Abstract
Intraductal carcinoma (IDC) is the current designation for a salivary gland neoplasm previously referred to as “low-grade salivary duct carcinoma” and “low-grade cribriform cystadenocarcinoma,” among others. IDC is conceptually believed to be similar to ductal carcinoma in-situ of the breast. Although IDC is one entity in the current WHO Classification of Head and Neck Tumors, recent studies have suggested that at least three subtypes exist: a low-grade, intercalated duct-like variant with frequent RET rearrangements; a high-grade apocrine variant with complex, salivary duct carcinoma-like genetics; and a mixed variant. We sought to characterize an unusual form of low-grade, purely apocrine IDC. Three cases of apocrine-type IDC with low-grade histology were retrieved from the authors’ consultation files. Immunohistochemistry for androgen receptor, GCDFP-15, S100, smooth muscle actin, and p40 was performed. A custom, targeted next generation sequencing (NGS) panel including 1425 cancer‐related genes was also done on all cases. All three cases developed in the parotid glands of men, aged 51, 63, and 73 years (mean, 62 years). All cases consisted of large, rounded macrocysts surrounded by smaller nests which were lined by cells with abundant granular eosinophilic cytoplasm and large round nuclei with prominent nucleoli. Pleomorphism was mild, the mitotic rate was low, and necrosis was absent. No cases had any invasive foci or areas of intercalated duct-like morphology. By immunohistochemistry, all cases were diffusely positive for androgen receptor and GCDFP-15, surrounded entirely by an intact layer of small myoepithelial cells positive for S100, smooth muscle actin, and p40. Targeted NGS results were obtained from two cases: both harbored HRAS mutations and copy number losses in TP53, while one case each harbored mutations in PIK3CA, SPEN, and ATM. Fusions were absent in both cases. All three patients were treated by surgery alone, and are currently free of disease (follow up 12–190 months). This study confirms the existence of a low-grade, purely apocrine form of IDC. In its pure form, i.e., without an intercalated duct-type component, low-grade apocrine IDC is genetically similar to high-grade salivary duct carcinoma, with frequent HRAS and PI3K pathway mutations. Despite its molecular similarities to the aggressive salivary duct carcinoma, low-grade apocrine IDC appears to behave in a very indolent manner, supporting is classification as a non-invasive neoplasm, and underscoring the need to distinguish these tumors from each other.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraductal carcinoma (IDC) is the current terminology used by the WHO Classification of Head and Neck Tumors for rare salivary gland neoplasm that has been previously referred to as “low-grade salivary duct carcinoma,” “salivary duct carcinoma in situ,” and “low-grade cribriform cystadenocarcinoma [1].” IDC occurs predominantly in the parotid gland, usually presenting as a slow-growing mass in patients of a wide age range (25–93 years, mean 63) [1, 2]. IDC is conceptually thought to be analogous to ductal carcinoma in-situ of the breast, with purely intraductal tumors believed to be very indolent with no capacity for metastasis.
Although IDC is included in the WHO Classification as a monomorphic entity, recent studies have suggested that it consists of at least three subtypes. The most common form of IDC is comprised of cells that resemble intercalated ductal cells and are strongly positive for S100. This form of IDC is almost always low-grade, only rarely demonstrates invasion, and frequently harbors RET rearrangements. On the other hand, there is a less common apocrine form of IDC that is negative for S100, is usually high-grade, very frequently shows invasion, and has complex genetic alterations resembling those of high-grade salivary duct carcinoma. Finally, the least common type has mixed intercalated duct and apocrine features; in low numbers, this variant appears to be prognostically and genetically similar to the intercalated duct-like subtype.
We sought to shed additional light on IDC by presenting a clinical, pathologic, and genetic analysis of a group of low-grade, purely apocrine, non-invasive IDCs.
Methods
Case Selection
Cases of low-grade, apocrine IDC were selected from the authors’ consultation files. All cases were reviewed by the corresponding author (J.A.B.) and were confirmed to meet diagnostic criteria for IDC as detailed in the 2017 World Health Organization Classification of Head and Neck Tumours [1].
