Abstract
In 1983, a monoclonal antibody, Ki67, was generated, that labeled the nuclei of proliferating non-neoplastic and neoplastic cells. The name Ki67 derived from the city of Kiel (Ki) where the antibody was produced in the university department of pathology and refers to the number of the original clone (67). Systematic assessment of the proliferative activity of tumors using Ki67 started in the 1990s, when Ki67, which only worked on frozen tissue, was complemented by the antibody MIB-1 that also worked in formalin-fixed tissues. Pancreatic neuroendocrine neoplasms (PanNENs) were the first endocrine tumors whose proliferative activity was assessed with Ki67. This approach was so successful that Ki67 was included as prognostic marker in the 2000 and 2004 WHO classifications of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs). In 2010, the WHO classification of GEP-NENs introduced a three-tiered grading, originally proposed by ENETS in 2006 that was mainly based on the Ki67 index. As it has subsequently been shown that the Ki67 index is the most reliable factor in the prognostic evaluation of GEP-NENs, especially of PanNENs, the 2017 WHO classification of PanNENs requires its use and strongly recommends exact assessment of the proportion Ki67-labeled cells as basis for the calculation of the Ki67 index. Problems in assessing the Ki67 index include intertumoral and intratumoral staining heterogeneity and counting methods. Despite such problems, the Ki67 index has emerged as indispensable for the prognostic and therapeutic stratification of the majority of GEP-NENs and can barely be replaced by counting mitoses. In future, however, it can be anticipated that the Ki67 cut-offs experience refinement in relation to the type of tumor, its location, and its response to therapy. It is also possible that the prognostic risk of an individual tumor is calculated for each Ki67 unit and not for an “a priori” fixed Ki67 class.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the essential issues in oncology is to predict tumor progression and patient survival. Traditionally, tumor type, stage, and mitotic activity are the main prognostic criteria. Tumor growth is correlated with mitotic activity; however, because of technical problems (e.g., section thickness, staining, sample size), it is often difficult to determine the mitotic count of a tumor exactly. This is much easier using the proliferation marker Ki67, which has therefore gained increasing significance in recent years, particularly in slowly growing tumors such as gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs). The predictive value of Ki67 depends very much on the precise assessment of the marker’s immunohistochemical expression in tumor tissues. This review briefly reflects on the history of Ki67 and its inclusion into the GEP-NEN classifications and focuses then on the various issues in conjunction with the accurate and reproducible determination of Ki67 as predictive indicator of tumor growth and prognosis.
Short history of Ki67
In 1983, Gerdes, Schwab, Lemke, and Stein reported upon a new monoclonal antibody called Ki67, which was generated by immunizing mice with nuclei of the Hodgkin lymphoma cell line L428. This antibody was found to label nuclei of proliferating cells including tumor cells and recognizes a nuclear protein (encoded by the MKI 67 gene) which is involved in nuclear remodeling during proliferation and is thought to control heterochromatin organization and enable mitotic chromosomes to move independently from each other [11, 52]. However, as the exact function of the protein is still not fully understood, the initial name Ki67 is kept. This name derived from the city of Kiel (Ki) where Gerdes and his group worked in the university departments of pathology and biochemistry, and the number 67 referred to the original clone in the 96-well plate. In their report, the authors concluded that this antibody may be a potent tool for easy and quick assessment of the proportion of proliferating cells in a tumor [12]. The original antibody worked only on frozen tissues and was therefore subsequently replaced by antibodies, such as the monoclonal MIB-1, which were found to react with Ki67 in formalin-fixed and paraffin-embedded tissues. Using the MIB-1 antibody, it was possible to perform large-scale studies in archive material to assess the proliferative activity in different neoplasms [21, 49].
The systematic assessment of the proliferative activity of pancreatic neuroendocrine neoplasms (PanNENs) started in the 1990s [23, 38, 62], and the first two studies using the MIB-1 antibody on formalin-fixed and paraffin-embedded PanNENs were published in 1996 by two independent Italian groups [23, 38]. As it became apparent that the Ki67 index, along with the mitotic count, may become a determinant of the prognosis of GEP-NENs, the Ki67 index was included as prognostic parameter into the WHO classifications of the gastrointestinal (in 2000) and pancreatic NENs (in 2004). Both classifications originated from a classification concept proposed by Capella, Solcia, Heitz, Höfler, and Klöppel in 1995 [6, 19]. The subsequent WHO classification of GEP-NENs [43], that was published in 2010, included a three-tiered grading system, mainly based on the Ki67 categories, defined by the European Neuroendocrine Tumor Society (ENETS) in 2006 and 2007 [45, 46] (Fig. 1). Since then, the Ki67 index has become essential for grading GEP-NENs and has been firmly established in the reporting protocols and guidelines used by ENETS [33], the North American Neuroendocrine Tumor Society (NANETS) [60], and the American Joint Committee on Cancer (AJCC) [28]).
