Abstract
The origin of a primary or metastatic carcinoma in the pelvic area is sometimes difficult to establish, in particular the distinction between those originating in the bladder and the prostate. A candidate marker is the HOXB13 gene, essential for prostate development. Some studies have shown expression of HOXB13 protein by immunohistochemistry in the nuclear compartment of benign prostate luminal epithelium and prostate carcinoma. Forty-two cases of biopsies and resection specimens of the prostate and urinary bladder, metastatic lymph nodes, and pelvic masses were retrieved from our databases. In all cases, doubt persisted regarding prostatic versus urothelial origin. All cases were stained for CK7, p63, p504s, PSA, CK20, and HOXB13. Chromogranin A, CD56, and synaptophysin were used when neuroendocrine differentiation was suspected. HOXB13 staining was negative or only weakly positive in all carcinomas of urothelial origin. Three of four carcinomas with neuroendocrine differentiation did not express HOXB13. The fourth carcinoma, in a patient with a history of prostate carcinoma, was positive. In two cases with a synchronous prostatic and urothelial carcinoma, HOXB13 was exclusively expressed in the prostatic carcinoma. Our results demonstrate that HOXB13 expression identifies prostatic origin of a carcinoma with good sensitivity (89 %) and very good specificity (100 %). HOXB13 is a specific and sensitive marker for prostate cells and a valuable diagnostic tool, especially when poorly differentiated or neuroendocrine tumors are encountered. These results justify testing of HOXB13 as a prostate-specific carcinoma marker in larger cohorts for a more thorough evaluation of its sensitivity and specificity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The HOXB13 gene is a member of the homeobox gene family and encodes a transcription factor which is essential for posterior abdominal vertebrate development during embryogenesis. The HOXB13 protein contains a homeodomain (approximately 60 amino acids), coded by a highly conserved DNA sequence (approximately 180 bp) with which it can bind DNA. Prostate development depends on HOXB13 function. HOXB13 maintains a high expression level into adulthood in the normal prostate. Recently, Ewing et al. showed that the HOXB13 G84E variant is associated with a significantly increased risk of hereditary prostate cancer [1].
Few studies have confirmed the expression of HOXB13 in the nuclear compartment of benign prostate luminal epithelium and prostate carcinoma cells by immunohistochemistry (IHC) [2]. Expression of HOXB13 in cells with urothelial origin has not been reported. HOXB13 might be a sensitive marker for prostatic cells (benign or neoplastic) and, as such, a valuable diagnostic tool for genitourinary tract carcinomas, particularly in case of doubt regarding urothelial or prostatic origin.
The distinction between tumors of bladder and prostatic origin in biopsy or resection specimens can be extremely challenging. Immunohistochemical markers such as CK7 and p63 are expressed in normal and tumoral urothelial tissue. PSA is expressed in prostatic tissue, and p504s shows cytoplasmic expression in prostate carcinoma. These markers are helpful, but sometimes insufficient to determine the origin of a neoplasm in the genitourinary tract, notably when a carcinoma with a neuroendocrine component or a poorly differentiated carcinoma is encountered.
There are limited IHC data for HOXB13. The aim of this study was to assess the sensitivity and specificity of IHC HOXB13 staining as a marker of prostate carcinoma. Furthermore, the utility of this marker was evaluated for its ability to distinguish the origin of carcinomas in the genitourinary tract.
Materials and methods
Tissue selection
The initial antibody screening was performed with a tissue microarray (TMA) of normal tissues (prostate, rectum, colon, small intestine, stomach, liver, bladder, ureter, kidney, testis, breast, pancreas, thyroid, adrenal gland, lymph node, and skin) from the Department of Pathology of the University Hospital de la Pitié-Salpêtrière. Ten cases were selected for each organ from our archives. Each tissue sample was fixed in formalin and paraffin-embedded (FFPE), and three 0.6-mm cores were included in the TMA. Ten cases each of confirmed prostate carcinoma and of confirmed urothelial carcinoma were also tested.
A search in the patient data files of the Department of Pathology allowed us to identify cases for which the pathologist had requested IHC because of difficulties in determining the origin of a carcinoma, in particular of urothelial or prostatic origin. From the period between January 1998 and March 2013, 40 cases were retrieved. Two cases were contributed by the Department of Pathology of the Indiana University School of Medicine. Overall, 42 cases of biopsy or resection specimens of the prostate and urinary bladder, metastatic lymph nodes, and samples of pelvic masses were retrieved. For diagnostic reasons, all of these cases had been subjected to IHC using antibodies against CK7, p63, p504s, and PSA. In cases with a suspicion of neuroendocrine differentiation, antibodies against Chromogranin A, synaptophysin, and CD56 were also applied. One representative block from each case was used for the study. Two whole tissue sections from each case were used; one was stained by hematoxylin–eosin saffron, the other by IHC. The gold standard for the diagnosis was the signed reports, all signed by the same senior uropathologist (EC), except for the two cases from Indianapolis which were signed by LC, with diagnosis confirmed by EC.
