Abstract
HoxB13 is a transcription factor involved in defining of posterior endodermal derivatives, including prostate and rectum. While it is used as a marker of prostatic adenocarcinoma, it has not been studied systematically in neuroendocrine neoplasms. Thus, we performed HoxB13 immunohistochemistry in tissue microarrays and the whole sections of 232 neuroendocrine neoplasms. These included 34 paragangliomas (PGs), 20 cauda equina neuroendocrine tumors (CENETs), 123 well-differentiated neuroendocrine tumors (WDNETs), and 55 neuroendocrine carcinomas (NECs). WDNETs were additionally analyzed with SATB2, and colorectal WDNETs with CDX2 and serotonin immunohistochemistry. In total, HoxB13 immunoreactivity was observed in 95% (19/20) CENETs, 10.6% (13/123) WDNETs, and 12.9% (7/54) NECs. No PGs were positive. Large intestine WDNETs expressed HoxB13 in 68.4% (13/19); five negative tumors originated in cecum and one in rectum. In rectum, 92.9% (13/14) WDNETs expressed HoxB13. HoxB13 was 92.9% sensitive and 100% specific, showing 100% positive predictive value for the rectal origin of WDNET. In NECs, HoxB13 was positive in 15.4% (2/13) GIT tumors and 80% (4/5) prostatic NECs, but in none of urinary bladder NECs (0/8). SATB2 was positive in 17.1% (21/123) WDNETs, including 78.9% (15/19) of colorectal WDNETs, 71.4% (5/7) appendiceal WDNETs, and 2.9% (1/34) small intestine WDNETs. All 4 SATB2-negative large bowel tumors originated in the cecum. When both markers combined, HoxB13+/SATB2+ immunoprofile was seen exclusively in rectal WDNETs (positive predictive value 100%), while HoxB13−/SATB2+ immunoprofile was highly suggestive of the appendiceal origin (positive predictive value 71.4%). Therefore, HoxB13 can be useful as an immunohistochemical marker of rectal WDNETs and prostatic NECs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine neoplasms (NENs) encompass a spectrum of entities characterized by the presence of neurosecretory vesicles and the expression of neurosecretory-related proteins such as synaptophysin and chromogranin A, adhesive molecules (CD56, CD57), or transcription factors related to neuroendocrine differentiation (ASCL1, INSM1) [1]. Entities belonging to NENs include paragangliomas (PGs), well-differentiated neuroendocrine tumors (WDNETs), neuroendocrine carcinomas (NECs), and cauda equina NETs (CENETs). In WDNETs and PGs, biological behavior is difficult to predict based on histological features as even WDNETs smaller than 10 mm can metastasize [2]. Indeed, WDNETs present with metastatic disease in 10–20%, and the identification of the primary origin is of therapeutic and prognostic significance [3]. Therefore, multiple immunohistochemical markers of the primary origin have been studied in the past, including CDX2 [4], Pitx2 [5], NKX6.1 [6], ISL1 [7], TTF1 [7], and SATB2 [3, 8].
HoxB13 is a transcription factor of the HOX family that contains the homeobox DNA-binding domain, encoded by the HOXB13 gene on chromosome 17q21.32. In total, the human genome includes 39 paralogous HOX genes, numbered 1 to 13 and arranged on 4 chromosomes in clusters A–D. On the chromosomes, the genes are arranged sequentially in 3′ to 5′ direction, with low-numbered genes located in 3′ regions, followed by higher-numbered HOX genes in 5′ direction. HOX genes are responsible for anteroposterior axis patterning, position information, and the development of different organs during embryogenesis [9]. The HOX genes located at the 3′ end of the genome are typically expressed in the front part of the developing embryo. In contrast, the HOX genes at the 5′ end, which include HOXB13, play a critical role in determining the identity of the tail end and the associated embryonic structures [9, 10]. HoxB13 is expressed early in the tail bud of the embryo [10] and helps to regulate the proliferation of neuromesodermal progenitors that are responsible for building the caudal structures [11]. Furthermore, HOXB13 helps to define the posterior endoderm derivatives, such as the prostate, colon, and rectum [9, 12]. HOXB13 expression persists in the colon, rectum, and prostate of adult individuals [13, 14] and is often used as a marker for prostatic adenocarcinoma [14, 15]. Additional studies also identified HOXB13 as a positive marker of CENETs [16,17,18].