Next-Generation Sequencing (NGS)
Targeted NGS was performed from formalin-fixed, paraffin-embedded tissues blocks as previously described [3]. Briefly, DNA and RNA were isolated using Qiagen Allprep kits (Qiagen, Germantown, MD) and a custom panel of DNA probes was used to produce an enriched library containing all exons from more than 1425 cancer-related genes, which were sequenced on Illumina NextSeq 550 instrument. DNA and RNA sequence analyses were performed using custom germline, somatic and mRNA bioinformatics pipelines run on the UTSW Bio-High Performance Computer cluster and optimized for detection of single nucleotide variants, indels and known gene fusions. Median target exon coverage for the assay is 900X with 94% of exons at > 100X. The minor allele frequency limit of detection is 5% for single nucleotide variants and 10% for indels and known gene fusions. Variants are not reported in exons covered < 100X. Somatic variants were identified on the basis of their variant allele frequencies (VAF), as well as their presence in databases of germline variants including dbSNP and gnomAD. All variants were reviewed in the Integrated Genomics Viewer (IGV) software prior to reporting.
Immunohistochemistry
Immunohistochemistry was performed on all cases. Using standard techniques, staining was performed for S100 (Ventana Medical Systems, Inc. Tucson, AZ), p40 (Biocare), smooth muscle actin (SMA) (Ventana), androgen receptor (Ventana), and gross cystic disease fluid protein (GCDFP)-15 (Ventana). All immunohistochemical signals were visualized using the Ultra view polymer detection kit (Ventana) on a Ventana BenchmarkXT autostainer.
Results
Three cases of low-grade apocrine IDC were retrieved, and they are summarized in Table 1. They all occurred in the parotid glands of men, aged 51, 63, and 73 years (mean, 62 years). All three presented as masses, two of which were specifically noted to be slowly-growing. The tumors were grossly cystic (Fig. 1), and measured 1.6, 1.8, and 2.1 cm (mean, 1.8 cm) in greatest dimension. All three patients were recognized and diagnosed prospectively as low-grade intraductal carcinomas and were treated with surgery alone. All patients are currently alive with no evidence of disease, with 12, 63, and 190 mon of follow-up.
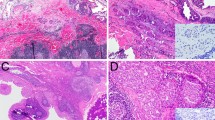
Histologically, all three cases had similar appearances. The tumors were dominated by large, dilated, cystic spaces, though each case also had scattered smaller microcysts or cribriform nests adjacent to the dominant macrocysts (Fig. 2a). All of the tumor cysts and nests were rounded with smooth edges (Fig. 2b). In each case, the tumor cysts and nests were lined by cells with abundant, granular, eosinophilic cytoplasm and large, round nuclei with macronucleoli (Fig. 2c). Cytoplasmic “snouting” was evident in each tumor, and one case had prominent large, brightly eosinophilic cytoplasmic granules (Fig. 2d). The luminal cells were variably arranged as a monolayer, as micropapillae or papillae, or in a “Roman bridge”-like architecture with ductal spaces. On close inspection, every tumor cyst or nest was surrounded by an inconspicuous layer of smaller, flattened cells with minimal amphophilic to clear cytoplasm. Although the luminal cells had larger nuclei, each cell type had minimal pleomorphism, and the mitotic rates were low (1–3 per 10 high power fields, mean 1.7). Intraluminal secretions were seen in each case, but necrosis was absent. All cases had adjacent degenerative changes in the form of fibrosis and/or hemorrhage. None of the tumors had any areas of irregular growth, single cells, or desmoplasia to suggest an invasive component. None of the tumors had evidence of a precursor lesion (e.g., pleomorphic adenoma or sclerosing polycystic adenoma). There was no component of intercalated duct-type tumor cells in any case.
At low power, this low-grade apocrine intraductal carcinoma consists of variably sized cysts and nests with intraluminal proliferations (a). The large cysts and small nests are rounded a have smooth edges, suggestive of a non-invasive process (b). The cases exhibited apocrine features in the form of abundant granular eosinophilic cytoplasm with apical snouts and decapitation secretions (c). In keeping with their apocrine differentiation, the tumor cells had large round nuclei with prominent nucleoli. However, pleomorphism and mitotic activity was minimal. This case also had unusual hypereosinophilic cytoplasmic granules similar to those seen in sclerosing polycystic adenoma (d)
By immunohistochemistry, the luminal tumor cells were uniformly, strongly positive for androgen receptor and GCDFP-15 and negative for p40, S100, and SMA. Each tumor was surrounded by a continuous, intact layer of small myoepithelial cells that were diffusely positive for p40 and SMA, and focally positive for S100 (Fig. 3).