Calculation of the Ki67 index
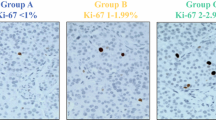
The monoclonal antibody Ki67 identifies a 359-kD non-histone nuclear protein that is involved in the control and timing of cell proliferation and expressed in all phases of the cell cycle, with a maximum in the G2 and M phases. This implicates that the mitotic counts are distinctly lower than the Ki67 labeling, depending on the number of cells which are in the G1 phase. Exact figures do not yet exist, but the ratio may be about 1:10 in a given area. During its expression, the Ki67 antigen is redistributed from the interior of the nucleus or the nucleolus to the perichromosomal layer and heterochromatin in the nucleus [39] (Fig. 2). For the calculation of the Ki67 index, all the immunohistochemically labeled nuclei, regardless of the staining intensity or whether the nuclei show a speckled expression pattern or are diffusely stained, need to be counted for the scoring process [1]. The number of stained nuclei is then expressed as a percentage (index) of immunoreactive cells. It is recommended to count between 500 and 1000 tumor cells in the highest labeled area (hot spot). Among the methods used to determine the Ki67 index, a manual count of a camera-captured, printed image (CCPI) has appeared to be the most reliable procedure [41, 57] showing good reproducibility, although the whole evaluation process takes on the average between 10 and 15 min (Fig. 3). This recommendation is based on the results of a study comparing CCPI with three other counting methods. “Eye-ball” estimation was the fastest method (average time < 1 min) but with the poorest reliability and reproducibility. Manual eye count proved to be a rather quick way to determine the Ki67 index, averaging between 5 and 8 min but had also a poor reproducibility. Automated count was the most expensive and least practical method with major impact on turnaround time (limited by the accessibility of machine and personnel) but, more importantly, had significant inaccuracies in over-counting nonendocrine cells such as lymphocytes, endothelial cells, and stromal cells. Also molding of tumor nuclei, overly thick sections and background pigment may contribute to miscalculations [41]. However, these statements are probably not the “last word,” since in a paper by Tang, automated counting was shown to have comparable accuracy as counting on camera-captured printed images [57].
Problems in assessing Ki67 labeling
During recent years, immunostaining procedures for Ki67 have generally been improved and standardized by the wide use of automated staining machines. However, there may be still differences between laboratories, since it was recently shown that interlaboratory variability in tissue processing and fixation, including the use of different reagents and pretreatments, may be the reason for interlaboratory differences [5], especially in low-proliferating tumors. It can be expected that commercially available staining optimizers and external standardization of the staining protocols by quality measures may help to further improve reliability and reproducibility.
Other problems in assessing Ki67 staining and calculating a reproducible Ki67 index are loss of antigenicity over time, intratumoral heterogeneity, and the interpretation of “pale brown” tumor nuclei as positive. Ki67 may lose its antigenicity with tissue age in paraffin-embedded formalin-fixed blocks, with a 10% signal loss in staining intensity over a period of 4.5 years [9]. Intratumoral heterogeneity may be observed in an individual tumor or among different metastatic sites, or may become obvious in higher grade metastasis developing in the course of disease progression (Fig. 4). This problem is endogenous to tumors and can only be solved by the availability of sufficient tumor tissue for evaluation. The problem of interpreting “pale staining” nuclei as positive is difficult to solve, as it will always remain to some extent a subjective issue. However, if in a slide with a Ki67 immunostaining of high quality, all nuclei with sharp borders are counted that display an intense or less intense, but homogeneous or speckled staining pattern, the counts have an excellent reproducibility in our experience.
Ki67 immunostaining reveals intratumoral proliferative heterogeneity in a lymph node metastasis from a G1 ileal neuroendocrine tumor, with a G1 area in the upper half of the picture and G3 area at the bottom (Courtesy of Dr. Silvia Uccella, Department of Medicine and Surgery, University of Insubria, Varese, Italy)
Intratumoral heterogeneity is another issue of the reliability of a Ki67 index in liver metastases from PanNETs when diagnosed by a radiologically guided core needle biopsy. However, it has been shown that Ki67 staining of core biopsies usually provides an adequately reliable method of proliferation assessment for prognosis of metastatic NETs to the liver [64].
Ki67 index and its prognostic significance in pancreatic NENs
In recent years, it has been shown that Ki67 index is the most significant factor in the prognostic evaluation of PanNENs. This has been revealed by a number of studies using the 2010 WHO classification [34, 44, 50] which, on the basis of the proliferative activity, grades well-differentiated PanNENs, called pancreatic neuroendocrine tumors (PanNETs), into either G1 PanNETs (Ki67 index ≤ 2%) or G2 PanNETs (Ki67 index 2–20%), and poorly differentiated PanNENs, called pancreatic neuroendocrine carcinomas (PanNECs), into G3 category with a Ki67 index > 20% [43]. In one of these studies, it was suggested to use a cut-off of 5% for the G1 PanNETs, since a higher risk of progression was observed when 5% was used instead of 2% [50]. However, there is so far not sufficient evidence of differences in clinical management based on this higher cut-point to justify changing it.
