Abstract
Background
The Ki-67 index is an established prognostic marker for recurrence after resection of pancreatic neuroendocrine tumors (PanNETs) that groups tumors into three categories: low grade (< 3%), intermediate grade (3–20%), and high grade (> 20%). Given that the majority of resected PanNETs have a Ki-67 less than 3%, this study aimed to stratify this group further to predict disease recurrence more accurately.
Methods
The Ki-67 index was pathologically re-reviewed and scored by a pathologist blinded to all other clinicopathologic variables using tissue microarray blocks made in triplicate. All patients who underwent curative-intent resection of non-metastatic PanNETs at a single institution from 2000 to 2013 were included in the study. The primary outcome was recurrence-free survival (RFS).
Results
Of 113 patients with well-differentiated PanNETs resected, 83 had tissue available for pathologic re-review. The Ki-67 index was lower than 3% for 72 tumors (87%) and between 3 and 20% for 11 tumors (13%). Considering only Ki-67 less than 3%, the tumors were further stratified by Ki-67 into three groups: group A (< 1%, n = 43), group B (1–1.99%, n = 23), and group C (2–2.99%, n = 6). Compared with group A, groups B and C more frequently had advanced T stage (T3: 44% and 67% vs 12%; p = 0.003) and lymphovascular invasion (50% and 83% vs 23%; p = 0.007). Groups B and C had similar 1- and 3-year RFS, both less than group A. After combining groups B and C, a Ki-67 of 1–2.99% was associated with decreased RFS compared with group A (< 1%). This persisted in the multivariable analysis (hazard ratio [HR] 8.6; 95% confidence interval [CI] 1.0–70.7; p = 0.045), with control used for tumor size, margin-positivity, lymph node involvement, and advanced T stage.
Conclusions
PanNETs with a Ki-67 of 1–2.99% exhibit distinct biologic behavior and earlier disease recurrence than those with a Ki-67 lower than 1%. This new stratification scheme, if externally validated, should be incorporated into future grading systems to guide both surveillance protocols and treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreatic neuroendocrine tumors (PanNETs) are rare, accounting for only 1–2% of all pancreatic neoplasms, with approximately 1000 new cases diagnosed annually in the United States.1,2 These tumors are typically indolent in nature, can be hormonally functional or nonfunctional, and can have low or high malignant potential. Many are discovered incidentally on cross-sectional imaging for other diagnoses.3 Heterogeneity in the clinical presentation of PanNETs creates unique challenges for their management, particularly in deciding on the extent of surgical resection or on surgical resection versus surveillance.4,5
The World Health Organization (WHO) classifies pancreatic neuroendocrine lesions into two main categories: well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs).4,6 Grading of NETs is based on mitotic count and the Ki-67 index.4,6 The Ki-67 index stratifies PanNETs into low-grade tumors (Ki-67 < 3%), intermediate-grade tumors (Ki-67 3–20%), and high-grade carcinomas (Ki-67 > 20%).1,7 Findings have shown the Ki-67 index, in particular, to be highly correlated with clinical outcome, perhaps more so than other known histopathologic features.8,9,10,11
Approximately 90% of diagnosed PanNETs are considered well-differentiated tumors (low-to-intermediate grade) and primarily treated with surgical resection.2,4,12 However, the clinical behavior of these well-differentiated tumors is heterogeneous, with recurrences reported for 10–54% of patients, and the median overall survival (OS) ranges from 51 to 79 months.3,12,13,14 With the majority of resected PanNETs (80%) well-differentiated, and with the Ki-67 index lower than 3% all grouped together, the current WHO stratification scheme is limited in its ability to differentiate outcomes within this low-grade PanNET subset.13,15,16 Thus, our study aimed to further risk-stratify patients with well-differentiated, low-grade PanNETs (Ki-67 index < 3%), with the intent of using Ki-67 to discriminate outcomes better by predicting disease recurrence and biologic behavior.
Methods
Study Population and Pathologic Assessment
All patients with primary, non-metastatic PanNETs who underwent surgical resection at Emory University from 1 January 2000 to 31 December 2013 were identified. Pathologic re-review was undertaken. Only patients who had surgery with curative intent and with available tissue samples for pathologic re-review were included in the study. For the purpose of this study, only patients with well-differentiated tumors and a Ki-67 lower than 3% (low grade) were included in the analysis. Patients with distant metastases or other malignancies, multifocal disease, R2 resections, or mortality within 30 days after surgery were excluded from the analysis.