In our previous study focused on CENETs, we observed a consistent immunoreactivity of HoxB13 in a limited subgroup of large intestine WDNETs [17]. As there are no available data on HoxB13 expression in NENs of other locations, we performed an immunohistochemical study on tissue microarrays (TMAs) and whole sections (WS) of different NENs to assess its diagnostic utility. Furthermore, we compared the results with immunohistochemistry of SATB2, a well-established marker of large intestine WDNETs, and assessed coexpression of enterochromaffin markers CDX2 and serotonin in colorectal WDNETs.
Materials and Methods
The cases of PGs, WDNETs, CENETs, and NECs diagnosed between 2005 and 2021 were collected from the archives of the pathology departments of the authors, and the original diagnosis was reviewed prior to case inclusion. The study was conducted in accordance with the Declaration of Helsinki, and the ethical committee of the first author’s institution approved the study (Ethical committee of University Hospital Hradec Kralove, reference no. 202101P06 and 202109P01). The formalin-fixed paraffin-embedded blocks of tumors were used to construct tissue microarrays (TMAs). In the study, 8 TMAs were constructed using the TMA Master II system (3DHISTECH Ltd., Budapest, Hungary). For each case, two representative tissue cores (1.5 mm in diameter) were transferred from a donor block into the recipient block. Additional cases were studied as whole sections (WS). The TMAs have been used in other studies [5, 17], and the selection of WS cases was mostly consistent with WS cases in a previous study [5].
In total, 232 unique tumors were analyzed for HoxB13. The composition of the cohort is summarized in Table 1. SATB2 was analyzed only in PGs, CENETs, and WDNETs. CDX2 and serotonin were analyzed only in large bowel WDNETs.
Tissue blocks, including TMAs, were cut into 2-µm-thick sections for routine H&E staining and additional studies. HoxB13 immunohistochemistry was performed after heat-induced epitope retrieval (24 min, Ventana CC1 solution, Ventana/Roche, Tucson, AZ, USA) with clone F-9 (1:100, SCBT Inc., Dallas, TX, USA), with a section of a normal prostate included on slide as a positive control (Fig. 1A). For SATB2 (EP281, 1:50, Zeta Corp. Arcadia, CA, USA), different pretreatment was chosen (32 min, Ventana CC1 solution, Ventana/Roche, Tucson, AZ, USA) with an appendix as an on-slide positive control. The procedure was carried out on a Benchmark Ultra stainer manufactured by Ventana/Roche, using Ventana OptiView DAB IHC Detection Kit. CDX2 (DAK-CDX2, 1:50, Dako, Glostrup, Denmark) and serotonin (5HT-H209, 1:40, Dako, Glostrup, Denmark) detection was performed in large bowel WDNETs, using Dako OMNIS platform and Dako EnVision Flex visualization system. Both methods use avidin–biotin complex method, horseradish peroxidase as enzyme, and DAB (3,3ʹ-diaminobenzidine) as chromogen. The slides were evaluated by two pathologists independently (M.M. and V.S. or J.S.), using the H-score, as published previously [19], along with the overall percentage of positive cells. Cases with H-score ≥ 1 were considered positive. Only the TMA cases with the tumor constituting at least one tissue core were included. Cases with positive versus negative result discrepancies between two observers were discussed during a joint session at a multiheaded microscope, and the consensus was reached. The average values of two observers of both H-score and the overall positive cell percentage in each case were used for analysis.
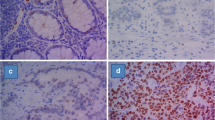
HoxB13 immunoreactivity in normal tissues. A Normal tissue of the prostate used as a positive on-slide control (× 200). B Positivity of normal rectal intestinal epithelium (× 200). C HoxB13 was occasionally observed in metaplastic intestinal epithelium of the stomach but not in normal gastric epithelium (× 300). D Rare focus of low-grade dysplasia in gastric intestinal metaplasia also showed HoxB13 positivity (× 400)
Statistical analysis was performed using the SigmaPlot 14 software. The Shapiro–Wilk test was used to assess the distribution of the data. Parametric values were described with mean and standard deviation (SD); nonparametric values were described with median and interquartile range (IQR). Two-tailed variants of the Student t-test, analysis of variance (ANOVA), and their respective nonparametric versions, the Mann–Whitney test (MW) and the Kruskal–Wallis test (KW), were used to analyze the data. p-values < 0.025 (Bonferroni correction) were considered significant.