The last step of our investigation was to perform HOXB13 staining on confirmed prostate cancer and normal prostatic tissue samples, in order to confirm the reproducibility of staining on a larger scale. We applied this staining to TMAs, which we had already used for another study with FFPE tissues from our department.
Immunohistochemistry and scoring
Tissue sections were deparaffinized and rehydrated. The HOXB13 antibody (sc-28333, Santa Cruz Biotechnology, Dallas, TX, USA) was chosen in view of its prior use in other publications. It is a mouse monoclonal antibody raised against amino acids 1–284 of HOXB13 of human origin. The antibody dilution used in this study was 1:100.
The stained tissues were scored by the two pathologists (JV and EC). Tissues or tumors that showed nuclear staining of at least 10 % of the cells were recorded as positive, while cases with staining of less than 10 % of the cells were classified as negative. The intensity of the staining was estimated as weak (1) or strong (2).
Results
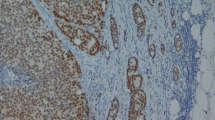
Staining scores of all normal tissues are presented in Table 1. Normal urothelial tissue sometimes showed weak nuclear staining in the basal cell layer of normal urothelium (Fig. 1). The ten cases of confirmed prostate carcinoma were strongly positive (Fig. 2); in the ten cases of urothelial carcinoma, no or very weak focal staining was noted (Fig. 3).
The patients of the 42 biopsy or resection specimens of prostate and bladder cancer, metastatic lymph nodes, and pelvic masses (for more details, see Table 2) had a median age of 70 years (range, 42–87). Two patients had repeated resections, of which only the first was taken into consideration in this study. Most of these cases were transurethral resections of the prostate or bladder (n = 22). In some cases, only biopsy material from either the prostate or bladder was available (n = 7). In two cases, the exact site of the resection was unknown, but both bladder and prostate tissue were recognized in the histological slides. Also included in this series were lymph node metastases of carcinomas of undetermined origin, pelvic masses, one liver metastasis, and other samples from the genitourinary region, for which doubt regarding the primary origin of the tumor persisted. Detailed results are listed in Table 2.
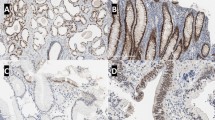
Two cases (13 %) were retrospectively confirmed as urothelial carcinomas, with no or very weak, focal labeling with HOXB13 (Fig. 4). Of the four cases with neuroendocrine differentiation, three did not express HOXB13. In one case, a final diagnosis of prostate carcinoma with neuroendocrine differentiation was confirmed; this case was strongly positive, and the patient had a prior history of prostate carcinoma (Fig. 5). In the two other cases, a minor component (<2 %) of urothelial carcinoma was observed. Based on these findings, we considered the third case as a neuroendocrine urothelial carcinoma, despite the fact that no urothelial carcinoma was found.
In two cases with concurrent prostate and bladder cancer, HOXB13 was exclusively expressed in the prostate cancer (Fig. 6). Both cases were not challenging from a diagnostic point of view but confirm our observations (see Table 3). Sensitivity of HOXB13 staining for determining a prostatic origin was 89 % with 100 % specificity (Table 4).
In all tested normal prostate tissues, only the luminal prostatic cells were strongly stained by the HOXB13 antibody, while the basal cells never displayed any staining (Fig. 7). In the 120 cases of normal prostatic tissue, we observed strong nuclear staining of HOXB13 in the secretory cells in all cases. All 400 cases of prostate carcinoma on the TMA also showed strong nuclear staining without any cytoplasmic staining. Additional information about Gleason scores, pT stage, and surgical margins is provided in Table 5.
Discussion
Reliable markers are needed in order to distinguish the tissue of origin in cases of poorly differentiated carcinomas, especially in the genitourinary tract. Several markers have been studied, such as HOXB13, which is expressed in normal as well as tumor tissue of the prostate [2]. There is little data on HOXB13 expression at the protein level; our study is the first to test HOXB13 expression in tissues other than the prostate by IHC, with particular emphasis on its use in diagnosis.
Our results demonstrate that HOXB13 staining is highly specific for prostate tissue. We show that normal prostate or prostate cancer tissue strongly expresses HOXB13; in all other normal tissues, except for two cases of the rectum, only weak focal expression was found or no staining at all (1). This is helpful in difficult cases of carcinoma in the genitourinary tract, notably when doubt remains regarding prostatic or urothelial origin. Even cases with neuroendocrine differentiation, or cases with distant metastasis, which are suspected to be of genitourinary origin, could be successfully resolved.