For this study, tissue microarrays were created in triplicate from the archived formalin-fixed, paraffin-embedded archived tissue blocks. Standard immunohistochemistry was performed. The slides were stained for Ki-67 (clone MIB-1; DAKO Agilent Pathology Solutions, Santa Clara, CA, USA) using antigen retrieval and the Leica Bond Autostainer (Leica Biosystems, Wetzlar, Germany) and counterstained with hematoxylin. The tissue microarray slides were scanned at × 40 magnification on the Leica Aperio AT2 bright field instrument (Leica Biosystems) for computerized quantitation image analysis. The Ki-67 proliferation index was determined as the percentage of tumor cells with Ki-67 immunoreactive nuclei. Depending on the tumor cellularity, the number of total cells counted ranged from 600 to 1800. An overall score for each sample was calculated as the sum of the expression percentage of each triplicate divided by 3. An experienced and dedicated gastrointestinal pathologist at Emory University, blinded to all other clinicopathologic variables for each tissue sample, supervised the analysis. Only the results from re-review of Ki-67 expression were used for analysis.
Study Variables
Retrospective chart review captured pertinent demographic, preoperative, intraoperative, pathologic, and postoperative data. Preoperative comorbidities were defined using the Charlson Comorbidity Scoring System, and staging was assigned as per the American Joint Committee on Cancer 7th edition guidelines.5 Data regarding neoadjuvant and adjuvant therapy, disease recurrence, and survival were additionally collected. Recurrence of disease was specifically determined according to review of patient medical records, radiographic reports on surveillance imaging, and/or biopsy results. Institutional review board approval was obtained before data collection.
Statistical Analyses
Descriptive and comparative analyses were performed on the entire cohort. Recurrence-free survival (RFS) was calculated from the date of operation to the date of recurrence diagnosis. All statistical analyses were performed using SPSS version 23.0 (Armonk New York Software, IBM Inc., Armonk, New York, USA). Chi square analyses and Fisher’s exact tests were used to compare categorical variables, and Student’s t test was used for continuous variables, where indicated. Uni- and multivariable Cox regression analyses were performed to assess the association of individual pathologic factors with RFS. Kaplan–Meier survival plots for RFS were created, and variables were compared using log-rank tests. Statistical significance was predefined as a p value lower than 0.05.
Results
Patient Variables
Of 265 patients with gastrointestinal/pancreatic neuroendocrine tumors and tissue available for pathologic re-review, 98 (37%) had primary PanNETs, and 83 (31%) had well-differentiated, non-metastatic pancreatic tumors that underwent curative-intent resection. After exclusion of 11 patients with a Ki-67 index of 3% or higher, 72 (27%) patients who had primary resected PanNETs with a Ki-67 index lower than 3% were available for analysis.
The baseline demographics and clinicopathologic features of this study cohort are summarized in Table 1. The mean age was 55 years. Nearly half of the patients (46%, n = 33) were male, and 74% (n = 53) were white. The mean tumor size was 3.2 cm, and 26% (n = 19) of the PanNETs were considered functional tumors. The location for 51% of the tumors (n = 37) was in the body or tail of the pancreas, and 71% (n = 51) were resected via an open procedure. Whereas 64 (89%) underwent R0 resections, only 8 (11%) underwent R1 resections. No R2 resections were performed in our study cohort, and 90% of all recurrences were at distant sites. Only 5 of the 72 patients received systemic therapy, 3 of which were treated neoadjuvantly.
Pathologic Re-review and Ki-67 Data
Among the 72 well-differentiated PanNETs with a Ki-67 index lower than 3%, Ki-67 was further stratified into three initial groups after pathologic re-review: group A (< 1%: n = 43, 60%), group B (1–1.99%: n = 23, 32%), and group C (2–2.99%: n = 6, 8%) (Table 2). Representative immunohistochemical KI-67 slides for this grouping are shown in Fig. 1. These groups were well-matched in terms of baseline demographic and operative variables including age, race, comorbidities, location of PanNET, tumor size, and operative blood loss (p > 0.05). However, the groups displayed key pathologic differences. Groups B and C were characterized by advanced T stage (44 and 67%, respectively) compared with group A (12%) (p = 0.003), as well as by an increased incidence of lymphovascular invasion (LVI) (83% in group C vs 23% in Group A) (p = 0.007) (Table 2).