Results
HoxB13 in WDNETs
HoxB13 immunoreactivity was observed in 95% (19/20) CENETs, 10.6% (13/123) WDNETs, and 12.9% (7/54) NECs. No PGs were positive. Detailed results of immunohistochemistry are shown in Table 2. All the HoxB13 + WDNETs (Figs. 2 and 3) were localized in the large intestine, while no WDNETs from other locations showed HoxB13 positivity. Large intestine WDNETs (rectum n = 14, cecum n = 5) expressed HoxB13 in 68.4% (13/19); five of the negative tumors originated in cecum (four primaries and one liver metastasis) and one in rectum. Thus, 92.9% (13/14) of rectal WDNETs expressed HoxB13. One rectal WDNET was included as both WS and TMA; the samples showed concordant immunoreactivity. In the group of WDNETs, HoxB13 was 92.9% sensitive and 100% specific for the rectal origin, showing 100% positive predictive value for the rectal origin of WDNET. HoxB13 + NECs included 2 GI tract NECs (H-scores 2.5 and 7.5) and 5 lower urinary tract NECs (H-score range 7.5 to 300). When HoxB13 H-score of positive cases was compared, we observed no significant differences between CENETs, WDNETs, and NECs (p = 0.4, ANOVA).
HoxB13 in NECs of Lower Genitourinary Tract
Since the HoxB13 expression is well characterized in prostate acinar cell adenocarcinomas, we were interested, if HoxB13 immunohistochemistry might be helpful in diagnosis of lower urinary tract NEC (n = 14; Figs. 4 and 5). These included 8 bladder NECs (7 primaries, 1 metastasis), 5 prostate NECs (4 tumors with small cell morphology and one Gleason 10 adenocarcinoma with neuroendocrine differentiation), and one NEC of undetermined primary site (bladder versus prostate). We observed HoxB13 in 80% (4/5) of prostatic NECs (H-scores 10, 115, 117.5, and 300) while no expression was seen in the bladder NECs (n = 8). In the NEC of undetermined site, HoxB13 was also positive (H-score 7.5), suggestive of the prostatic origin. Interestingly, NEC with H-score 300 also showed strong and diffuse NKX3.1 positivity in archive slides (Fig. 4A–C).
HoxB13 immunoreactivity in NECs of lower urinary tract. A Carcinoma of the prostate showing high-grade neuroendocrine morphology (× 200, H&E). B The same tumor maintained high expression of NKX3.1 (× 200) and C HoxB13 H-score 300 (× 200). D Another neuroendocrine carcinoma of the prostate showing only weak and patchy HoxB13 positivity (× 400)
HoxB13 immunoreactivity in NECs of lower urinary tract. A Areas of prostatic adenocarcinoma (× 200, H&E) and B areas of neuroendocrine carcinoma in the same biopsy sample (× 200). C Prostatic adenocarcinoma from A showing HoxB13 immunoreactivity (× 200). D The same focus of NEC from B, showing HoxB13 positivity (x 200). E In contrast, all urinary bladder NECs were HoxB13-negative (× 200). F Complete loss of HoxB13 was also observed in one prostatic NEC and this contrasted with maintained immunoreactivity of adenocarcinoma component (× 100)
Comparison of SATB2 and HoxB13 Immunoreactivity in WDNETs
To assess the diagnostic utility of HoxB13, we analyzed the expression of SATB2 in the study group of WDNETs. In WDNETs, SATB2 was positive in 17.1% (21/123) WDNETs, including 78.9% (15/19) of colorectal WDNETs (Fig. 6), 71.4% (5/7) appendiceal WDNETs, and 2.9% (1/34–nodal metastasis) small intestine WDNETs. In the colorectal subset, all 4 SATB2-negative tumors originated in the cecum (three primary tumors and one liver metastasis), while only one cecal tumor was SATB2 + , compared to SATB2 positivity in all (14/14) rectal cases. Therefore, SATB2 was 100% sensitive and 93.6% specific for the diagnosis of rectal WDNET, and it showed 66.7% positive predictive value for the rectal origin of WDNET. We observed no difference in H-scores between WDNETs of different locations (p = 0.16, ANOVA). Four cecal tumors were negative for both HoxB13 and SATB2, while the only HoxB13-negative rectal WDNET was SATB2-positive. When both markers combined, HoxB13 + /SATB2 + immunoprofile was seen exclusively in rectal WDNETs (positive predictive value 100%), while HoxB13 − /SATB2 + immunoprofile was highly suggestive of appendiceal origin (positive predictive value 71.4%). We observed no immunoreactivity of PGs or CENETs for SATB2.