Targeted NGS results are summarized in Table 2. Sequencing was successful in two of three cases. The remaining case could not be sequenced due to inadequate DNA and RNA quality, likely due to the age of the tumor block (15 years). Both successfully sequenced low-grade apocrine IDCs harbored HRAS p.Gly13Arg mutations. In addition, one case also harbored a PIK3CA mutation, and the other case had mutations in the tumor suppressors ATM and SPEN. There were additional mutations and copy number changes of unknown significance, listed in Table 2. Fusions were not found by RNA sequencing in either case. Copy number analysis was performed as part of the targeted NGS, which showed both cases lost a single copy of TP53, and one featured a loss in ATM.
Discussion
Salivary gland tumor classification has long been recognized as one of the most challenging areas in head and neck pathology. Over the past decade, emerging molecular findings have helped shed light on the nature of these tumors, refined their classification, and aided in their diagnoses [4]. Despite the great strides made in refining salivary gland tumor classification, however, there are still rare salivary gland lesions that remain to be fully characterized, especially at the molecular level.
Intraductal carcinoma (IDC) is the current WHO designation for a peculiar, rare salivary gland neoplasm that is thought to be analogous to ductal carcinoma in-situ of the breast. IDC has an interesting history. It was first described as intraductal carcinoma by Chen, et al. in the oral cavity [5], with a handful of subsequent case reports in the major and minor salivary glands [6,7,8]. Delgado et al. published the first series of 10 cases in 1996, proposing the term “low-grade salivary duct carcinoma [9].” Brandwein-Gensler et al. followed in 2004 with a series of 16 cases using the same terminology [2]. The 2005 WHO Classification of Head and Neck Tumors included this entity for the first time, but controversially changed the terminology to “low-grade cribriform cystadenocarcinoma [10].” In 2006 Weinreb et al. described the first three low-grade cases that were explicitly noted to show apocrine differentiation, including one that progressed to a high-grade invasive carcinoma [11], and in 2008 Simpson et al. published three cases of purely intraductal high-grade salivary duct carcinoma which they referred to as “salivary duct carcinoma in-situ [12].” In the 2017 WHO Classification, terminology officially reverted to IDC, with the acknowledgement that cases could be either low- or high-grade [1]. Despite the multiple name changes, what remained evident was that this tumor was very indolent when its growth was entirely intraductal.
The lack of consensus on the terminology of this tumor largely reflected confusion regarding what relationship, if any, IDC has to high-grade invasive salivary duct carcinoma. Advancements in immunohistochemical and molecular techniques have recently provided much-needed clarity to this issue. Salivary duct carcinoma is now established to be a high-grade tumor that exhibits apocrine differentiation by definition; this is now routinely confirmed with immunohistochemical markers including androgen receptor and GCDFP-15 [13, 14]. Salivary duct carcinoma is negative for S100. At the molecular level, salivary duct carcinoma has been shown to harbor multiple complex genetic alterations, including frequent mutations in TP53, PI3K pathway genes, RAS genes, EGFR, ATM, MET, among others [15,16,17,18]. Salivary duct carcinoma is typically negative for fusions, except those carcinomas that arose from pleomorphic adenomas which do retain PLAG1 or HMGA2 rearrangements.
These same techniques described above have recently been applied to IDC in studies by Weinreb et al. and Skalova et al. [19,20,21]. Based on these studies, it is now evident that most cases of IDC, including those previously called “low-grade salivary duct carcinoma,” are S100-positive, androgen receptor/GCDFP-15 negative tumors that are not apocrine but rather recapitulate intercalated ductal cells. These intercalated duct-like IDCs only rarely demonstrate invasion, and they frequently harbor fusions involving RET: NCOA4-RET and TRIM27-RET [19,20,21]. This form of IDC does not appear to be related to salivary duct carcinoma. In contrast, there is a much less common form of IDC that, like salivary duct carcinoma, is high-grade and truly apocrine, with an androgen receptor/GCDFP-15 positive, S100 negative immunophenotype. Not surprisingly, the apocrine form of IDC is genetically similar to salivary duct carcinoma, with frequent PIC3CA and HRAS mutations and no RET fusions. Moreover, apocrine IDC is usually associated with widespread tumor invasion. These findings suggest that the apocrine variant of IDC may be the precursor lesion to a subset of invasive salivary duct carcinomas. There is a third variant of IDC that has mixed intercalated duct-like and apocrine features. In this form, each component has its expected S100/androgen receptor immunoprofile, but the apocrine component is histologically low-grade. Interestingly, in small numbers, this mixed variant seems to be genetically similar to the intercalated duct-type of IDC, with the TRIM27–RET fusion being particularly common [19,20,21,22]. Lastly, there is a single small study describing an “oncocytic variant” of IDC that is S100-positive and is likely a variant of intercalated duct-type IDC, but it has not been genetically characterized [23].