Recently, the distinction between well and poorly differentiated PanNENs applying the 2010 WHO classification has become difficult for those few well-differentiated PanNENs whose Ki67-index exceeds 20%. Although these tumors retain their well-differentiated neuroendocrine growth pattern, their Ki67 index greater than 20% classifies them into the G3 category of poorly differentiated neuroendocrine neoplasms (NEC) [4]. Though the prognosis of these tumors is worse than that of G2 PanNETs, it is still better than that of PanNECs. Moreover, they retain the features (i.e., hormone expression and hormonal syndromes) characterizing PanNETs and appear to lack genetic abnormalities (i.e., changes in expression and mutation of TP53 and RB1) associated with PanNECs [56, 63]. In the new 2017 WHO classification, these PanNENs therefore constitute a new category, called PanNET G3 (Table 1). Their clear distinction from PanNECs is currently the topic of a number of articles [4, 20, 29, 56].
Ki67 index and its prognostic significance in gastric NENs
Gastric NENs are a heterogeneous group of tumors showing different clinicopathological features and behavior [25]. They include NETs arising in both the oxyntic and antral mucosa as well as NECs and mixed adenoneuroendocrine carcinomas (MANECs) which have been recently proposed to be renamed as mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs) [24]. With the exception of very rare antral NETs, that produce gastrin, somatostatin, or serotonin, all other gastric NETs arise in the oxyntic (corpus) mucosa and are composed of histamine-producing enterochromaffin-like (ECL) cells [25]. ECL cell NETs are a heterogeneous group of neoplasms in terms of pathogenesis, biology, and prognosis. In 1993, the group of Rindi, Capella, and Solcia, considering the morphology of the peritumoral oxyntic mucosa, the presence of antral G cell hyperplasia, the presence of hypergastrinemia, the setting of MEN1, and the presence of hypo/achlorhydria, divided ECL cell NETs into the following three categories: type 1, type 2, and type 3 [47]. These different types of ECL-NETs are known to show per se different clinical and, more importantly, prognostic features [22, 42], with type 1 NETs showing the best prognosis and type 3 the worst. As for other GEP sites, gastric NENs are graded using Ki67 labeling and mitotic count. However, it has been demonstrated that Ki67 is more sensitive in identifying intermediate grade gastric NENs than mitotic count [22], since counting of mitoses has its limits in small biopsy specimens, that contain insufficient tissue to evaluate 10HPF. For this reason, tumor grading is mainly based on Ki67 index and this correlates well with prognosis. An even better Ki67 cut-off to distinguish G1 from G2 neoplasms seems to be 3% [22]. Further improvement of the prognostic stratification of patients is achieved, if grading is combined with Rindi’s clinicopathological classification [22, 36, 47]. Both approaches should therefore be used for proper diagnostic assessment of a gastric ECL cell NET, especially in a small biopsy.
The majority of G1-NETs of gastric type 1 have a good prognosis and require only endoscopic treatment, when they are small, confined to the mucosa, and arising in the setting of type A chronic atrophic gastritis. The clinical relevance of the few type 1 ECL cell NETs showing a Ki67 index > 3% is still not clear. Grozinsky-Glasberg demonstrated that metastatic type 1 ECL cell gastric NETs showed an average Ki67 labeling index of 6.8% which was statistically higher than the average Ki67 index (1.9%) of the whole series used as control [14]. However, the Ki67 index alone does not seem to be the most important parameter determining the metastatic potential, as this tumor property is also closely related to tumor size and depth of tumor infiltration of the gastric wall [14, 21].
Gastric type 2 NETs that arise in MEN1-ZES patients, and especially gastric type 3 NETs, that occur sporadically in normal gastric mucosa, show a more aggressive biology and often need, depending on tumor stage, further treatment including gastric resection [51].
The stomach, like the pancreas, also harbors NETs whose Ki67 index exceeds 20%. These peculiar tumors, which in adaptation of the recently proposed nomenclature for PanNETs may be called gastric G3-NETs, were only found among gastric type 3 (sporadic) ECL cell NETs and showed a prognosis that was worse than in “conventional” G2-NETs, but still better than in gastric poorly differentiated NENs that have been called NECs [22] or gastric type 4 NENs [18]. NECs and MiNENs (MANECs) arise more frequently in the cardial or antral region [25] and show similar morphological, immunohistochemical, and prognostic features to those of their counterparts arising in other organs of the digestive system.