Enterochromaffin Features of Colorectal WDNETs
To assess the relationship between SATB2/HoxB13 immunoreactivity and enterochromaffin phenotype, we analyzed the expression of CDX2 and serotonin in whole sections or TMAs of large bowel tumors. CDX2 immunoreactivity was seen in all 5 cecal tumors (H-scores range 90 to 275), while none of the rectal tumors was positive. In contrast, serotonin immunoreactivity was seen in 9 colorectal WDNETs, including 4 cecal tumors (H-score ranges 110 to 276.5) and 5 rectal tumors (H-scores 2.5, 7.5, 11, 179.5, and 297.5). The only HoxB13-negative rectal tumor showed weak serotonin immunoreactivity (H-score 7.5). The comprehensive data about coexpression of all the markers are included in Supplementary Table 1.
HoxB13 in Adjacent Normal Tissues
HoxB13 immunoreactivity was only rarely observed in normal tissues; large bowel epithelium stained in 14 cases, with 4, 3, and 7 samples showing strong, moderate, and weak patchy reactivity respectively (Fig. 1B). An equivocal weak positivity was observed in the bronchiolar epithelium in the 2 cases and in gland necks of the gastric mucosa in one sample. Interestingly, HoxB13 was weakly positive in 40% (4/10) cases of gastric intestinal metaplasia (Fig. 1C). Of these, two positive metaplastic cases showed low-grade dysplasia (Fig. 1D). Consistent positivity was observed in normal prostatic glands and prostatic adenocarcinomas.
Discussion
To our best knowledge, this is the first systematic study of HoxB13 immunohistochemistry in neuroendocrine neoplasms of different primary sites. WDNETs in the large intestine occur most commonly in the appendix, followed by the rectum and the colon [20]. Prostate specific acidic phosphatase (PSAP) and Special AT-rich sequence-binding protein 2 (SATB2) can be used as a marker of rectal WDNETs [3, 7], although PSAP can be observed also in some midgut WDNETs [3] and SATB2 was also detected in WDNETs of the appendix and other parts of the GI tract [3, 21]. The majority of colonic and appendiceal tumors shows enterochromaffin differentiation, while most rectal lesions show L-cell origin [22]. Enterochromaffin tumors may also occur in the rectum and tend to show poorer prognosis compared to L-cell type WDNETs [22]. Both rectal subtypes may be differentiated using serotonin antibody, as the L-cell type is usually negative, while showing PSAP positivity in almost 70% of the cases [20]. There are also clinical differences among WDNETs of different sites as the colic tumors show significantly higher lymph node metastatic rate compared to rectal lesions [2], and almost 30–40% of colic WDNETs present initially with metastatic disease [20]. Although rectal WDNETs are usually considered indolent, metastatic involvement of regional lymph nodes was observed in 33.9% (85/251) tumors, including 9.2% (9/98) of WDNETs smaller than 10 mm [2]. Thus, immunohistochemical confirmation of primary rectal origin in metastatic WDNET can be of practical importance.
Transcription factor SATB2 has been studied extensively in rectal WDNETs, with varying positivity rates (81–96% [3, 7, 21]). Appendiceal WDNETs are also known to be SATB2-positive [3, 7], although the intensity is reportedly lower [3]. Among other NENs, SATB2 was reported in various NECs and a subset of extraadrenal paragangliomas [3]. In our study, we observed 100% positivity rate of rectal WDNETs. In contrast, only 20% (1/5) cecal tumors were SATB2-positive, showing exactly the same positivity rate (20%, 1/5) like in previous report [21]. The positivity rate of appendiceal tumors was similar to the previously published study [3], and we observed no differences in the percentages or H-scores of the tumors. No expression was seen in extraadrenal PGs [17], and this might be due to the use of TMAs, as the immunoreactivity in PGs was reportedly limited to rare cells [3]. The only other SATB2+ WDNET in the study was a lymph node metastasis of small intestinal WDNET, showing only weak immunoreactivity in 10% of cells. While HoxB13 showed lower sensitivity, missing out one SATB2+ rectal tumor, it showed 100% specificity, compared to 93.6% specificity of SATB2.