Re-stratification of Ki-67 based on histologic analysis by a pathologist blinded to all other clinicopathologic variables using tissue microarray blocks made in triplicate. Patients with low-grade, Ki-67 < 3% were further stratified into 3 initial groups based on immunohistochemical expression: Group A (Ki-67 < 1%), Group B (Ki-67 1–1.99%), and Group C (Ki-67 2–2.99%)
Recurrence-Free Survival Analysis
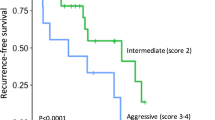
The median follow-up period was 39 months (interquartile range [IQR] 7.1–60.3). In the Kaplan-Meir analysis, the 3-year RFS was 97% for group A, 71% for group B, and 67% for C (p = 0.018) (Fig. 2a).
Kaplan-Meier survival curve for recurrence-free survival in re-stratified Ki-67 index groups. a There is no statistically significant difference between Group B (Ki-67 1–1.99% (n = 23)) and Group C (Ki-67 2–2.99% (n = 6)), but both are associated with worse RFS compared to Group A (Ki-67 < 1% (n = 43)). Log-rank p value = 0.018; b The combined Groups B and C (Ki-67 1–2.99% (n = 29)) show a 27% decrease in 3-year RFS compared with Group A (Ki-67 < 1% (n = 43)). Log-rank p value = 0.005. c After excluding functional and syndromic PanNETs (n = 20), the combined Groups B and C (Ki-67 1–2.99% (n = 20)) show a 23% decrease in 3-year RFS compared with Group A (Ki-67 < 1% (n = 32)). Log-rank p value = 0.017
Ki-67 Index as a Guide for Re-stratification
Given the similarity in pathologic characteristics and RFS between groups B and C, these groups were combined to form two final subsets of patients with well-differentiated, low-grade PanNETs for subsequent survival analysis: group A with a Ki-67 lower than 1% (n = 43, 60%) and groups B and C with a Ki-67 of 1–2.99% (n = 29, 40%). Analysis showed that group A had a 3-year RFS of 97%, whereas groups B and C had a decreased 3-year RFS of 70% (p = 0.005) (Fig. 2b).
A subgroup analysis also was performed excluding the 20 patients of the study group with functional and syndromic PanNETs. The results indicated a 23% decrease in 3-year RFS (96% vs 73%) for the combined groups B and C compared with group A (p = 0.017) (Fig. 2c).
Predictors for Recurrence-Free Survival
In the univariable Cox regression analysis, a Ki-67 index of 1% to 2.99% (HR 7.1; 95% CI 1.5–33.8; p = 0.014), tumor size, final resection status, perineural invasion (PNI), lymph node positivity, and advanced T stage each was associated with worse RFS (Table 3). In the multivariable analysis, R1 resection and Ki-67 (HR 8.6; 95% CI 1.0–70.7; p = 0.045) remained independently significant, even when these other adverse clinicopathologic factors were taken into account (Table 3).
Discussion
Although well-differentiated, low-grade PanNETs are uniformly noted to have improved recurrence and overall survival compared with high-grade PanNETs (i.e., pancreatic NECs), significant heterogeneity in outcomes among these well-differentiated tumors remains.12,13 The absence of a reliable risk stratification scheme to discriminate among well-differentiated, low-grade PanNETs has hindered ability to predict disease recurrence adequately after surgical resection.13,17 Furthermore, grouping up to 80% of resected PanNETs as well-differentiated (grade 1 or 2) limits ability to guide individualized postoperative management and surveillance.2,13,18,19
Findings have shown the Ki-67 index to be a particularly sensitive histopathologic marker for a more aggressive clinical course.9,20 However, the current method of using broad Ki-67 categories to define PanNETs may obscure the true prognostic value of this variable, particularly for the low-grade cohort.10 Indeed, our findings suggest that even among well-differentiated, low-grade PanNETs, a Ki-67 index of 1–2.99% is independently associated with an eightfold increase in risk of disease recurrence compared with tumors that have a Ki-67 index lower than 1%, even after the study has accounted for other adverse clinicopathologic variables.