Ki67 index and its prognostic significance in duodenal NENs
Duodenal NENs are a heterogeneous group of tumors including well-differentiated and poorly differentiated neoplasms that differ in terms of clinical presentation, localization, and immunophenotype [18]. In a large series of duodenal NENs, recently published by Vanoli and collaborators, four different groups were distinguished among the well-differentiated neoplasms including gastrinomas (associated with the Zollinger-Ellison syndrome [48]), nonfunctioning NETs (lacking any association with endocrine symptoms but expressing gastrin or somatostatin [48]), ampullary nonfunctioning somatostatin-producing NETs, and gangliocytic paraganliomas [18, 59]. Using a cut-off of 2.5% for distinguishing G1 and G2 tumors, the Ki67 grading failed to separate the four tumor groups but was found to be a predictor of lymph node metastasis. Moreover, at univariate analysis, the Ki67 index was well associated with disease-specific survival, but at multivariate analysis failed to discriminate between the disease-free survival of G1 and G2 tumors. From this study, it appeared that a multiparametric approach including NET size, site, and proliferative activity is useful to identify the metastatic cases but has limited significance for disease-specific survival.
Ki67 index and its prognostic significance in ileal and appendiceal NENs
The vast majority of ileal NENs are serotonin-producing NETs, while NECs are extremely rare. Ileal NETs are peculiar tumors because of their ability to metastasize early to regional lymph nodes and/or the liver despite a low proliferation index (most tumors are G1). It is therefore conceivable that tumor grading fails to predict the metastatic potential of these tumors [7, 8, 13, 26, 32, 35]. However, tumor grading, mainly based on Ki67 proliferation, has been demonstrated to be correlated with prognosis either by using the cut-offs proposed by ENETS/WHO or the cut-offs of 1% [2, 10]. Panzuto has demonstrated that Ki67 grading was statistically associated with tumor progression and patients’ survival, but at multivariate analysis, the best predictive Ki67 cut-off in discriminating G1 versus G2 tumors was 5% [35]. Interestingly, these authors also calculated the increasing risk for tumor progression and tumor death for each increasing Ki67 unit which was 14 and 18%, respectively. This underlines the biological concept that Ki67 should be considered as a continuous variable and the calculation of prognostic risk for each increasing Ki67 unit may be superior to the separation of tumors using fixed Ki67 categories. Although the prognostic classification of ileal NETs based on individual parameters such proliferative activity alone has been demonstrated to have a clinical power [3, 35, 54], a new multiparametric approach including a NET nomogram seems more promising for improved prognostic stratification of patients [8, 30].
Appendiceal NETs are the most peculiar tumors among the intestinal NENs. Despite frequent infiltrative growth into the sub serosal tissues, lymph node metastases are rare and liver metastases are virtually absent. Patients with these tumors, among them many children, have an excellent outcome after appendectomy. The Ki67 labeling index which is generally low has no predictive power regarding stage and outcome [61]. For this reason, therapeutic strategies exceeding appendectomy and including hemicolectomy should not be based on tumor grading but rather on tumor stage [37].
Ki67 index and its prognostic significance in rectal NENs
Rectal NENs include both well and poorly differentiated neoplasms. Their number increased significantly in recent decades, probably due to the increased use of diagnostic colonoscopy [40, 51]. Rectal NECs are highly aggressive cancers with dismal prognosis and show the same clinicopathologic features of NECs at other sites. Rectal NETs show a broad range of clinical features ranging from indolent and asymptomatic to aggressive and metastatic cases. Most rectal NETs are composed of L cells producing glicentin and/or PP or PYY [18]. Several papers demonstrated that tumor size and level of wall invasion are prognostic factors, so that small (< 10 mm) mucosal/submucosal rectal NETs can be treated by endoscopic polypectomy alone [40]. Grading rectal NETs on the basis of their Ki67 index reveals that about 90% of the tumors falls into the G1 category [15,16,17, 27, 31, 53, 58] and shows a better survival than G2 NETs [16]. Recently, a Ki67 cut-off of 3% was proposed, as it seems to be better in predicting metastatic dissemination than a cut-off of 2% [55]. Tumor grading has been proved to be a prognostic marker in univariate analysis [16, 53], but it was not an independent factor at the multivariate analysis [53]. It has therefore been suggested that the best approach for stratifying patients into different prognostic categories is a multiparametric evaluation considering grade together with tumor size, lympho-vascular invasion, level of wall infiltration, and immunophenotype (L cell versus EC cell NET) [16, 53]. G1 rectal NETs with a size less than 10 mm, absence of lympho-vascular and muscular layer infiltration, and L cell phenotype require only endoscopic resection, while larger G2 NETS, especially when of EC cell type and deeply infiltrating the rectal wall in the presence of lympho-vascular invasion, need surgical resection.