While it is not uncommon to see focal intraductal spread associated with invasive salivary duct carcinoma, purely apocrine IDCs without any invasion appear to be exceptionally rare. Almost all cases previously published as IDC, low-grade salivary duct carcinoma, or low-grade cribriform cystadenocarcinoma were reportedly S100-positive, and histology images from those publications largely demonstrate intercalated duct-like morphology [2, 9, 10]. Two of three cases reported in 2006 by Weinreb et al. as IDC with apocrine features now appear to be the mixed IDC variant, with reported S100 and androgen receptor positivity [11]. The three cases reported by Simpson et al. were non-invasive, but they were all high-grade [12]. All cases of apocrine IDC in the 2018 study by Weinreb et al. were high-grade and demonstrated widespread invasion [19]. We reported three new cases of purely apocrine, non-invasive, low-grade IDC. Despite their low-grade histologic features, targeted NGS revealed that they are surprisingly very similar to high-grade invasive salivary duct carcinoma at the genetic level, with oncogenic mutations in HRAS, PIK3CA, SPEN, and ATM, but no fusions identified. This would seem to place low-grade apocrine IDC with its high-grade counterpart in the current IDC classification scheme (see Table 3). The relationship (if any) between low-grade and high-grade apocrine IDC counterparts remain to be determined. These may represent a morphologic spectrum, and it is possible that a subset of low-grade apocrine IDC may progress to high-grade apocrine IDC via events that remain to be identified. While there are no standardized criteria for grading IDC, the uniformly indolent behavior of all forms and grades suggest that histologic grade is not clinically important for pure IDCs. Indeed, although the numbers are limited, our experience supports that the low-grade apocrine variant, like other forms of purely IDC, is effectively treated with complete tumor excision alone. Therefore, to avoid overtreatment, it is most important to recognize when any salivary gland carcinoma, regardless of type or grade, is purely intraductal.
Low-grade apocrine IDC may be mistaken for salivary duct carcinoma on the basis of its apocrine histologic appearance and strong positivity for androgen receptor and GCDFP-15. Low-grade apocrine IDC, however, lacks the mitotic activity, pleomorphism, and comedo-type necrosis that characterizes salivary duct carcinoma. Low-grade apocrine IDC, like other types of IDC, grows as large cysts with adjacent rounded nests. The contour of these cysts and nests is smooth, and there is no evidence of invasion of stroma, salivary parenchyma, nerves, or vessels. Importantly, low-grade apocrine IDC, also like all forms of IDC, is entirely surrounded by an intact layer of myoepithelial cells. These cells are usually visible by routine histology, but are highlighted by immunostains for p40, SMA, or S100. For IDC of any type or grade, it is mandatory to submit the lesions entirely to rule out any invasive component. It is not usually necessary to perform myoepithelial stains on every block, but it is advisable to do so in any irregular area where invasion is suspected.
A recent study by Bishop et al. on sclerosing polycystic adenoma (SPA, previously known as sclerosing polycystic adenosis), has some parallels to the current study [3]. Indeed, most cases of SPA have foci closely resembling apocrine intraductal carcinoma within them, which can range from low- to high-grade. These areas are androgen receptor and GCDFP-15 positive, and are surrounded by a layer of myoepithelial cells, virtually identical to apocrine IDC. Like apocrine IDC, SPA harbors salivary duct carcinoma-like genetic alterations in the form of PTEN, PIKCA, and PIK3R1 mutations. These mutations are present even in those SPAs that lack apocrine ductal proliferation. Also like IDC, SPA behaves in a very indolent manner, with only a single reported case of invasive carcinoma arising from it [24]. Interestingly, the cells of one of the low-grade apocrine IDCs reported here had prominent, brightly eosinophilic cytoplasmic granules, a finding that is characteristically seen in almost all cases of SPA. On the other hand, the study cases lacked other features of SPA, namely other neoplastic elements such as small ducts and acini. Nevertheless, the many similarities strongly suggest that apocrine IDC (both low- and high-grade) and SPA may be related entities.
