Sharma A, Schmitt NC, Johnson JT, Ferris RL, Duvvuri U, Kim S (2016) A 20-year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg 142:489–495
Omholt K, Karsberg S, Platz A, Kanter L, Ringborg U, Hansson J (2002) Screening of N-ras codon 61 mutations in paired primary and metastatic cutaneous melanomas: mutations occur early and persist throughout tumor progression. Clin Cancer Res 8:3468–3474
Nikiforov YE, Nikiforova MN, Gnepp DR, Fagin JA (1996) Prevalence of mutations of ras and p53 in benign and malignant thyroid tumors from children exposed to radiation after the Chernobyl nuclear accident. Oncogene 13:687–693
Acknowledgments
The authors would like to thank Ms. Syue-Fong Hua for her technical support and Pei-Wen Yang, PhD, for statistical assistance.
Funding
This study was supported by grants from the National Taiwan University Hospital (nos. 106-M3739, 106-003612, 107-N3971, and 107-N4038).
Author information
Authors and Affiliations
Contributions
MSH, YTJ, and YJK designed the research study. MSH and YHL performed the research. MSH and YHL analyzed the data. MSH and YJK wrote the paper.
Corresponding author
Ethics declarations
Clinical data of these cases were obtained from their medical records. This study (201812013RINA) was approved by the Research Ethics Committee of National Taiwan University Hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hsieh, MS., Lee, YH., Jin, YT. et al. Clinicopathological study of intraductal carcinoma of the salivary gland, with emphasis on the apocrine type. Virchows Arch 477, 581–592 (2020). https://doi.org/10.1007/s00428-020-02823-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02823-7