Conclusion and perspectives
During the last 20 years, Ki67 labeling and grading have become essential for prognostic assessment of many GEP-NENs (Table 2). The cut-offs currently proposed by ENETS/WHO to separate the G1, G2, and G3 NEN-categories work well and are used together with tumor type, site, and stage, to stratify patients in different prognostic categories. In future, however, it can be anticipated that the Ki67 cut-offs will be refined in relation to tumor type, its location, and its response to therapy. For foregut and hindgut NENs, including gastric, duodenal, pancreatic, and rectal neoplasms, the best Ki67 cut-off in distinguishing G1 and G2 NETs seems to be 3%, while for ileal NETs it is 5%. In addition, it may be worthwhile to consider the use of multiparametric approaches which include the Ki67 index and combine it with other clinicopathologic parameters. Finally, the biological view of Ki67 expression as a continuous variable in the proliferation process should be taken into account and may lead to a new approach to predict the outcome of the patients by calculating the prognostic risk for each increasing Ki67 unit. This approach could then replace the separation of tumor categories on the basis of “a priori” fixed Ki67 cut-offs that are currently in use.
Change history
26 December 2017
The authors of the above article have correct the caption for Figure 3 – namely the second sentence – clarifying the information depicted within the picture correcting the Ki67 index percentage. Figure 3 and the corrected caption can be found below.
References
Adsay V (2012) Ki67 labeling index in neuroendocrine tumors of the gastrointestinal and pancreatobiliary tract: to count or not to count is not the question, but rather how to count. Am J Surg Pathol 36:1743–1746. https://doi.org/10.1097/PAS.0b013e318272ff77
Ahmed A, Turner G, King B, Jones L, Culliford D, McCance D, Ardill J, Johnston BT, Poston G, Rees M, Buxton-Thomas M, Caplin M, Ramage JK (2009) Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer 16:885–894. https://doi.org/10.1677/erc-09-0042
Araujo PB, Cheng S, Mete O, Serra S, Morin E, Asa SL, Ezzat S (2013) Evaluation of the WHO 2010 grading and AJCC/UICC staging systems in prognostic behavior of intestinal neuroendocrine tumors. PloS one 8:e61538. https://doi.org/10.1371/journal.pone.0061538
Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, Sigel C, Klimstra DS (2015) The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 39:683–690. https://doi.org/10.1097/PAS.0000000000000408
Blank A, Wehweck L, Marinoni I, Boos LA, Bergmann F, Schmitt AM, Perren A (2015) Interlaboratory variability of MIB1 staining in well-differentiated pancreatic neuroendocrine tumors. Virchows Arch 467:543–550. https://doi.org/10.1007/s00428-015-1843-3
Capella C, Heitz PU, Höfler H, Solcia E, Klöppel G (1995) Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch 425:547–560
Clift AK, Faiz O, Al-Nahhas A, Bockisch A, Liedke MO, Schloericke E, Wasan H, Martin J, Ziprin P, Moorthy K, Frilling A (2016) Role of staging in patients with small intestinal neuroendocrine tumours. J Gastrointest Surg 20:180–188; discussion 188. https://doi.org/10.1007/s11605-015-2953-6
Clift AK, Faiz O, Goldin R, Martin J, Wasan H, Liedke MO, Schloericke E, Malczewska A, Rindi G, Kidd M, Modlin IM, Frilling A (2017) Predicting the survival of patients with small bowel neuroendocrine tumours: comparison of 3 systems. Endocr Connect 6:71–81. https://doi.org/10.1530/ec-16-0114
Combs SE, Han G, Mani N, Beruti S, Nerenberg M, Rimm DL (2016) Loss of antigenicity with tissue age in breast cancer. Lab Invest 96:264–269. https://doi.org/10.1038/labinvest.2015.138
Cunningham JL, Grimelius L, Sundin A, Agarwal S, Janson ET (2007) Malignant ileocaecal serotonin-producing carcinoid tumours: the presence of a solid growth pattern and/or Ki67 index above 1% identifies patients with a poorer prognosis. Acta Oncol 46:747–756. https://doi.org/10.1080/02841860701218659
Cuylen S, Blaukopf C, Politi AZ, Muller-Reichert T, Neumann B, Poser I, Ellenberg J, Hyman AA, Gerlich DW (2016) Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature 535:308–312. https://doi.org/10.1038/nature18610
Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13–20