Previous studies have attempted to determine the optimal Ki-67 index cutoff point to predict outcomes for low-to-intermediate grade PanNETs.21 For example, Lowe et al.9 demonstrated that a Ki-67 index above 10% rather than above 3% better predicts lymph node metastases and poor overall survival (OS). Another study by Hamilton et al.22 found that a Ki-67 index higher than 9% predicts a higher likelihood of disease recurrence and worse OS. Finally, a Ki-67 index cutoff of 7.5% was the recommendation of Goodell et al.23 However, these studies included both low- and intermediate-grade PanNETs in their analyses instead of focusing solely on the well-differentiated, low-grade subset. Because our findings suggest heterogeneity in disease recurrence when the Ki-67 index is used for stratification, even for such low-grade PanNETs, it may be inappropriate to increase the Ki-67 cutoff to classify more patients into the low-grade group. Partly due to the rarity of PanNETs, studies that focus on this subset with a Ki-67 lower than 3% currently are limited.
In our study, the PanNET patients with a Ki-67 lower than 1% had improved RFS compared with the patients who had a Ki-67 index of 1–2.99%, with an RFS of 97% at 3 years versus 70%, respectively. Although no studies to our knowledge have focused on the effect of the Ki-67 index on RFS in well-differentiated, low-grade PanNETs, a study by Boyar Cetinkaya et al.3 did show a significant difference in 5-year OS for patients who had PanNETs with a Ki-67 lower than 2% (75.2%) versus a Ki-67 of 3–20% (55.8%) (p = 0.04).3This difference continued in the analysis of 10-year OS in this study, with a Ki-67 lower than 2% related to a 68.9% survival compared with a 46.5% survival for a Ki-67 of 3–20% (p = 0.03).
Another study by Miller et al.19 also analyzed OS for patients whose NETs were graded on the basis of the WHO/European Neuroendocrine Tumor Society (ENETS) stratification scheme. In this study, those classified as having low-grade tumors had an OS of 87% compared with 83 and 50% for the patients with intermediate- and high-grade tumors, respectively.19 However, this study did not focus solely on PanNETs, but rather on all gastrointestinal NETs.
Finally, a study by Panzuto et al.24 also identified the Ki-67 index as a risk factor for disease progression in PanNETs. In their study, compared with low-grade PanNETs, intermediate-grade (Ki-67 3–20%) and high-grade (Ki-67 > 20%) PanNETs were associated respectively with 3.43-fold (p < 0.001) and 1.5-fold (p = 0.074) increases in risk for progression (Ki-67 ≤ 2%).24
To our knowledge, this study is the first to evaluate the use of the Ki-67 index to further risk stratify and predict outcomes for well-differentiated, low-grade PanNETs. These findings may be used to guide future management of low-grade PanNETs given the unclear current National Comprehensive Cancer Network (NCCN) guidelines regarding surgical resection versus observation for small well-differentiated, low-grade PanNETs.15 These results could perhaps be extrapolated to support surgical resection of PanNETs 2 cm in size or smaller if the measured Ki-67 index is higher than 1% on a preoperative biopsy because tumors with a Ki-67 index higher than 1% may display more aggressive behavior. Specifically, this study demonstrated higher recurrence rates after resection for such tumors compared with those smaller than 1%. Moreover, postoperative surveillance could be increased to include imaging at shorter intervals rather than intervals of 1 year or longer, as per the current standard of care.15 Ultimately, this proposed stratification scheme could be included in future adjuvant trials for the development of targeted postoperative therapy, as the armamentarium for PanNETs continues to improve.
This study had various limitations, including its retrospective design using data from a single institution with a relatively small number of patients who had tissue available for analysis. Pathologic re-review was performed only for Ki-67, which left data on other important histopathologic factors such as lymphovascular and perineural invasion dependent on the original surgical pathology reports, from which they were frequently missing. Moreover, the creation of tissue microarrays from random areas in each tumor may have underestimated the Ki-67 index because hot-spot analysis generally is used in the clinical setting. The applicability of our findings is further limited by the numerous and unstandardized methods that currently exist for measuring Ki-67 proliferation and their variable reported accuracy and reliability.23,25
In conclusion, our data suggest that even within well-differentiated, low-grade PanNETs, the Ki-67 index is a strong predictor of disease recurrence. This may have implications for its incorporation into future grading systems, treatment recommendations, and surveillance protocols as the management for PanNETs continues to evolve.