Unfortunately, especially in poorly differentiated cases, distinguishing high-grade prostate carcinoma from poorly differentiated urothelial carcinoma is exceedingly difficult, especially on limited biopsy material. The treatments for prostate carcinoma and urothelial carcinoma are completely different. Advanced prostate carcinoma is commonly treated with hormonal therapy, while high-stage, metastatic urothelial carcinoma is treated with chemotherapy. Therefore, it is of major importance to distinguish between the different origins of poorly differentiated carcinomas in the genitourinary tract, or distant metastatic localizations in patients with a clinical history of urothelial carcinoma and/or prostate carcinoma. An accurate diagnosis is essential for optimal patient care.
Morphology is helpful, with a cribriform pattern and prominent nucleoli, sheet, and cords of uniform cells in favor of prostate carcinoma. However, for some poorly differentiated carcinomas or carcinomas with neuroendocrine differentiation, the diagnosis can be very challenging. The presence of a coexisting low-grade papillary urothelial tumor or urothelial carcinoma in situ would favor a diagnosis of high-grade urothelial carcinoma, but the possibility of synchronous prostate carcinoma would have to be excluded, since both tumor types occur in elderly men. Morphological characteristics and clinical manifestations can overlap between the carcinomas, but their distinction has significant implications on staging and therapy.
Immunohistochemistry is often needed to resolve differential diagnoses. Several immunohistochemical markers have been described to distinguish between prostatic and urothelial tissue. PSA and PSAP are highly specific for prostate epithelium [3]. In our experience, PSA staining may be weak and focal. Therefore, strong nuclear HOXB13 staining should be an improvement on PSA for diagnostic use. Another useful marker is p504s (AMACR), also known as alpha-methyl-acyl-CoA racemase, expression of which is upregulated in prostate carcinoma, and which has given good results for prostatic-specific staining, even in metastatic lesions [4, 5]. Recently, a new marker, NKX3.1, has been tested. This antibody shows high sensitivity for nontreated, metastatic prostate carcinoma, with 98.6 % of the cases stained in some studies. Nevertheless, it occasionally also stains lobular breast carcinoma [3].
For urothelial carcinoma, many excellent markers are available, such as high molecular weight cytokeratins, which are expressed in 65 % of urothelial carcinomas but in only 6 % of prostate carcinomas [6]. For example, CK7 and CK20 are expressed in 40–60 % of urothelial carcinomas; often urothelial carcinomas coexpress both cytokeratins. The relatively new biomarker p63 has also shown utility in the distinction between genitourinary carcinomas of different origin. It is a member of the p53 family, predominantly expressed in the basal layer of the urothelium, and has shown reactivity in squamous and urothelial carcinomas [7, 8]. Staining p63 is negative in 2–17 % of high-grade urothelial carcinomas, while positive in 0–3 % of high-grade prostate carcinoma [9–13]. Placental S100 (S100P) staining has also recently been noted in 78 % of urothelial carcinomas; a combination of S100 and p63 stained 95 % of urothelial carcinomas [14].
Recent studies have shown that a new marker, GATA3, is expressed in 67–80 % of urothelial carcinomas, but not in prostate carcinoma [15, 16]. GATA3 has the potential to serve as a useful immunohistochemical marker that distinguishes between urothelial carcinoma and other genitourinary tumors, such as prostate carcinoma and renal cell carcinoma [17]. Another advantage of GATA3 is its homogenous staining. Chang et al. demonstrated that GATA3 performs better than Uroplakin III, which is considered an antigen highly specific for urothelium [18]. It has a sensitivity of 31–60 % for primary invasive urothelial carcinomas and 53 % for metastatic urothelial carcinomas. One problem with Uroplakin III as a distinguishing marker is that its expression decreases with increasing grade and stage of urothelial carcinomas, while poorly differentiated cases do not express Uroplakin III at all. Furthermore, Uroplakin III staining is difficult for pathologists to interpret, as it is heterogeneous [10, 17, 18].
In daily practice, classical IHC markers used for distinguishing tumor origin in the genitourinary tract, such as p504S, PSA, and PSAP, are positive in cases of prostatic origin, and negative in cases of urothelial origin, while the inverse is true for p63 and CK7. None of these markers have 100 % sensitivity and specificity; for example, about 12 % of urothelial carcinomas are p504S positive [9]. In particular, poorly differentiated cases can lose immunohistochemical expression of several antigens.