Gonzalez RS, Liu EH, Alvarez JR, Ayers GD, Washington MK, Shi C (2014) Should mesenteric tumor deposits be included in staging of well-differentiated small intestine neuroendocrine tumors? Mod Pathol 27:1288–1295. https://doi.org/10.1038/modpathol.2013.232
Grozinsky-Glasberg S, Thomas D, Strosberg JR, Pape UF, Felder S, Tsolakis AV, Alexandraki KI, Fraenkel M, Saiegh L, Reissman P, Kaltsas G, Gross DJ (2013) Metastatic type 1 gastric carcinoid: a real threat or just a myth? World J Gastroenterol 19:8687–8695. https://doi.org/10.3748/wjg.v19.i46.8687
Hong SM, Kim YS, Moon JS, Kim JN, Oh MK, Kwon SO, Jeong SY, Hong SW, Kang YK (2013) Prognostic significance of Ki-67 expression in rectal carcinoid tumors. Korean J Gastroenterol 61:82–87
Jernman J, Valimaki MJ, Louhimo J, Haglund C, Arola J (2012) The novel WHO 2010 classification for gastrointestinal neuroendocrine tumours correlates well with the metastatic potential of rectal neuroendocrine tumours. Neuroendocrinology 95:317–324. https://doi.org/10.1159/000333035
Kim GU, Kim KJ, Hong SM, Yu ES, Yang DH, Jung KW, Ye BD, Byeon JS, Myung SJ, Yang SK, Kim JH (2013) Clinical outcomes of rectal neuroendocrine tumors≤ 10 mm following endoscopic resection. Endoscopy 45:1018–1023. https://doi.org/10.1055/s-0033-1344860
Klöppel G (2011) Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 18(Suppl 1):S1–16. https://doi.org/10.1530/erc-11-0013
Klöppel G, Perren A, Heitz PU (2004) The gastroenteropancreatic neuroendocrine cell system and its tumors. The WHO classification. Ann NY Acad Sci 1014:13–27
Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, Agaimy A, Sipos B, Zamboni G, Weichert W, Esposito I, Pfarr N, Klöppel G (2017) Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod Pathol. https://doi.org/10.1038/modpathol.2016.217
Kreipe H, Heidebrecht HJ, Hansen S, Rohlk W, Kubbies M, Wacker HH, Tiemann M, Radzun HJ, Parwaresch R (1993) A new proliferation-associated nuclear antigen detectable in paraffin-embedded tissues by the monoclonal antibody Ki-S1. Am J Pathol 142:3–9
La Rosa S, Inzani F, Vanoli A, Klersy C, Dainese L, Rindi G, Capella C, Bordi C, Solcia E (2011) Histologic characterization and improved prognostic evaluation of 209 gastric neuroendocrine neoplasms. Hum Pathol 42:1373–1384. https://doi.org/10.1016/j.humpath.2011.01.018
La Rosa S, Sessa F, Capella C, Riva C, Leone BE, Klersy C, Rindi G, Solcia E (1996) Prognostic criteria in nonfunctioning pancreatic endocrine tumours. Virchows Arch 429:323–333
La Rosa S, Sessa F, Uccella S (2016) Mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr Pathol 27:284–311. https://doi.org/10.1007/s12022-016-9432-9
La Rosa S, Vanoli A (2014) Gastric neuroendocrine neoplasms and related precursor lesions. J Clin Pathol 67:938–948. https://doi.org/10.1136/jclinpath-2014-202515
Lardiere-Deguelte S, de Mestier L, Appere F, Vullierme MP, Zappa M, Hoeffel C, Noaves M, Brixi H, Hentic O, Ruszniewski P, Cadiot G, Panis Y, Kianmanesh R (2016) Toward a preoperative classification of lymph node metastases in patients with small intestinal neuroendocrine tumors in the era of intestinal-sparing surgery. Neuroendocrinology 103:552–559. https://doi.org/10.1159/000441423
Li P, Wu F, Zhao H, Dou L, Wang Y, Guo C, Wang G, Zhao D (2015) Analysis of the factors affecting lymph node metastasis and the prognosis of rectal neuroendocrine tumors. Int J Clin Exp Pathol 8:13331–13338
Lloyd RV, Osamura RY, Klöppel G, Rosai J (2017) WHO Classification of Tumours of Endocrine Organs. IARC Press, Lyon
Milione M, Maisonneuve P, Spada F, Pellegrinelli A, Spaggiari P, Albarello L, Pisa E, Barberis M, Vanoli A, Buzzoni R, Pusceddu S, Concas L, Sessa F, Solcia E, Capella C, Fazio N, La Rosa S (2017) The clinicopathologic heterogeneity of grade 3 gastroenteropancreatic neuroendocrine neoplasms: morphological differentiation and proliferation identify different prognostic categories. Neuroendocrinology 104:85–93. https://doi.org/10.1159/000445165
Modlin IM, Gustafsson BI, Pavel M, Svejda B, Lawrence B, Kidd M (2010) A nomogram to assess small-intestinal neuroendocrine tumor (‘carcinoid’) survival. Neuroendocrinology 92:143–157. https://doi.org/10.1159/000319784
Nakamura K, Osada M, Goto A, Iwasa T, Takahashi S, Takizawa N, Akahoshi K, Ochiai T, Nakamura N, Akiho H, Itaba S, Harada N, Iju M, Tanaka M, Kubo H, Somada S, Ihara E, Oda Y, Ito T, Takayanagi R (2016) Short- and long-term outcomes of endoscopic resection of rectal neuroendocrine tumours: analyses according to the WHO 2010 classification. Scand J Gastroenterol 51:448–455. https://doi.org/10.3109/00365521.2015.1107752