We were interested if the lack of HoxB13 might reflect the enterochromaffin phenotype. All cecal tumors were CDX2-positive, and majority (80%) produced serotonin. In contrast, although serotonin could be detected in 35.7% (5/14) rectal WDNETs, we observed consistent negativity of CDX2 in rectal tumors. The only HoxB13-negative rectal tumor showed scarce serotonin producing cells, compared to another two rectal WDNETs with strong serotonin immunoreactivity and maintained HoxB13. This is in line with a previous study that was unable to demonstrate CDX2 in a large cohort (n = 56) of rectal WDNETs, while serotonin could be identified in 16% of the tumors [23]. Similar consistent lack of CDX2 was also demonstrated in two additional cohorts including in total 30 rectal WDNETs [24, 30]. Thus, the results suggest that both CDX2 and HoxB13 are related to the respective midgut and hindgut site of primary origin rather than enterochromaffin differentiation.
High specificity of HoxB13 for rectal WDNETs is not surprising, given the role of HoxB13 in embryonal development of caudal endoderm [9, 12]. In fact, immunohistochemical positivity of HoxB13 in normal prostatic tissue and colorectal epithelium has been observed in the past [14], similar to our study. Weak HoxB13 positivity in other normal tissues, including the stomach, thyroid, and small intestine, has been also reported [14]; however, this contradicts both publicly available data on HOXB13 mRNA and protein expression in Human Protein Atlas (HPA) [13] and our results. Indeed, we only observed equivocal staining in rare gland neck cells from one stomach sample but no immunoreactivity of the other structures of normal stomach mucosa, thyroid, or small intestine adjacent to the analyzed tumors.
Due to the well-established expression of HoxB13 in prostate and prostatic adenocarcinoma [9, 14, 15, 25], we were interested in whether HoxB13 immunohistochemistry could be helpful in distinguishing neuroendocrine carcinomas of the prostate and lower urinary tract. In both locations, NECs usually occur as a component of conventional high-grade prostatic adenocarcinomas [26, 27] or urothelial carcinomas [28, 29], sometimes after previous treatment [26], although primary NECs unrelated to treatment or without identifiable component of conventional carcinoma have been described as well [27, 29]. Without appropriate clinical history and radiological findings, the distinction may be impossible, as both NECs tend to lose normal urothelial and prostatic markers [27, 29]. In our study, we observed HoxB13 positivity in 80% (4/5) of prostatic NECs, compared to the lack of HoxB13 in any of the 8 urinary bladder NECs. In contrast, only 25% (1/4 cases, positive case showing strong positivity) prostatic NECs were positive in the previous report [14], and another study demonstrated even lower positivity rate (11.1%, 1/9 cases, positive case with H-score 2) [25]. Furthermore, loss of HoxB13 mRNA expression and the signature of the HOX gene was a hallmark of neuroendocrine transdifferentiation of prostatic adenocarcinomas [25]. However, neuroendocrine transdifferentiation in prostatic adenocarcinoma represents a continuum, and tumors with both neuroendocrine and androgen-dependent luminal phenotype do exist [27]. Of the 4 HoxB13-positive neuroendocrine carcinomas in our study, one tumor morphologically corresponded to Gleason 10 adenocarcinoma with neuroendocrine differentiation (H-score 117.5), and another case (H-score 300) was a de novo carcinoma with intact NKX3.1 expression (Fig. 4A-C). Two remaining cases (H-scores 10 and 115) showed standard neuroendocrine morphology and immunoprofile. However, we cannot exclude that neuroendocrine reprogramming was incomplete, and tumors retained some degree of luminal differentiation.
Conclusions
Our study demonstrated excellent sensitivity and specificity of HoxB13 immunohistochemistry for the diagnosis of rectal WDNETs. SATB2 showed higher sensitivity but lower specificity for rectal origin. However, coexpression of both markers was seen exclusively in rectal WDNETs indicating its diagnostic potential. Interestingly, our study observed higher positivity rate of HoxB13 in prostatic NECs compared to the literature. While this suggests that HoxB13 may be useful for identifying a subset of prostatic NEC, the small number of prostatic tumors in our study and the previous contradictory results require further validation of its utility as a marker of prostatic NECs.