References
Kimura W, Tezuka K, Hirai I. Surgical management of pancreatic neuroendocrine tumors. Surg Today. 2011;41:1332–43.
Shanahan MA, Salem A, Fisher A, et al. Chromogranin A predicts survival for resected pancreatic neuroendocrine tumors. J Surg Res. 2016;201:38–43.
Boyar Cetinkaya R, Vatn M, Aabakken L, Bergestuen DS, Thiis-Evensen E. Survival and prognostic factors in well-differentiated pancreatic neuroendocrine tumors. Scand J Gastroenterol. 2014;49:734–41.
Dickson PV, Behrman SW. Management of pancreatic neuroendocrine tumors. Surg Clin North Am. 2013;93:675–91.
Li J, Lin JP, Shi LH, et al. How reliable is the Ki-67 cytological index in grading pancreatic neuroendocrine tumors? A meta-analysis. J Dig Dis. 2016;17:95–103.
Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909–18.
Bosman FT, World Health Organization, International Agency for Research on Cancer. WHO Classification of Tumours of the Digestive System. 4th ed. International Agency for Research on Cancer Lyon, 2010.
Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437–47.
Lowe K, Khithani A, Liu E, et al. Ki-67 labeling: a more sensitive indicator of malignant phenotype than mitotic count or tumor size? J Surg Oncol. 2012;106:724–7.
Jamali M, Chetty R. Predicting prognosis in gastroentero-pancreatic neuroendocrine tumors: an overview and the value of Ki-67 immunostaining. Endocr Pathol. 2008;19:282–8.
Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–92.
Burns WR, Edil BH. Neuroendocrine pancreatic tumors: guidelines for management and update. Curr Treat Options Oncol. 2012;13:24–34.
Strosberg JR, Cheema A, Weber JM, et al. Relapse-free survival in patients with nonmetastatic, surgically resected pancreatic neuroendocrine tumors: an analysis of the AJCC and ENETS staging classifications. Ann Surg. 2012;256:321–5.
Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis, and recent trend toward improved survival. Ann Oncol. 2008;19:1727–33.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Salama A, Badawy O, Mokhtar N. Ki-67 is a powerful tool for grading neuroendocrine tumors among Egyptian patients: a 10-year experience. J Cancer Res Clin Oncol. 2014;140:653–61.
Liu TC, Hamilton N, Hawkins W, Gao F, Cao D. Comparison of WHO Classifications (2004, 2010), the Hochwald grading system, and AJCC and ENETS staging systems in predicting prognosis in locoregional well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2013;37:853–9.
Nadler A, Cukier M, Rowsell C, et al. Ki-67 is a reliable pathological grading marker for neuroendocrine tumors. Virchows Arch. 2013;462:501–5.
Miller HC, Drymousis P, Flora R, Goldin R, Spalding D, Frilling A. Role of Ki-67 proliferation index in the assessment of patients with neuroendocrine neoplasias regarding the stage of disease. World J Surg. 2014;38:1353–61.
McCall CM, Shi C, Cornish TC, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37:1671–7.
Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557–77.
Hamilton NA, Liu TC, Cavatiao A, et al. Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery. 2012;152:107–13.
Goodell PP, Krasinskas AM, Davison JM, Hartman DJ. Comparison of methods for proliferative index analysis for grading pancreatic well-differentiated neuroendocrine tumors. Am J Clin Pathol. 2012;137:576–82.
Panzuto F, Boninsegna L, Fazio N, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol. 2011;29:2372–7.
Tang IP, Singh S, Krishnan G, Looi LM. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses: a rare case. J Laryngol Otol. 2012;126:1284–6.
Acknowledgements
Funding in part was provided by the Katz Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopez-Aguiar, A.G., Ethun, C.G., Postlewait, L.M. et al. Redefining the Ki-67 Index Stratification for Low-Grade Pancreatic Neuroendocrine Tumors: Improving Its Prognostic Value for Recurrence of Disease. Ann Surg Oncol 25, 290–298 (2018). https://doi.org/10.1245/s10434-017-6140-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6140-8