Norlen O, Stalberg P, Oberg K, Eriksson J, Hedberg J, Hessman O, Janson ET, Hellman P, Akerstrom G (2012) Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg 36:1419–1431. https://doi.org/10.1007/s00268-011-1296-z
O’Toole D, Kianmanesh R, Caplin M (2016) ENETS 2016 consensus guidelines for the management of patients with digestive neuroendocrine tumors: an update. Neuroendocrinology 103:117–118. https://doi.org/10.1159/000443169
Panzuto F, Boninsegna L, Fazio N, Campana D, Pia Brizzi M, Capurso G, Scarpa A, De Braud F, Dogliotti L, Tomassetti P, Delle Fave G, Falconi M (2011) Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol 29:2372–2377. https://doi.org/10.1200/jco.2010.33.0688
Panzuto F, Campana D, Fazio N, Brizzi MP, Boninsegna L, Nori F, Di Meglio G, Capurso G, Scarpa A, Dogliotti L, De Braud F, Tomassetti P, Delle Fave G, Falconi M (2012) Risk factors for disease progression in advanced jejunoileal neuroendocrine tumors. Neuroendocrinology 96:32–40. https://doi.org/10.1159/000334038
Pape UF, Jann H, Müller-Nordhorn J, Bockelbrink A, Berndt U, Willich SN, Koch M, Röcken C, Rindi G, Wiedenmann B (2008) Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 113:256–265
Pape UF, Niederle B, Costa F, Gross D, Kelestimur F, Kianmanesh R, Knigge U, Oberg K, Pavel M, Perren A, Toumpanakis C, O’Connor J, Krenning E, Reed N, O’Toole D (2016) ENETS consensus guidelines for neuroendocrine neoplasms of the appendix (excluding goblet cell carcinomas). Neuroendocrinology 103:144–152. https://doi.org/10.1159/000443165
Pelosi G, Bresaola E, Bogina G, Pasini F, Rodella S, Castelli P, Iacono C, Serio G, Zamboni G (1996) Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: a comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum Pathol 27:1124–1134
Pelosi G, Rindi G, Travis WD, Papotti M (2014) Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol 9:273–284. https://doi.org/10.1097/jto.0000000000000092
Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, Taal B, Pascher A (2016) ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology 103:139–143. https://doi.org/10.1159/000443166
Reid MD, Bagci P, Ohike N, Saka B, Erbarut Seven I, Dursun N, Balci S, Gucer H, Jang KT, Tajiri T, Basturk O, Kong SY, Goodman M, Akkas G, Adsay V (2015) Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod Pathol 28:686–694. https://doi.org/10.1038/modpathol.2014.156
Rindi G, Azzoni C, La Rosa S, Klersy C, Paolotti D, Rappel S, Stolte M, Capella C, Bordi C, Solcia E (1999) ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology 116:532–542
Rindi G, Arnold R, Bosman FT, Capella C, Klimstra DS, Kloppel G et al. (2010) Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds) WHO Classification of Tumours of the Digestive system, 4th edn. IARC, Lyon, pp 13–14
Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A, Doglioni C, Delle Fave G, Fischer L, Fusai G, de Herder WW, Jann H, Komminoth P, de Krijger RR, La Rosa S, Luong TV, Pape U, Perren A, Ruszniewski P, Scarpa A, Schmitt A, Solcia E, Wiedenmann B (2012) TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst 104:764–777. https://doi.org/10.1093/jnci/djs208
Rindi G, Klöppel G, Ahlman H, Caplin M, Couvelard A, de Herder WW, Eriksson B, Falchetti A, Falconi M, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B, participants aaoFCC (2006) TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 449:395–401
Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B (2007) TNM staging of midgut and hindgut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 451:757–762
Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E (1993) Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology 104:994–1006
Rosentraeger MJ, Garbrecht N, Anlauf M, Raffel A, Knoefel WT, Wiedenmann B, Klöppel G (2016) Syndromic versus non-syndromic sporadic gastrin-producing neuroendocrine tumors of the duodenum: comparison of pathological features and biological behavior. Virchows Arch 468:277–287. https://doi.org/10.1007/s00428-015-1890-9
Rudolph P, Kellner U, Chassevent A, Collin F, Bonichon F, Parwaresch R, Coindre JM (1997) Prognostic relevance of a novel proliferation marker, Ki-S11, for soft-tissue sarcoma. A multivariate study. Am J Pathol 150:1997–2007
Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, Panzuto F, Pederzoli P, delle Fave G, Falconi M (2010) Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 23:824–833. https://doi.org/10.1038/modpathol.2010.58
Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G (2011) Management of early gastrointestinal neuroendocrine neoplasms. World J Gastrointest Endosc 3:133–139. https://doi.org/10.4253/wjge.v3.i7.133
Sobecki M, Mrouj K, Camasses A, Parisis N, Nicolas E, Lleres D, Gerbe F, Prieto S, Krasinska L, David A, Eguren M, Birling MC, Urbach S, Hem S, Dejardin J, Malumbres M, Jay P, Dulic V, Lafontaine D, Feil R, Fisher D (2016) The cell proliferation antigen Ki-67 organises heterochromatin. eLife 5:e13722. https://doi.org/10.7554/eLife.13722
Sohn JH, Cho MY, Park Y, Kim H, Kim WH, Kim JM, Jung ES, Kim KM, Lee JH, Chan HK, Park DY, Joo M, Kim S, Moon WS, Kang MS, Jin SY, Kang YK, Yoon SO, Han H, Choi E (2015) Prognostic significance of defining L-cell type on the biologic behavior of rectal neuroendocrine tumors in relation with pathological parameters. Cancer Res Treat 47:813–822. https://doi.org/10.4143/crt.2014.238
Strosberg JR, Weber JM, Feldman M, Coppola D, Meredith K, Kvols LK (2013) Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J Clin Oncol 31:420–425. https://doi.org/10.1200/jco.2012.44.5924
Sugimoto S, Hotta K, Shimoda T, Imai K, Yamaguchi Y, Nakajima T, Oishi T, Mori K, Takizawa K, Kakushima N, Tanaka M, Kawata N, Matsubayashi H, Ono H (2016) The Ki-67 labeling index and lymphatic/venous permeation predict the metastatic potential of rectal neuroendocrine tumors. Surg Endosc 30:4239–4248. https://doi.org/10.1007/s00464-015-4735-3
Tang LH, Basturk O, Sue JJ, Klimstra DS (2016) A practical approach to the classification of WHO grade 3 (G3) well-differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC) of the pancreas. Am J Surg Pathol 40:1192–1202. https://doi.org/10.1097/pas.0000000000000662
Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS (2012) Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. Am J Surg Pathol 36:1761–1770. https://doi.org/10.1097/PAS.0b013e318263207c
Tsukamoto S, Fujita S, Yamaguchi T, Yamamoto S, Akasu T, Moriya Y, Taniguchi H, Shimoda T (2008) Clinicopathological characteristics and prognosis of rectal well-differentiated neuroendocrine tumors. Int J Color Dis 23:1109–1113. https://doi.org/10.1007/s00384-008-0505-1
Vanoli A, La Rosa S, Klersy C, Grillo F, Albarello L, Inzani F, Maragliano R, Manca R, Luinetti O, Milione M, Doglioni C, Rindi G, Capella C, Solcia E (2017) Four neuroendocrine tumor types and neuroendocrine carcinoma of the duodenum: analysis of 203 cases. Neuroendocrinology 104:112–125. https://doi.org/10.1159/000444803
Vinik AI, Woltering EA, Warner RR, Caplin M, O’Dorisio TM, Wiseman GA, Coppola D, Go VL (2010) NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas 39:713–734. https://doi.org/10.1097/MPA.0b013e3181ebaffd
Volante M, Daniele L, Asioli S, Cassoni P, Comino A, Coverlizza S, De Giuli P, Fava C, Manini C, Berruti A, Papotti M (2013) Tumor staging but not grading is associated with adverse clinical outcome in neuroendocrine tumors of the appendix: a retrospective clinical pathologic analysis of 138 cases. Am J Surg Pathol 37:606–612. https://doi.org/10.1097/PAS.0b013e318275d1d7
von Herbay A, Sieg B, Schurmann G, Hofmann WJ, Betzler M, Otto HF (1991) Proliferative activity of neuroendocrine tumours of the gastroenteropancreatic endocrine system: DNA flow cytometric and immunohistological investigations. Gut 32:949–953
Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM, Shi C, Sharma R, Laheru D, Edil BH, Wolfgang CL, Schulick RD, Hruban RH, Tang LH, Klimstra DS, Iacobuzio-Donahue CA (2012) Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 36:173–184. https://doi.org/10.1097/PAS.0b013e3182417d36
Yang Z, Tang LH, Klimstra DS (2011) Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol 35:853–860. https://doi.org/10.1097/PAS.0b013e31821a0696
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work does not violate any ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Klöppel, G., La Rosa, S. Ki67 labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch 472, 341–349 (2018). https://doi.org/10.1007/s00428-017-2258-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2258-0