Data Availability
The results of immunohistochemistry of individual tumors together with the clinical data and histology images (in the form of “*.svs” formatted image files) used and analyzed during the current study are available from the corresponding author on reasonable request. The results of immunohistochemistry of all analyzed colorectal tumors are included as Supplementary Table 1.
References
Rindi G, Wiedenmann B Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol 8: 54-64, 2011.
Kojima M, Ikeda K, Saito Net al. Neuroendocrine Tumors of the Large Intestine: Clinicopathological Features and Predictive Factors of Lymph Node Metastasis. Front Oncol 6: 173, 2016.
Bellizzi AM SATB2 in neuroendocrine neoplasms: strong expression is restricted to well-differentiated tumours of lower gastrointestinal tract origin and is most frequent in Merkel cell carcinoma among poorly differentiated carcinomas. Histopathology 76: 251–264, 2020.
Lin X, Saad RS, Luckasevic TM, Silverman JF, Liu Y Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol 15: 407-414, 2007.
Soukup J, Manethova M, Faistova Het al. Pitx2 is a useful marker of midgut-derived neuroendocrine tumours - an immunohistochemical study of 224 cases. Histopathology 81: 799-807, 2022.
Tseng IC, Yeh MM, Yang CY, Jeng YM NKX6-1 Is a Novel Immunohistochemical Marker for Pancreatic and Duodenal Neuroendocrine Tumors. Am J Surg Pathol 39: 850-857, 2015.
Zhao LH, Chen C, Mao CYet al. Value of SATB2, ISL1, and TTF1 to differentiate rectal from other gastrointestinal and lung well-differentiated neuroendocrine tumors. Pathol Res Pract 215: 152448, 2019.
Bellizzi AM Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: what can brown do for you? Hum Pathol 96: 8–33, 2020.
Brechka H, Bhanvadia RR, VanOpstall C, Vander Griend DJ HOXB13 mutations and binding partners in prostate development and cancer: Function, clinical significance, and future directions. Genes Dis 4: 75-87, 2017.
Zeltser L, Desplan C, Heintz N Hoxb-13: a new Hox gene in a distant region of the HOXB cluster maintains colinearity. Development 122: 2475-2484, 1996.
Aires R, de Lemos L, Novoa Aet al. Tail Bud Progenitor Activity Relies on a Network Comprising Gdf11, Lin28, and Hox13 Genes. Dev Cell 48: 383–395 e388, 2019.
Sreenath T, Orosz A, Fujita K, Bieberich CJ Androgen-independent expression of hoxb-13 in the mouse prostate. Prostate 41: 203-207, 1999.
Uhlen M, Fagerberg L, Hallstrom BMet al. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015.
Varinot J, Cussenot O, Roupret Met al. HOXB13 is a sensitive and specific marker of prostate cells, useful in distinguishing between carcinomas of prostatic and urothelial origin. Virchows Arch 463: 803-809, 2013.
Kristiansen I, Stephan C, Jung Ket al. Sensitivity of HOXB13 as a Diagnostic Immunohistochemical Marker of Prostatic Origin in Prostate Cancer Metastases: Comparison to PSA, Prostein, Androgen Receptor, ERG, NKX3.1, PSAP, and PSMA. Int J Mol Sci 18, 2017.
Asa SL, Mete O, Schuller U, Ramani B, Mirchia K, Perry A Cauda Equina Neuroendocrine Tumors: Distinct Epithelial Neuroendocrine Neoplasms of Spinal Origin. Am J Surg Pathol, 2022.
Soukup J, Manethova M, Kohout Aet al. Cauda equina neuroendocrine tumors show biological features distinct from other paragangliomas and visceral neuroendocrine tumors. Virchows Arch, 2022.
Bockmayr M, Korner M, Schweizer L, Schuller U Cauda equina paragangliomas express HOXB13. Neuropathol Appl Neurobiol 47: 889-890, 2021.
Lee JP, Hung YP, O’Dorisio TM, Howe JR, Hornick JL, Bellizzi AM Examination of PHOX2B in adult neuroendocrine neoplasms reveals relatively frequent expression in phaeochromocytomas and paragangliomas. Histopathology 71: 503-510, 2017.