In summary, this study confirms the existence of a rare form of purely intraductal, low-grade salivary adenocarcinoma that is entirely apocrine. Morphology and NGS results support that this lesion is distinct from intercalated duct-type and mixed-type IDC, but is genetically and molecularly similar to high-grade apocrine IDC and salivary duct carcinoma. Clinical follow-up, on the other hand, suggests that like other forms of IDC, low-grade apocrine IDC is very indolent in the absence of invasive growth.
References
Loening T, Leivo I, Simpson RHW, et al. Intraductal carcinoma. In: el-Naggar AK, Chan JKC, Grandis JR, et al., editors. WHO Classification of Head and Neck Tumours. Lyon, France: IARC Press; 2017. p. 170–1.
Brandwein-Gensler M, Hille J, Wang BY, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28:1040–4.
Bishop JA, Gagan J, Baumhoer D, et al. Sclerosing polycystic “adenosis” of salivary glands: a neoplasm characterized by PI3K pathway alterations more correctly named sclerosing polycystic adenoma. Head Neck Pathol. 2019.
Skalova A, Vanecek T, Simpson RHW, et al. Molecular advances in salivary gland pathology and their practical application. Diagn Histopathol. 2012;18:388–96.
Chen KT. Intraductal carcinoma of the minor salivary gland. J Laryngol Otol. 1983;97:189–91.
Anderson C, Muller R, Piorkowski R, et al. Intraductal carcinoma of major salivary gland. Cancer. 1992;69:609–14.
Tatemoto Y, Ohno A, Osaki T. Low malignant intraductal carcinoma on the hard palate: a variant of salivary duct carcinoma? Eur J Cancer B Oral Oncol. 1996;32B:275–7.
Watatani K, Shirasuna K, Aikawa T, et al. Intraductal carcinoma of the tongue: report of a case. Int J Oral Maxillofac Surg. 1991;20:175–6.
Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma: a distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996;78:958–67.
Brandwein-Gensler MS, Gnepp DR. Low-grade cribriform cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, et al., editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumors. Lyon, France: IARC Press; 2005. p. 233.
Weinreb I, Tabanda-Lichauco R, Van der Kwast T, et al. Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol. 2006;30:1014–21.
Simpson RH, Desai S, Di Palma S. Salivary duct carcinoma in situ of the parotid gland. Histopathology. 2008;53:416–25.
Williams L, Thompson LD, Seethala RR, et al. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015;39:705–13.
Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11:288–94.
Dalin MG, Desrichard A, Katabi N, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22:4623–33.
Ku BM, Jung HA, Sun JM, et al. High-throughput profiling identifies clinically actionable mutations in salivary duct carcinoma. J Transl Med. 2014;12:299.
Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(Suppl 1):E1838–47.
Jaehne M, Roeser K, Jaekel T, et al. Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103:2526–33.
Weinreb I, Bishop JA, Chiosea SI, et al. Recurrent RET gene rearrangements in intraductal carcinomas of salivary gland. Am J Surg Pathol. 2018;42:442–52.
Skalova A, Vanecek T, Uro-Coste E, et al. Molecular profiling of salivary gland intraductal carcinoma revealed a subset of tumors harboring NCOA4-RET and novel TRIM27-RET fusions: a report of 17 cases. Am J Surg Pathol. 2018;42:1445–55.
Skalova A, Ptakova N, Santana T, et al. NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors: is "intraductal" correct? Am J Surg Pathol. 2019;43:1303–13.
Lu H, Graham RP, Seethala R, et al. Intraductal carcinoma of salivary glands harboring TRIM27-RET fusion with mixed low grade and apocrine types. Head Neck Pathol. 2019.
Nakaguro M, Urano M, Suzuki H, et al. Low-grade intraductal carcinoma of the salivary gland with prominent oncocytic change: a newly described variant. Histopathology. 2018;73:314–20.
Canas Marques R, Felix A. Invasive carcinoma arising from sclerosing polycystic adenosis of the salivary gland. Virchows Arch. 2014;464:621–5.
Funding
This study was funded by the Jane B. and Edwin P. Jenevein M.D Endowment for Pathology at UT Southwestern Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that he/she has no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB 112017-073), which did not require informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bishop, J.A., Gagan, J., Krane, J.F. et al. Low-grade Apocrine Intraductal Carcinoma: Expanding the Morphologic and Molecular Spectrum of an Enigmatic Salivary Gland Tumor. Head and Neck Pathol 14, 869–875 (2020). https://doi.org/10.1007/s12105-020-01128-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-020-01128-0