Although our study is limited by the small set of 42 cases with diagnostic uncertainty, we show that HOXB13 staining has good sensitivity (89 %) for prostate carcinoma and very high specificity (100 %). These data were confirmed by a supplementary study using normal prostatic tissue, as well as confirmed prostate cancer tissue, retrieved from radical prostatectomies. These results should be confirmed in larger studies. Nevertheless, these results are very promising and permitted us to reevaluate several very challenging cases.
As described above, sometimes very weak, focal staining of HOXB13 was observed in the basal layer of the urothelium or colonic mucosa. We observed two cases of normal rectum that exhibited strong staining in the crypts. As a consequence, HOXB13 might not be the ideal marker to distinguish between colon and prostate cancer. However, Alcian blue or PAS staining would be helpful in those cases, as well as CK20 and CDX-2 staining.
The major difficulty in uropathology remains the differential diagnosis between bladder and prostate cancer. In the differential diagnosis between poorly differentiated prostate carcinoma and urothelial carcinoma, we suggest that PSA, PSAP, p504s, CK7, and p63 remain the first screening markers. If PSA, PSAP, and p504s are negative, HOXB13 might direct the diagnosis towards prostate carcinoma. If all prostate markers are negative, it is extremely unlikely that the tumor is of prostatic origin.
References
Ewing CM, Ray AM, Lange EM et al (2012) Germline mutations in HOXB13 and prostate-cancer risk. NEJM 366:2
Kim YR, Oh KJ, Park RY et al (2010) HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling. Mol Cancer 9:124
Gurel B, Ali TZ, Montgomery EA et al (2010) NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J Surg Pathol 34(8):1097–1105
Jiang Z, Woda BA, Rock KL et al (2001) P504S: a new molecular marker for the detection of prostate carcinoma. Am J Surg Pathol 25(11):1397–1404
Luo J, Zha S, Gage WR et al (2002) Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res 62(8):2220–2226
Genega EM, Hutchinson B, Reuter VE, Gaudin PB (2000) Immunophenotype of high-grade prostatic adenocarcinoma and urothelial carcinoma. Mod Pathol 13(11):1186–1191
Compérat E, Camparo P, Haus R et al (2006) Immunohistochemical expression of p63, p53 and MIB-1 in urinary bladder carcinoma. A tissue microarray study of 158 cases. Virchows Arch 448(3):319–324
Chastain EC, Oliva IV, Osunkoya AO (2012) Utility of p63 and high molecular weight cytokeratin in the distinction between urothelial carcinoma with prostatic stromal invasion and urothelial carcinoma with colonisation of prostatic ducts and acini. Pathology 44(3):199–203
Ud Din N, Qureshi A, Mansoor S (2011) Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol 54:59–62
Kaufmann O, Volmerig J, Dietel M (2000) Uroplakin III is a highly specific and moderately sensitive immunohistochemical marker for primary and metastatic urothelial carcinomas. Am J Clin Pathol 113:683–687
Kunju LP, Mehra R, Snyder M, Shah R (2006) Prostate-specific antigen, high-molecular-weight cytokeratin (clone 34betaE12), and/or p63: an optimal immunohistochemical panel to distinguish poorly differentiated prostate adenocarcinoma from urothelial carcinoma. Am J Clin Pathol 125:675–681
Langner C, Ratschek M, Tsybrovskyy O, Schips L, Zigeuner R (2003) p63 immunoreactivity distinguishes upper urinary tract transitional-cell carcinoma and renal-cell carcinoma even in poorly differentiated tumors. J Histochem Cytochem 51:1097–1099
Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L (2000) p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol 157:1769–1775
Hodges KB, Lopez-Beltran A, Emerson RE, Montironi R, Cheng L (2010) Clinical utility of immunohistochemistry in the diagnoses of urinary bladder neoplasia. Appl Immunohistochem Mol Morphol 18(5):401–410
Chang A, Amin A, Gabrielson E et al (2012) Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol 36(10):1472–1476
Miyamoto H, Izumi K, Yao JL et al (2012) GATA binding protein 3 is down-regulated in bladder cancer yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum Pathol 43:2033–2040
Koga F, Kawakami S, Fujii Y et al (2003) Impaired p63 expression associates with poor prognosis and uroplakin III expression in invasive urothelial carcinoma of the bladder. Clin Cancer Res 9(15):5501–5507
Matsumoto K, Satoh T, Irie A et al (2008) Loss expression of uroplakin III is associated with clinicopathologic features of aggressive bladder cancer. Urology 72(2):444–449
Acknowledgments
The authors thank Annette Lesot for perfect technical assistance.
Conflict of interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varinot, J., Cussenot, O., Roupret, M. et al. HOXB13 is a sensitive and specific marker of prostate cells, useful in distinguishing between carcinomas of prostatic and urothelial origin. Virchows Arch 463, 803–809 (2013). https://doi.org/10.1007/s00428-013-1495-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-013-1495-0