Volante M, Grillo F, Massa Fet al. Neuroendocrine neoplasms of the appendix, colon and rectum. Pathologica 113: 19-27, 2021.
Li Z, Yuan J, Wei Let al. SATB2 is a sensitive marker for lower gastrointestinal well-differentiated neuroendocrine tumors. Int J Clin Exp Pathol 8: 7072-7082, 2015.
Kim JY, Kim KS, Kim KJet al. Non-L-cell immunophenotype and large tumor size in rectal neuroendocrine tumors are associated with aggressive clinical behavior and worse prognosis. Am J Surg Pathol 39: 632-643, 2015.
Koo J, Zhou X, Moschiano E, De Peralta-Venturina M, Mertens RB, Dhall D The immunohistochemical expression of islet 1 and PAX8 by rectal neuroendocrine tumors should be taken into account in the differential diagnosis of metastatic neuroendocrine tumors of unknown primary origin. Endocr Pathol 24: 184-190, 2013.
Yang MX, Coates RF, Ambaye Aet al. NKX2.2, PDX-1 and CDX-2 as potential biomarkers to differentiate well-differentiated neuroendocrine tumors. Biomark Res 6: 15, 2018.
Cheng S, Yang S, Shi Y, Shi R, Yeh Y, Yu X Neuroendocrine prostate cancer has distinctive, non-prostatic HOX code that is represented by the loss of HOXB13 expression. Sci Rep 11: 2778, 2021.
Beltran H, Tomlins S, Aparicio Aet al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res 20: 2846-2850, 2014.
Abdulfatah E, Fine SW, Lotan TL, Mehra R De novo neuroendocrine features in prostate cancer. Hum Pathol 127: 112-122, 2022.
Cheng L, Jones TD, McCarthy RPet al. Molecular genetic evidence for a common clonal origin of urinary bladder small cell carcinoma and coexisting urothelial carcinoma. Am J Pathol 166: 1533-1539, 2005.
Wang G, Xiao L, Zhang Met al. Small cell carcinoma of the urinary bladder: a clinicopathological and immunohistochemical analysis of 81 cases. Hum Pathol 79: 57-65, 2018.
La Rosa S, Rigoli E, Uccella S, Chiaravalli AM, Capella C CDX2 as a marker of intestinal EC-cells and related well-differentiated endocrine tumors. Virchows Arch 445: 248-254, 2004
Acknowledgements
We would like to thank Dr. Boris Rychly (Unilabs sro., Bratislava, SK), Dr. Marketa Trnkova (AeskuLab k.s., Prague, CR), Dr. Ludmila Michnova (Military University Hospital Prague, Prague, CR), and Maria Wozniakova (University Hospital Ostrava and Faculty of Medicine, University of Ostrava) for kindly providing cases of CENETs from the archives of their institutions.
Funding
This study was supported by the project BBMRI-CZ LM2023033, Charles University Cooperation Program, research area DIAG and METD, and Project of Czech Ministry of Defense MO 1012. The work was supported by the European Regional Development Fund-Project BBMRI-CZ Biobank network—a versatile platform for the research of the etiopathogenesis of diseases, No: EF16_013/0001674.
Author information
Authors and Affiliations
Contributions
JS, AR, and FG conceived and designed the study; JS, MM, and VS procured and reviewed the cases included in the study; TC and DN procured clinical data of the tumors and histological material of CENETs and helped with the study design; FG procured clinical data; HH implemented, validated, and performed the immunohistochemical procedures; MM, JS, and VS analyzed the results of the study; JS performed statistical analysis. All authors contributed to writing and reviewing the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki and with the approval of the Ethics Committee of University Hospital Hradec Kralove (reference no. 202101P06 and no. 202109P01).
Competing Interests
The authors declare no competing interests.
Disclaimer
Funding sources were not involved in study design, collection, analysis, or interpretation, in the writing of the report, or in the decision to submit the article for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soukup, J., Manethova, M., Stejskal, V. et al. Immunoreactivity of HOXB13 in Neuroendocrine Neoplasms Is a Sensitive and Specific Marker of Rectal Well-Differentiated Neuroendocrine Tumors. Endocr Pathol 34, 333–341 (2023). https://doi.org/10.1007/s12022-023-09779-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-023-09779-9